Abstract
A study was conducted to evaluate the potential of autologous bone marrow‐derived cells in comparison with buffy coat of autologous blood for rapid cutaneous wound healing in rabbit model. Three square full‐thickness skin excisional wounds were created in 15 selected experimental animals (rabbit) divided randomly into three groups. The wound was treated with autologous bone marrow cells in plasma (group 1), buffy coat of blood in plasma (group 2) and autologous plasma as control (group 3). Wounds were observed for 30 days for granulation tissue formation, biochemical, histomorphological and histochemical evaluation. In this study, granulation tissue appeared significantly lesser in wounds of group 3 animals followed by group 2 and 1 animals. Neovascularisation, granulation tissue formation, denser, thicker and better arranged collagen fibres, reticulin fibres and elastin fibres formation was more in group 1 as compared with other groups. It was concluded that the application of bone marrow‐derived nucleated cells into the wound margins resulted in early and significantly faster rate of complete healing as compared with buffy coat of autologous blood and autologous plasma (control). This approach may be beneficial in various surface wounds that heal at a slower rate and recommended for healing of various complicated wound in future.
Keywords: Autologous bone marrow, Buffy coat, Rabbits, Wound healing
INTRODUCTION
Wound healing is a complicated and well‐orchestrated process that involves three classical phases namely, inflammatory, proliferative and maturation phase. Despite appreciable advances in understanding the basic principles of wound healing, problems in wound healing continue to cause significant morbidity and suffering in animals. The conventional stages of wound healing include irrigation, mechanical and chemical debridement, the use of antiseptics and antimicrobials, adherent and non adherent dressings and miscellaneous topical applications. In majority cases, the optimal way to treat such wounds is management as an open wound 1, 2. Second‐intention wound healing occurs by wound contraction, reepithelialisation and formation of granulation tissue (3). Subsequently, granulation tissue develops within 5–7 days from underlying connective tissue to cover a wound 4, 5. Although, topical application of different drugs and medicines or agents has shown to favour healing (6), the subject is still controversial (7). Extensive research in this aspect has been conducted for treatment of open wounds and it has been found that topical medicaments either do not affect wound healing or inhibit it 5, 8, 9. Wounds are dynamic and treatment for healing may need to be tailored during the sequence of the healing through different phases. It is therefore critical to select ideal agents thoughtfully and scientific rationale for optimal healing environment.
Bone marrow cells are found to be useful for the skin healing process 10, 11. The bone marrow cells contain inflammatory progenitor cells and mesenchymal stem cells which are capable of differentiating into numerous cell types. Bone marrow‐derived stem cells have a unique ability to renew themselves by mitotic division as well as they can differentiate into wide spectrum of cells as it is seen during embryonic development (12). In adult, stem and progenitor cells are involved in different repair process and also maintain the normal turnover of regenerative organs like skin. Because of their plasticity to differentiate, and the fact that bone marrow cells are not lineage restricted, these cells can be easily adjusted when transplanted on foreign tissues. Bone marrow‐derived nucleated cells (BMNCs) are also capable of producing various cytokines and growth factors which are also triggering factor to accentuate the healing process (13). It is therefore assumed that the bone marrow cells may take part in active inflammation for the repair and healing of chronic non healed wounds (14). Besides, BMNCs fill the dermis of the skin and it has been reported that it altered and/or senescent the chronic wounds. Thus the bone marrow cells seem to be a logical candidate for the treatment of chronic wounds especially for the cutaneous wounds 10, 11, 15, 16.
The aim of this study was therefore to evaluate the potential of autologous bone marrow‐derived cells in comparison with buffy coat of autologous blood for rapid cutaneous wound healing in rabbit.
MATERIALS AND METHODS
Animals
Fifteen clinically healthy rabbits of either sex, weighing between 2 and 3 kg and aged between 3 and 9 months were selected for the current experiment. The animals were maintained individually at room temperature of 12:12‐hour dark and light circle along with green vegetables, gram and water ad libitum. The Institute Animal Ethics Committee of West Bengal University of Animal and Fishery Sciences, India approved this study.
Experimental design
Fifteen selected experimental animals were divided randomly into three groups (n = 5 for each group; Table 1). Three square full‐thickness skin excisional wounds were created at the prepared site and bone marrow aspiration was carried out aseptically under xylazine hydrochloride at 5 mg/kg body weight intramuscularly and ketamine hydrochloride at 35 mg/kg body weight (17). Thus each group was evaluated for a total of 15 wounds. Post‐operatively, a broad spectrum antibiotic enrofloxacin at 5 mg/kg body weight once daily for five consecutive days and an analgesic meloxicam at 0·2 mg/kg intramuscularly were administered to all the animals for 3 consecutive days from the day of operation.
Table 1.
Different experimental groups and their respective treatment
| Group | Number of animals | Treatment |
|---|---|---|
| 1 | 5 | Bone marrow in the autologous plasma |
| 2 | 5 | Buffy coat in the autologous plasma |
| 3 | 5 | Autologous plasma (control) |
Bone marrow aspiration, separation and count of BMNCs
Under general anaesthesia with xylazine and ketamine as described previously, the anteromedial aspect of each proximal tibia was prepared aseptically. Bone marrow aspiration was carried out as per the method described by Crow and Walshaw (18) with some modifications. After excising the skin and muscle, a 20 gauge needle was inserted into the proximal medullary cavity of tibia after drilling the bone with micro motor dental drill. A 10 ml disposable syringe containing 5000 IU of heparin was used and negative pressure was applied by forcefully pulling back on syringe plunger. Approximately, 3 ml of bone marrow aspirate was collected from each tibia. Bone marrow nucleated cells were collected from the marrow aspirate by volume reduction centrifuge ‘buffy coat' protocol as per the method described by Kasten et al. (19) with some modifications. The mononuclear cells were isolated from bone marrow aspirant using density gradient centrifugation. After collecting (aspirating) fluid from bone marrow (0·5–1 ml), dilution was made with phosphate buffered saline (PBS) (pH 7·2) to make the volume up to 3 ml. The diluted fluid was layered onto Hisep® at the ratio of 1:3 (one part of Hisep and three parts of diluted fluid) and centrifuged for 30 minutes at 400 g. Following centrifugation, the mononuclear cell rich interface layer (buffy coat) was collected using a micropipette, transferred into clean sterile test tube and washed thrice with PBS. Viable cells were enumerated by the trypan blue dye exclusion method using Neubauer's counting chamber and 2 × 106 cells/ml was adjusted in sterile PBS for instillation (1 ml) into the experimental wound site.
Wound creation
After collection of bone marrow from the tibia, the hair from the dorsum (thoracolumber region) of the rabbits were shaved and cleaned for aseptic surgery. Three experimental wounds were outlined using a clean transparency sheet template and permanent marker. Three square full‐thicknesses 2 × 2 cm of skin including subcutaneous tissue were excised with surgical blade, two in one side with 2 cm gap and one in opposite side of dorsal midline. Haemorrhage, if any, was controlled by digital gauze pressure. All wounds were cleaned/irrigated and treated on the same day of wound creation as per treatment protocol described previously.
Evaluation of wound healing
The wounds were evaluated on the basis of clinical examination, macroscopical observations, histopathological and histochemical examination of biopsy specimens.
Clinical examination and macroscopic evaluation of the wound healing
Clinical parameters such as rectal temperature, heart rate and respiration rate were recorded on days 0, 1, 2 and 3 after the surgery to assess the general condition of the animal and of the wound.
Exudate
Quantity of exudate was evaluated subjectively and graded as: none: 1 (apparently dry wound); slight exudate: 2 (wound is moist but no oozing on pressing); moderate: 3 (wound is moist and there is slight oozing on pressing); extreme: 4 (exudate is evident and slight pressing leads to excessive exudation).
Type of exudates was graded as: dry cast: 1, slight: 2, fibrinious: 3 and purulent: 4
Peripheral swelling was recorded as: no swelling: 1; slight: 2, moderate: 3 and marked: 4
Evaluation of granulation tissue
Evaluation of granulation tissue was categorised as per Bigbie et al. (20) with some modifications as follows: no granulation tissue seen: 1; granulation tissue depressed below the skin edge: 2; granulation tissue proliferated to the level of skin edges: 3; granulation tissue elevated above skin edges: 4; granulation tissue elevated above skin edges, projecting over the advancing border of epithelium: 5.
Colour of granulation tissue: it was scored as: pale yellow: 1, pale red: 2 and pink: 3.
Time of appearance of granulation tissue was recorded as the first day when the granulation tissue was observed.
Gross morphology
Clinical assessment of healing was carried out during the course of wound dressing by observing the general appearance of the wound, granulation tissue, colour of the wound site, any discharge and smell from the wound area. Any other changes like evidence of abnormal granulation tissue were also keenly recorded.
Biochemical studies
Serum alkaline phosphatase (ALP), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were estimated according to the method described by Kind and King (21), Coyle and Arvan (22) and Reitman and Frankel (23), respectively. The above parameters were recorded in days 0, 3, 7, 28 and 30.
Collection of tissue samples and processing for histomorphological and histochemical study
For histomorphological and histochemical study, tissue samples were collected from the margin of the wound on days 7, 14, 21 and 30 post‐operation without affecting the healing process significantly. All the autopsy samples were preserved in 10% neutral buffer formalin.
The preserved tissues were processed routinely and microscopic sections of 5‐µ thickness were prepared. From each tissue, slides were prepared in quadruplet, one for histomorphology and other three for histochemistry. Histomorphological sections were subjected to routine Haematoxylin and eosin stain and histochemical sections were stained for collagen with Masson's trichrome stain (24), reticulin with Gridley's modification of the silver impregnation method (25) and elastin with Weigert's Resorcin–Fuschin method (26).
Collagen/elastin/reticulin formation. Absent – 0; small amount of thin collagen – 1; in between 1 and 3–2; diffuse thick collagen – 3; wavy collagen – 4.
Statistical method
Independent samples equality of mean test tested all parameters for each day due to two different treated groups by student ‘t’ and level of significance of test was either 5% or 1%. If significance of probability of rejection is more than 0·05, we concluded no significant difference between two treated groups. Statistical Package for Social Scientists (Windows version 10·0) was used for statistical analyses of the data.
RESULTS
Clinical examination and macroscopic evaluation of the wound healing
Clinical parameters
Heart rate, rectal temperature and respiration rate of all animals during the period of observation were within normal physiological range. No difference (P > 0·05) between the groups was observed for these parameters.
Exudate
Quantity of exudates
On day 1, some wounds were moist with slight oozing on pressure in most of the animals. By day 3 after operation, most of the wound became dry and by the day 7 all the wounds were completely dried. Slight exudation was observed in all the groups initially. The exudation was decreased gradually and no exudation remained after day 3 in any group. No significant (P > 0·05) variation was noticed among the different groups.
Type of exudates. No difference (P > 0·05) in the mean score of type of exudates among the different treatments at different time interval was found (Table 2).
Table 2.
Mean ± standard error values of score for type of exudates in excisional wound of rabbits of different treatment groups at different days of interval
| Treatment | Post‐wounding days | ||||||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 7 | Day 14 | Day 21 | Day 30 | |
| Group 1 | 1·50 ± 0·11 | 1·25 ± 0·12 | 1·14 ± 0·09 | 1·00 ± 0·01 | 1·00 ± 0·02 | 1·00 ± 0·11 | 1·00 ± 0·01 |
| Group 2 | 1·49 ± 0·11 | 1·23 ± 0·09 | 1·12 ± 0·06 | 1·00 ± 0·01 | 1·01 ± 0·00 | 1·00 ± 0·01 | 1·01 ± 0·01 |
| Group 3 | 1·56 ± 0·11 | 1·21 ± 0·12 | 1·04 ± 0·03 | 1·00 ± 0·01 | 1·00 ± 0·01 | 1·01 ± 0·00 | 1·01 ± 0·01 |
Peripheral swelling
There was no significant (P > 0·05) difference in the mean score among the different groups. Initially in few animals, there was slight to moderate swelling at the periphery but gradually it was subsided. Finally, by day 7 no wounds were visible with any degree of peripheral swelling.
Evaluation of granulation tissue
The mean values of score for granulation tissue and colour in excisional wounds of rabbits in various treatments groups at a particular time point were similar (P > 0·05). The time of appearance of granulation tissue seen was significantly earlier (P < 0·05) in group 1 when compared with other groups (Table 3).
Table 3.
Mean ± standard error values of day when granulation tissue was first seen in excisional wound of rabbits of different treatment groups at different days of interval *
| Category | Mean ± standard error |
|---|---|
| Group 1 | 3·22 ± 0·21b |
| Group 2 | 3·86 ± 0·41a |
| Group 3 | 4·82 ± 0·43a |
*Values with different superscript differ significantly (P < 0·05).
Gross morphology
The salient observations of the photographic view suggest that all the wounds created aseptically were almost equidimentional on day 0. Contraction of the wound was initiated by the day 3 and the wound become dry on day 7. Appreciable reduction of size of the wound of group 1 was observed on day 2 when compared with other groups. Moreover, complete granulation of the wound without scab along with prominent wound contraction in group 1 on day 21 was noticeably different from other groups. Remarkable epithelialisation and closure of wound in group 1 was distinguished on day 21. By the end of 1‐month study, compared with the other groups, complete healing and haring were discernible in group 1 only.
Biochemical studies
The mean of values of serum ALT, AST, ALP and γ‐glutamyltransferase (GGT) levels recorded in the rabbit of different groups at different time intervals have been shown (Table 4). It was found that this study did not affect (P > 0·05) the serum ALT, ALP, AST and GGT levels which were noticed to be within normal physiological range.
Table 4.
Serum ALT, AST and ALP (IU/l) level estimated during different periods of observation (n = 5 for each group)
| Days | Group 1 | Group 2 | Group 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ALT | AST | ALP | ALT | AST | ALP | ALT | AST | ALP | |
| 0 | 32.5 | 20.2 | 65.4 | 35.3 | 24.6 | 3.2 | 34.9 | 25.3 | 69.8 |
| 3 | 32.4 | 21.4 | 65.8 | 35.4 | 24.3 | 3.0 | 35.3 | 25.3 | 70.2 |
| 7 | 32.5 | 21.3 | 65.0 | 35.3 | 24.5 | 3.2 | 35.3 | 25.5 | 70.4 |
| 28 | 32.2 | 21.1 | 64.9 | 35.6 | 24.4 | 3.1 | 35.4 | 25.3 | 69.6 |
| 30 | 32.5 | 21.5 | 65.0 | 35.3 | 24.5 | 3.3 | 35.5 | 25.2 | 69.9 |
| Pulled standard error of the mean | 0.047 | 0.112 | 0.65 | 0.070 | 0.073 | 0.063 | 0.053 | 0.046 | 0.069 |
| P value | 0.284 | 0.240 | 0.125 | 0.842 | 0.855 | 0.515 | 0.138 | 0.473 | 0.116 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Histomorphological study
On day 7 in group 1 animals, the epidermal layer showed discontinuity in their line of union. Lymphocytic aggregression in dermal layer is moderate. A large amount of collagen fibres are running all throughout the layers of skin. Vascularisation in dermal layer is moderate. Fibroblastic cells are very numerous in group 2 animals, the epidermal layer showed proliferation of squamous epithelium. Below epidermal cells, collagenous stroma laid underneath the structures. The cellularity of mononuclear cells is moderate. Exudation in between collagen fibre and fibroblast are in proper volume indicating initiation of healing process. Vascularisation and proliferation of fibroblast all throughout the section are in normal range of healing. In group 3 animals, the uppermost epithelium showed discontinuity in many places. The fissure form below the epidermal layer is almost avascular although budding of collagenous patch in the inner layer of fissure has initiated, diminishing the gap around wound bridges. The collagen formation in dermal stroma is low to moderate. Inflammatory cells namely macrophages, neutrophil and lymphocyte are very scanty. A point of congestion is noticed at the dermal layer giving nutrition to the surrounding damaged cells (Figure 1).
Figure 1.
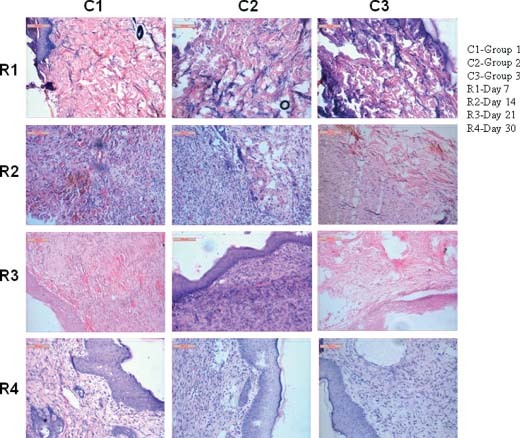
Photomicrographs showing histological disposition of healing tissues of skin at different days of interval.
On day 14 in group 1 animals, the tremendous amount of angiovascularisation exerts a very nice harmony repairing process. The collateral and branches neovessels have given ampoule chance to provide nutrition for proper functioning of regeneration procedure. Scavenger's cells (dermal macrophages) and lymphocytes are abundant in this lesion giving possibilities of quick healing mechanisms. In group 2 animals, the proliferating fibroblast and matured collagen fibres form the stroma of damaged wound. A portion of isolated fibrous encapsulation consisting of macrophages, lymphocytes and red cells along with fibrin network make a nidus for regenerating the skin wound. Fibroblast proliferation in dermal and epidermal layer is supported by newly formed capillaries. In group 3 animals, the epidermal layers showed discontinuity of bridging the wound. The blood vessels, collagen matrix and fibroblast are proliferating below the dermal layer to close the gap of the wound. The vascularisation by red cells and other inflammatory cells are scanty to moderate. The clumping of collagen fibre and swelling of the same indicate the work in exigency to close the gap for the wound. Below dermal layer, the cellular orientations are quite normal. Although, some patches of discontinuity arise because of improper healing (Figure 1).
On day 21 in group 1 animals, the epidermal layer showed a moderate amount of hyperplasia. Beneath the layer, the vascular beds are scattered nicely all throughout the section. Collagen formation and cellular infiltration are properly marked in this lesion. The newly formed capillaries invaded all epidermis and dermis even at hypodermal area showing proper alignment to close the gap of wound. In group 2 animals, the superficial squamous epithelium is swollen; hyperplastic and still they take a basic colour indicating the proliferation of epidermal layer. Below epidermal layer, the fibroblast cells are stout and robust in appearance attaching to newly formed blood capillary in different angle. Collagenous stroma is less numerous in respect of fibroblastic maturation. Cellular infiltrations by mononuclear cells are quite evident. In group 3 animals, the collagen fibres are in proliferating mode. Below the epidermal layer, the newly formed clumped fibroblasts invade the dermal part of skin. The proliferating cells are scanty and they are mainly mononuclear cells. The vascularisation needs to be hampered thus, causing the failure to gap the fissures (Figure 1).
On day 30 in group 1 animals, the squamous epithelium showed proper regeneration of superficial tissue (epidermis) with active nucleoli and mitotic activity. Beneath the epidermis, the vascularisation of the stromal tissue is moderate showing encouraging healing activity. Besides, fibroblast proliferation and collagenous tissue formation are moderate indicating healing process almost in a finishing stage. The roll of macrophages and other mononuclear cells is vividly visible. In group 2 animals, the epidermal layer showed a large number of hyperplastic epithelial regeneration. Keratin formations over the epidermis are moderate. Below epidermis, the layer of stratum germinativum showed moderate amount of angioinvasion with bulk collagen formation. The layer of fibroblast in stratum basali showed proliferating nature of regeneration. The nucleoli of epithelium and fibroblast showed enlargement in their nucleolus and cytoplasm is faintly granular. Below epidermis, connective tissue proliferation and blood vascularisation are moderate to normal indicating a proper healing process is going on. In group 3 animals, the epidermal layer showed enlarged robust squamous epithelium with a tendency to swollen up. The keratin formations in epidermal edges are very poor to moderate. Fibrovascular proliferations beneath epidermis are moderate indicating the positive healing effect. Although the number of macrophages and other inflammatory cells are lower than the previous two cases (Figure 1).
Histochemical study
Collagen
On day 7 in group 1 animals, collagen fibres are running in all directions to the affected wound. Inflammatory reactions are scanty. It is graded as 3. In group 2 animals, collagen fibres are moderate to thin although the amount in all throughout the section is scanty. It is graded as 2. In group 3 animals, amount of collagen is very small and architecture is thin. The cellular reactions are also minimal. It is graded as 1 (Figure 2).
Figure 2.
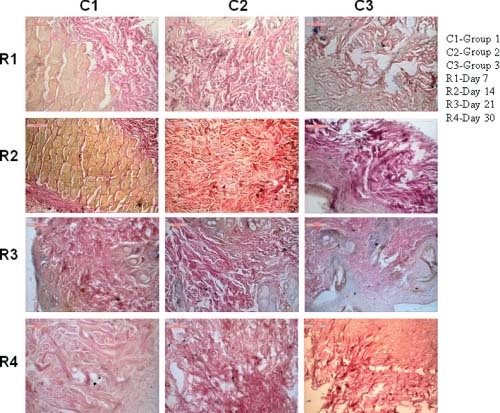
Photomicrographs showing collagen fibre disposition in different groups at different days of interval.
On day 14 in group 1 animals, collagen fibres are robust in nature, thick and wavy indicating a fine fibrous for initiating healing process. It is graded as 4. In group 2 animals, amount of collagen is moderate to high and their structures are robust, thick and less wavy. It is graded as 3. In group 3 animals, few collagen fibres are formed, their structure indicates poor response in healing aspect. It is graded as 1 (Figure 2).
On day 21 in group 1 animals, collagen fibres are thick, clumsy running in all directions in tortuous manner. Other related inflammatory process is quite. It is graded as 4. In group 2 animals, collagen fibres run in all throughout the section but they are thin and less robust. Cellular reactions are moderate. It is graded as 3. In group 3 animals, concentrations of collagen are thin, running in some part of section with moderate inflammatory reaction. It is graded as 2 (Figure 2).
On day 30 in group 1 animals, orientation of collagen fibres are wavy, stout and thick indicating satisfactory healing process. It is graded as 4. In group 2 animals, collagen fibres are moderate but structures are robust and wavy. Cellular reactions are quite satisfactory to fill the gap of wound. It is graded as 3. In group 3 animals, moderate to low response of collagen fibre proliferation which is almost flat, short and tortuous. In some portion, the cellular margins are left with some gap. It is graded as 2 (Figure 2).
Elastin
On day 7 in group 1 animals, the section depicted mild proliferation of elastin fibre around the hair follicle and dermal matrix. The area below dermis also showed interwoven bundle of elastin along with collagen. Cellular responses are mild proliferation of mononuclear cell in the layer of stratum germinativum. In group 2 animals, the section depicted elastin fibre proliferation on and around hair follicle, stratum germinativum and stratum spinosum together with lymphocytic and few macrophage infiltration. In group 3 animals, the section depicted elastin fibre proliferation in dermal and epidermal layer. The fibres are thin and scanty with minimal mononuclear cell infiltration (Figure 3).
Figure 3.
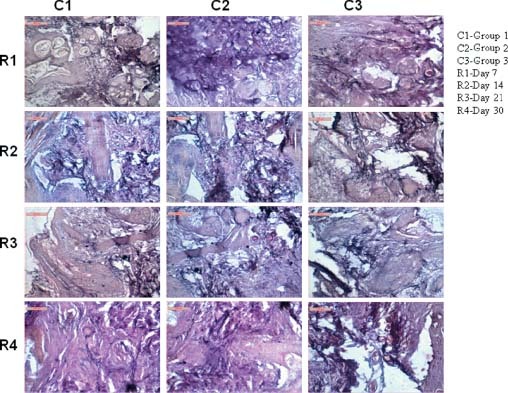
Photomicrographs showing elastin fibre disposition in different groups at different days of interval.
On day 14 in group 1 animals, the section depicted moderate proliferation of elastin on epidermal and subepidermal layer. The basal layer of stratum germinativum is also admixture with few elastin fibres. The elastin structures are moderately thick and running in different direction of dermal stroma. In group 2 animals, the section depicted elastin fibre proliferation and numerous to moderate in amount. Fibres are aggregated in some places as cluster while in dermal layer, most of the elastin fibres are solitary and comparatively thinner in appearance. Cellular reaction is moderate. In group 3 animals, the section depicted scanty elastin fibre proliferation in epidermal layer although in dermal layer, their proliferation makes a mesh work around the hair follicles and other glandular structure. Cellular reaction is minimal (Figure 3).
On day 21 in group 1 animals, the section depicted large number of elastin fibre running all throughout the skin parenchyma. The long, slender and tortuous elastin fibre encircled the hair follicle. In group 2 animals, the section depicted large number of elastin fibre admixtured with collagenous stroma running deep into the dermal layer. Some portion of stratum germinativum and stratum granulose showed proliferation of elastin in epidermal stroma. In group 3 animals, the elastin fibres are numerous in dermal layer along with faint collagenous stroma. Cellular reaction is very minimal (Figure 3).
On day 30 in group 1 animals, the section depicted fine network of collagen and elastin fibres, run all throughout the section along with blood vessels adventitious layer. The cellularity and vascularity of dermal stroma are fairly satisfactory. In group 2 animals, the section depicted clump of fibres formed in between dermal and epidermal layer. Some portion of elastin are also encircled the blood vessels and hair follicles. Mononuclear cell reaction in epidermal layer is found scanty. In group 3 animals, the section depicted network of elastin fibres especially encircling blood vessels and hair follicles. Other dermal stroma showed less number of elastin fibres in some part of stratum spinosum and stratum germinativum. Dermal layer showed moderate vascularisation along with collagen fibre formation (Figure 3).
Reticulin
On day 7 in group 1 animals, enormous number of reticulin fibre was found within the granulation tissue and number was more as compared with groups 1 and 2. In group 2 animals, distinct reticulin fibres were showed within the dermal component of the skin. Fibres were most prominent as compare with group 3. In group 3 animals, scanty amount of reticulin fibres were scatterly distributed within the granulation tissue (Figure 4).
Figure 4.
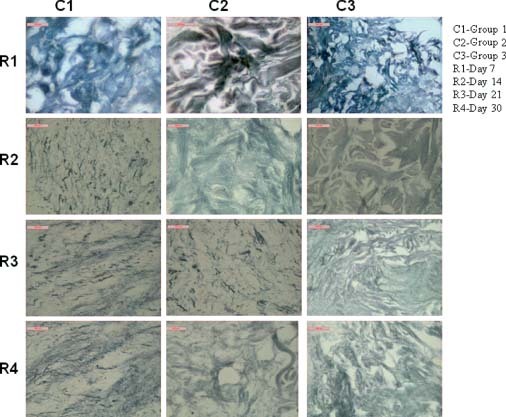
Photomicrographs showing reticulin fibre disposition in different groups at different days of interval.
On day 14 in group 1 animals, numerous reticulin fibres were present in the section. In group 2 animals, abundant reticulin fibre network was showed on day 14 of wound healing which was more as compared with day 7. In group 3 animals, it was showed that the reticulin fibres were distributed within the dermal component but the frequency of reticulin fibre was almost similar as compared with day 7 (Figure 4).
On day 21 in group 1 animals, reticulin fibres were scatterly distributed within the dermis of the healing tissue which was denser as compared with group 2. In group 2 animals, numerous reticulin fibres were distributed within the healing tissue and they are mostly distributed within the dermal component of the skin. In group 3 animals, in the control group, scanty numbers of reticulin fibres were appeared within the healing tissue (Figure 4).
On day 30 in group 1 animals, numerous small branched reticulin fibres were showed within the dermal component as compared with group 2. In group 2 animals, the section of day 30 healing tissue showed a large number of reticulin fibres distributed within the dermal component as compared with control group. In group 3 animals, the section taken from the control group on day 30 showed appearance of reticulin fibres within the dermal component. Quantitatively, the reticulin fibre was less as compare with groups 1 and 2 (Figure 4).
DISCUSSION
Bone marrow cells are found to contribute significantly to the skin healing process 10, 11. There is increased evidence of bone marrow cells actively taking part in the formation of skin cells such as fibroblasts and keratinocytes. Bone marrow cells are mainly involved in the formation of dermal fibroblast population during healing (10). Bone marrow mesenchymal cell/artificial dermis composite graft was found an effective therapy for skin regeneration in the patients with intractable dermatopathies (27). Bone marrow fluid was aspirated from the ilium and cultured in medium containing either fetal calf or autologous serum. The resulting cultured cells were placed in artificial dermis made of collagen sponge, and this composite graft was used to treat skin wounds. Bone marrow also contains mesenchymal stem cells (BMSCs) that secrete a large number of growth factors and cytokines, which are critical to proper repair and regeneration of damaged.
Considering the reported potential of bone marrow‐derived cells to secrete growth factors and cytokines and the ability to trans‐differentiate it to the healing tissue components, we hypothesised that there would be better healing of the wounds treated with bone marrow cells than with buffy coat of autologous blood or autologous plasma (control). It was assumed that excisional wounds treated with bone marrow cells would heal faster with more granulation tissue formation, epithelisation, rapid wound contraction, early histological maturation and complete healing than buffy coat of autologous blood or autologous plasma (control) group.
In this study, the mean values of rectal temperature, respiratory rate and heart rate in different treatment groups were within the physiological limits throughout the observation period, which suggested that the surgical wounds were free from infection.
Initially, some wounds were moist with slight oozing on pressure in most of the animals. By day 3 post‐operation, most of the wound became dry and by day 7 all the wounds were completely dried. Slight exudation was observed in all the groups initially. The exudation was decreased gradually and no exudation was remained after day 3 in all the groups. Wound surface became moist in initial days by escaping of fluid into the tissue, the migrating leucocytes and dead tissue leading to inflammatory exudates (28). Moist wound environment allows optimal healing by hastening autolytic debridement by endogenous enzymes that break down necrotic tissue, promoting granulation tissue formation and fast epithelialisation. However, the potential disadvantages for moist wound healing include bacterial colonisation of the wound surface, folliculitis and maceration of the wound border (29).
There was no significant difference (P > 0·05) in the mean score of swelling of the wound among the different groups. Initially in few animals there was slight to moderate swelling at the periphery but gradually it was subsided. Finally, by day 7 no wounds were visible with any degree of peripheral swelling. This observation was quite obvious as the procedure did not hamper the normal healing at all. Slight to moderate swelling could be attributed to the normal inflammatory mechanisms of the body in favour of tissue repair to bring the wound edges in apposition during wound healing (30). These findings were also corroborated with the observations of Borena et al. (31).
The granulation tissue of the wound is mainly composed of fibroblasts, collagen fibres and small new blood vessels. The collagen is the major component of extracellular tissue which provides support and strength (32). Granulation tissue is of paramount necessity for the healing of wounds because it is extremely resistant to infection and serves as a barrier against systemic infection, provides a surface over which epithelium is able to migrate, plays a vital role in wound contraction and contains the fibroblasts that produce the collagen for wound healing (28). In this study, granulation tissue appeared significantly lesser in wounds of group 3 animals followed by group 2 and 1 animals. Higher amount of granulation tissue formation appeared in group 1 animals indicating that bone marrow‐derived cells triggered granulation tissue genesis 33, 34 which also corroborated the histological findings of early deposition of relatively more granulation tissue with more cellularity in group 1 animals as compared with other two groups.
Histopathological assessment depicted mode and rate of healing in wounds with more precision than clinical and high resolution ultrasound (35). In this study, serial examinations were possible as the biopsies were collected at different stages of wound healing like on days 7, 14, 21 and 30 post‐operative days which helped in providing sequential evaluation of wound healing. In this study wider and more compact granulation tissue and neovascularisation were evident in group 1 animals as compared with wounds in group 2 and 3 animals because of marked infiltration with fibroblasts. As inflammation plays a vital role in combating infection and inducing the proliferation phase, it is essential for healing (36). In wound healing process there is a marked gradient of oxygen that exists within the wound with the centre of the wound most deficient. This gradient may be partially responsible for the branching and ingrowth of new blood vessels in the form of capillaries, from endothelial cells of wound periphery into the wound (28).
More neovascularisation, denser, thicker and better arranged collagen, reticulin and elastin fibres were also recorded in the sections of test wounds than control. Pittenger et al. (12) reported that bone BMSCs are multiotential stem cells capable of differentiation into numerous cell types including fibroblasts, bone, cartilage and muscles. When tissues are disrupted following injury, collagen is needed to repair the defect and restore anatomic structure and function (37). In summary, the application of BMNCs into the wound margins (group 1) resulted in early and significantly faster rate of complete healing as compared with buffy coat of autologous blood and autologous plasma (control). Histological and histochemical observations showed that the bone marrow cells augmented wound healing activity significantly by increasing cellular proliferation, formation of granulation tissue, neovascularisation, synthesis of collagen, epithelisation and early histological maturation in excisional wounds. The application of BMNCs offers several advantages such as simple and easy method, immediate application using simple processing procedure, no immunological reaction, cost effective, and so forth and may have tremendous prospect to enhance wound healing in routine clinical cases.
ACKNOWLEDGEMENTS
The authors sincerely acknowledges the help and facilities provided by the Dean, Faculty of Veterinary and Animal Sciences, West Bengal University of Animal and Fishery Sciences, Kolkata, India for the research work. The first author has done the experimental work. The other authors have conceptualized the work, analyzed the data and gone through the final version of the manuscript.
REFERENCES
- 1. Lee AH, Swaim SF, Yang ST, Wilken LO. Effects of gentamicin solution and cream on the healing of open wound. Am J Vet Res 1984;45:1487–92. [PubMed] [Google Scholar]
- 2. Yamada KM. Cell surface interaction with extracellular materials. Annu Rev Biochem 1983;52:761–99. [DOI] [PubMed] [Google Scholar]
- 3. Swaim SF, Hinkle SH, Bradley DM. Wound contraction: basic and clinical factors. Compendium 2001;23:20–4. [Google Scholar]
- 4. Layton CE. Nutritional support of the surgical patient. In: Harari J, editor. Surgical complications and wound healing in the small animal practice. Philadelphia, PA: Saunders, 1993:89–125. [Google Scholar]
- 5. Lee AH, Swaim SF, McGuire JA, Hughes KS. Effects of non‐adherent dressing materials on the healing of open wound in dogs. J Am Vet Med Assoc 1987;190:416–22. [PubMed] [Google Scholar]
- 6. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–46. [DOI] [PubMed] [Google Scholar]
- 7. Harari J. Surgical complication and wound healing in the small animal practice. Philadelphia, PA: Saunders, 1993:78–9. [Google Scholar]
- 8. Swaim SF. Wound healing. In: Swaim SF, editor. Small animal wound management. Baltimore: William & Wilkins, 1997:1–12. [Google Scholar]
- 9. Sardari K, Mirshahi A, Maleki M, Aslani MR, Barjasteh MN. Effects of topical Allicin on second intention wound healing in dogs (histological aspects). Comp Clin Pathol 2006;15:1177–81. [Google Scholar]
- 10. Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, Isik F. Contribution of bone marrow‐derived cells to skin: collagen deposition and wound repair. Stem Cells 2004;22:812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deng W, Han Q, Liao L, Li C, Ge W, Zhao Z, You S, Deng H, Murad F, Zhao RC. Engrafted bone marrow‐derived flk‐(11) mesenchymal stem cells regenerate skin tissue. Tissue Eng 2005;11: 110–9. [DOI] [PubMed] [Google Scholar]
- 12. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–7. [DOI] [PubMed] [Google Scholar]
- 13. Yongbo L, Deborah S, Dulchavsky PA, Gao X, Kwon D, Chopp M, Dulchavsky S, Gautam SC. Wound repair by bone marrow stromal cells through growth factor production. J Surg Res 2006;136:336–41. [DOI] [PubMed] [Google Scholar]
- 14. Neagos D, Mitran V, Chiraku G, Ciurab R, Lanku C, Cimpean A, Iordachescu D. Skin wound healing in a free floating fibroblast populated collagen lattice model. Roman J Biophy 2006;16: 157–68. [Google Scholar]
- 15. Kataoka K, Medina RJ, Kageyama T, Miyazaki M, Yoshino T, Makino T, Huh NH. Participation of adult mouse bone marrow cells in reconstitution of skin. Am J Pathol 2003;163:1227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakagawa H, Akita S, Fukui M, Fujii T, Akino K. Human mesenchymal stem cells successfully improve skin‐substitute wound healing. Br J Dermatol 2005;153:29–36. [DOI] [PubMed] [Google Scholar]
- 17. Kahn CM. Merck veterinary manual, 9th edn. Whitehouse Station, NJ: Merck and Co Inc, 2005:149–50. [Google Scholar]
- 18. Crow SE, Walshaw SQ. Manual of clinical procedures in the dog, cat and rabbit. Lippincott Williams and Wilkins, 1997:169–278. [Google Scholar]
- 19. Kasten P, Luginbuhl R, Feebuer K, Suda AJ, Egermann M, Gasser B, Beyen I. Instant mesenchymal stem cell therapy: how can we do it? Characterization and concentration of mesenchymal stem cells in vitro. Eur Cell Mater 2007;14:39. [DOI] [PubMed] [Google Scholar]
- 20. Bigbie RB, Schumacker J, Swaim SF, Purohit KC, Wright JC. Effect of amnion and yeast cell derivate on second intention healing in horses. Am J Vet Res 1991;52:1376–82. [PubMed] [Google Scholar]
- 21. Kind PRN, King EJ. Alkaline phosphatase activity determination in serum. J Clin Path 1954;7:322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coyle M, Arvan DA. Glucose 6‐phosphate dehydrogenase activity in erythrocytes. Absence of diurnal variation; Amer. J Clin Path 1969;51:777–9. [DOI] [PubMed] [Google Scholar]
- 23. Reitman S, Franke S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Amer J Clin Path 1957;28:56–63. [DOI] [PubMed] [Google Scholar]
- 24. Masson P. Trichrome staining and their preliminary techniques. J Tech Methods 1929;12:75–90. [Google Scholar]
- 25. Gridley MF. A modification of the silver impregnation method of staining reticulin fibers. Amer J Clin Path 1951;21:897–9. [DOI] [PubMed] [Google Scholar]
- 26. Mallony FB. Pathological techniques. Philadelphia, PA: Saunders Co, 1961. [Google Scholar]
- 27. Yoshikawa T, Mitsuno H, Nonaka I, Sen Y, Kawanishi K, Inada Y, Takakura Y, Okuchi K, Nonomura A. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg 2008;121:860–77. [DOI] [PubMed] [Google Scholar]
- 28. Swaim SF, Henderson RA. Small animal wound management. Philadelphia, PA: Lea and Fabiger, 1990:1–33. [Google Scholar]
- 29. Fossum TW, Hedlund CS, Hulse DA, Johnson AL, Seim HB, Willard MD, Carroll GL. Surgery of integumentary system. In: Hedlund CS, editor. Manual of small animal surgery, 2nd edn. St. Louis: Mosby, 2007:132–228. [Google Scholar]
- 30. Kumar A. Veterinary surgical techniques. New Delhi: Vikas Publishing House, 1996:151–65. [Google Scholar]
- 31. Borena BM, Pawde AM, Aithal HP, Amarpal, Kinjavdekar P, Singh R, Kumar D. Autologous bone marrow‐derived cells for healing excisional dermal wounds of rabbits. Vet Rec 2009;165:563–8. [DOI] [PubMed] [Google Scholar]
- 32. Singh SDJ, Krishna V, Manikani KL, Manjunatha BK, Vidya SM, Monohara YN. Wound healing activity of the leaf extracts and de‐oxyelephantopin isolated from Elephantopus scaber Linn. Indian J Pharmacol 2005;37:237–42. [Google Scholar]
- 33. Badiavas EV, Abedi M, Butmare J, Falanga V, Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol 2003;196:245–50. [DOI] [PubMed] [Google Scholar]
- 34. Badiavas EV, Falanga V. Treatment of chronic wound with bone marrow derived cells. Arch Dermatol 2003;139:510–6. [DOI] [PubMed] [Google Scholar]
- 35. Abaramo F, Martins P, Lloyd DH, Auxilia ST, Noli C, Leotta R, Pfeiffer D. An evaluation of methods for the assessments of healing of open wounds in the dog. Vet Dermatol 2004;15:13. 14989700 [Google Scholar]
- 36. Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. J Biochem Cell Biol 2004;36:1031–7. [DOI] [PubMed] [Google Scholar]
- 37. Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, Pecoraro RE, Rodeheaver G, Robson MC. Definition and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 1994;130:489–93. [PubMed] [Google Scholar]


