Abstract
Chronic wounds such as diabetic foot wounds are a tremendous burden to the health care system and often require a multidisciplinary approach to prevent amputations. Advanced technologies such as negative pressure wound therapy (NPWT) and bioengineered tissues have been successfully used in the treatment of these types of complex wounds. However, the introduction of NPWT with instillation (NPWTi) has provided an alternative treatment for treating complex and difficult‐to‐heal wounds. This article provides an overview of NPWT and the new NPWTi system and describes preliminary experience using NPWTi on patients with complicated infected diabetic foot wounds after surgical debridement and in a multidisciplinary setting.
Keywords: Diabetic foot wounds, Instillation therapy, Negative pressure wound therapy
Introduction
Chronic wounds form a tremendous burden to the health care system, accounting for about $20 billion in health care costs per year worldwide 1. Foot ulceration is the precursor to approximately 85% of all diabetic amputations, with an estimated 14–20% of patients with foot ulcers undergoing an amputation 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15. Infection of the ulcer further increases the risk of amputation. If these patients were initially treated by a multidisciplinary team, major amputations could be prevented in 80–90% of them with limb‐threatening ischaemia 16, 17, 18, 19, 20, 21 and in 95% with infection 22, 23, 24, 25. This is significant considering that amputations are related to high morbidity or mortality rates and a financial burden of up to $60 000 per patient.
The treatment of the diabetic foot wounds requires a multidisciplinary approach. The treatment of peripheral vascular disease, infection and pathological plantar pressure plays a significant role in the global management of complex wounds. The topical treatment of wounds using advanced wound dressings has not shown consistent results. More promising perspectives have been obtained in the treatment of neuropathic wounds owing to the introduction of bioengineered tissue in clinical practice 26, 27, 28, 29 and negative pressure wound therapy (NPWT) via Vacuum‐Assisted Closure Therapy (V.A.C.® Therapy; KCI USA, Inc., San Antonio, TX).
This NPWT system uses a reticulated open‐cell foam that is modelled to fit into the wound. The foam is subsequently covered and sealed with a semi‐occlusive film. The tubing pad is attached to a 2‐cm2 aperture, which is cut on the surface of the dressing, thereby allowing a connection with the foam, whereas the other end of the tubing system is attached to the therapy unit that delivers negative pressure to the wound and provides either continuous or intermittent pressure.
NPWT has been shown to help wound healing in various ways. Tests on animals have demonstrated that NPWT decreased bacterial burden in wounds, changing them from infected wounds to colonised wounds within 4–5 days of treatment 30. Other postulated mechanisms of action that might affect wound healing are the induction of an increased local wound perfusion 31, 32, the induction of microdeformations at the wound surface and the removal of infectious material and wound fluids, including inhibitory factors 31, 32, 33. These mechanisms may explain how NPWT stimulates granulation tissue formation in comparison with wet‐to‐moist dressings. NPWT has also been shown to be an effective treatment of both complicated and non‐complicated ulcerated wounds 30, 31, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44.
Prior to 2006, only two randomised controlled trials (RCTs) that had evaluated the clinical effectiveness of NPWT in the treatment of diabetic foot wounds were found in the literature 35, 37. In the first study, McCallon et al. included only ten patients but found faster healing and greater wound surface reduction when NPWT was used compared with gauze dressings 35. In the second study, Armstrong and Lavery 37 published a large multicentre RCT investigating the effectiveness of NPWT on open diabetic foot amputations. The control group was treated with advanced moist wound dressings according to standard guidelines of the participating centres. Treatment with NPWT resulted in a statistically significant reduction in healing time, a higher percentage of healed wounds and a potential reduction in the number of reamputations 37. However, less than 50% of the patients reached complete healing during the 112‐day follow‐up period. Besides treating ulcerated chronic wounds, it has been shown that NPWT is effective in improving the qualitative and quantitative take‐rate of skin grafts in venous leg ulcers 45 and several other wound types but not in diabetic foot ulcers 46, 47.
Previous authors, most notably Fleischmann as well as Svedman, have discussed the infusion of a variety of solutions into the wound 48, 49, 50, 51, 52, 53, 54, 55. The initial work of Fleischmann led to the development of the V.A.C.® Instill Wound Therapy System (KCI USA, Inc.), which combines NPWT with instillation (NPWTi) and consists of a vacuum port and an infusion port with two clamps to allow for separate infusion and vacuum periods. This process involves a ‘hold’ cycle during which fluid is held in the foam during a pause in NPWT.
During the last 2 years, a new device has been developed as the next generation of instillation therapy. The V.A.C.Ulta™ Therapy System (KCI USA, Inc.) is an integrated system that allows standard NPWT as well as an instillation option using V.A.C. VeraFlo™ Therapy (KCI USA, Inc.). With this combination system, it is possible to deliver topical wound cleansing solutions to the wound bed. Complex and difficult‐to‐heal‐wounds can be managed, avoiding the need for a separate NPWT unit. This new NPWTi system uses dressings (V.A.C. VeraFlo Dressing; KCI USA, Inc.) specifically designed for instillation therapy, which have an open pore structure that is similar to the black foam dressings used with NPWT. These dressings have reduced hydrophobic properties and provide greater mechanical strength for use during instillation therapy, which may help to prevent tearing at dressing changes.
It is well recognised that wound preparation plays a key role in creating an optimal environment for wound healing. Regular cleansing of the wound can help to address the barriers to healing by removing devitalised tissue, debris, infectious materials and exudate and help prepare the wound bed for closure 56. Compared with swabbing or bathing, wound irrigation is considered to be the most consistently effective method of wound cleansing 57.
Initially, this new instillation system was used adjunctively in infected ulcers using antiseptic solutions; however, its use has now been expanded to include cleansing regimens that can help to remove debris, exudate, infectious materials and healing inhibitors 51, 58. Several publications describe various clinical applications of adjunctive instillation therapy, most of which focus on the treatment of wound infection. The instillation of polyhexanide [polyhexamethylene biguanide (PHMB)] solution showed effective treatment of soft tissue necrotising fasciitis and osteomyelitis when used in combination with other treatments 59, 60. Lehner et al. also reported that the same regimen was an effective adjunctive therapy for acutely and chronically infected orthopaedic implants 61. Table 1 lists the studies that demonstrate the clinical advantages of using NPWTi.
Table 1.
Literature review of instillation therapy
| Title | Study type | Aim | Method | Findings | |
|---|---|---|---|---|---|
| Bernstein and Tam 62 | Combination of subatmospheric pressure dressing and gravity feed antibiotic instillation in the treatment of post‐surgical diabetic foot wounds | Case series of five patients | To evaluate the effect of NPWT with instillation of saline, polymyxin B and bacitracin in the treatment of diabetic foot wounds |
|
|
| Gabriel et al. 49 | Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds | Pilot study, NPWT with instillation: n = 15, control: n = 15 | To compare the effect of NPWT with instillation to treatment with moist gauze wound care in patients with complex, infected wounds |
|
|
| Timmers et al. 60 | Negative pressure wound treatment with polyvinyl alcohol foam and polyhexanide antiseptic solution instillation in posttraumatic osteomyelitis | Retrospective case–control cohort study of 30 patients | To evaluate the effect of NPWT plus instillation in patients with osteomyelitis of the pelvis or lower extremity |
|
|
| Schintler et al. 59 | The impact of V.A.C. Instill® in severe soft tissue infections and necrotising fasciitis | Case series of 15 patients treated with NPWT plus instillation | To evaluate the effect of NPWT with instillation on patients with skin and soft tissue infections |
|
|
| Lehner et al. 61 | First experiences with negative pressure wound therapy and instillation in the treatment of infected orthopaedic implants: a clinical observational study | 32 patients with an infected orthopaedic implant were included in this observational study | To observe the effect of NPWTi on orthopaedic implant retention following an acute or chronic infection |
|
|
| Wolvos 63 | The use of negative pressure wound therapy with an automated, volumetric fluid administration: an advancement in wound care | Pilot study including six patients treated with NPWTi with either Dressing A or Dressing B; Dressing A: n = 5, Dressing B: n = 1 | To introduce initial clinical experience with NPWTi and a comparison of two different dressings |
|
|
NPWT, negative pressure wound therapy; NPWTi, NPWT with instillation.
Case studies
The use of NPWTi in the treatment of diabetic foot ulcers is only recent. The following cases describe the preliminary experience using NPWTi on patients with complicated infected wounds after surgical debridement and in a multidisciplinary setting.
Case 1
A 68‐year‐old male with type 2 diabetes presented with a wet gangrene in the fourth and fifth toes of the right foot and compartment syndrome of the plantar aspect of the midfoot (Figure 1A). He presented a critical limb ischaemia treated with an angioplasty of superficial femoral artery and anterior tibial artery. An open amputation of the lateral rays was performed. Methicillin‐resistant Staphylococcus aureus (MRSA) was isolated and appropriate antibiotic therapy was used. During the postoperative period after debridement (Figure 1B) and bleeding control, NPWTi using polyhexanide (PHMB) was applied with a soak time of 15 minutes, followed by NPWT for 2 hours at −125 mmHg. NPWTi was carried out for 7 days with progression of granulation tissues to prepare the wound bed for dermal substitute application (Integra™ dermal regeneration template; Integra Lifescience, Plainsboro, NJ) (Figure 1C). The wound was completely healed at follow‐up (Figure 1D).
Figure 1.
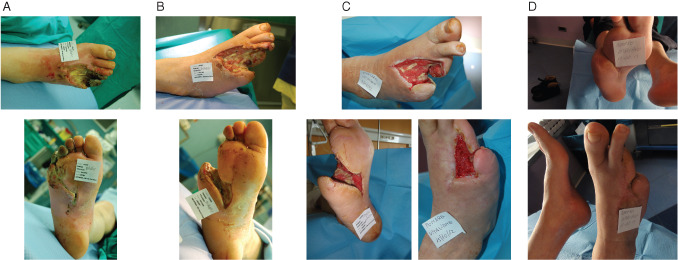
(A) Initial presentation, (B) 11 days after surgical debridement, (C) 14 days after treatment with negative pressure wound therapy with instillation (NPWTi) using polyhexamethylene biguanide (left) and 42 days after coverage with dermal substitute (right). (D) Wound was completely healed at follow‐up.
Case 2
A 42‐year‐old male with type 1 diabetes presented with wet gangrene of the fourth ray of the right foot and with progression to necrotising fasciitis of the lateral aspect of the leg (Figure 2A). Critical limb ischaemia was assessed, and after emergent surgical debridement (Figure 2B), a percutaneous angioplasty below the knee of the anterior and posterior tibial artery was performed. MRSA and Serratia marcescens were isolated and appropriately treated with complex antibiotic regimen. NPWTi using polyhexanide (PHMB) was applied with a soak time of 15 minutes, followed by NPWT for 2 hours at −125 mmHg (Figure 2C). Wound received dermal replacement therapy followed by first skin graft application (Figure 2D). The wound healed after application of second graft (Figure 2E).
Figure 2.
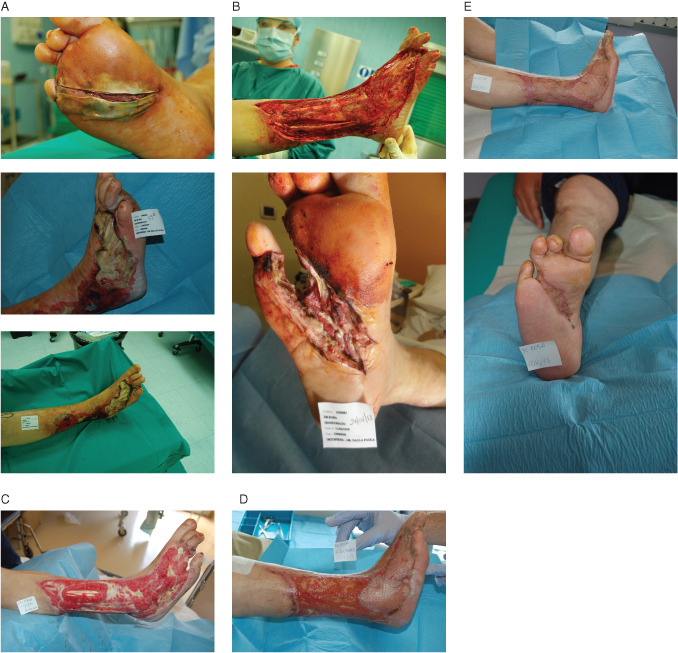
(A) Initial presentation, (B) 3 days after surgical debridement and revascularisation, (C) 15 days after treatment with negative pressure wound therapy with instillation (NPWTi) using polyhexamethylene biguanide, (D) 60 days after dermal replacement therapy and first skin graft and (E) 30 days after healing with second skin graft application.
Conclusion
NPWTi has become an alternative option for treating complex, chronic wounds, which is supported by several studies in the literature. By using the new NPWTi system with automated volumetric fluid instillation, we have demonstrated its successful use for the treatment of DFUs. Larger studies are required to further investigate the efficacy of NPWTi on DFUs.
Acknowledgements
Dr LDP presented as a faculty member during the 2012 International Surgical Wound Forum (ISWF), an annual educational event sponsored by Kinetic Concepts, Inc. (KCI). His article is part of a KCI‐funded educational supplement based on faculty presentations at 2012 and 2013 ISWF sessions related to wound care strategies with a focus on use of negative pressure wound therapy with instillation (i.e. V.A.C. Instill® Wound Therapy and V.A.C. VeraFlo™ Therapy; KCI, San Antonio, TX). KCI assisted with editorial review of the manuscript.
Dalla Paola L. Diabetic foot wounds: the value of negative pressure wound therapy with instillation.
References
- 1. Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999;22:382–7. [DOI] [PubMed] [Google Scholar]
- 2. Muller IS, de Grauw WJ, van Gerwen WH, Bartelink ML, van Den Hoogen HJ, Rutten GE. Foot ulceration and lower limb amputation in type 2 diabetic patients in Dutch primary health care. Diabetes Care 2002;25:570–4. [DOI] [PubMed] [Google Scholar]
- 3. Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, et al. The North‐West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community‐based patient cohort. Diabet Med 2002;19:377–84. [DOI] [PubMed] [Google Scholar]
- 4. Trautner C, Haastert B, Spraul M, Giani G, Berger M. Unchanged incidence of lower‐limb amputations in a German City, 1990–1998. Diabetes Care 2001;24:855–9. [DOI] [PubMed] [Google Scholar]
- 5. Henriksson F, Agardh CD, Berne C, Bolinder J, Lonnqvist F, Stenstrom P, Ostenson CG, Jonsson B. Direct medical costs for patients with type 2 diabetes in Sweden. J Intern Med 2000;248:387–96. [DOI] [PubMed] [Google Scholar]
- 6. Abbott CA, Vileikyte L, Williamson S, Carrington AL, Boulton AJ. Multicenter study of the incidence of and predictive risk factors for diabetic neuropathic foot ulceration. Diabetes Care 1998;21:1071–5. [DOI] [PubMed] [Google Scholar]
- 7. van Houtum WH, Lavery LA, Harkless LB. The impact of diabetes‐related lower‐extremity amputations in The Netherlands. J Diabetes Complications 1996;10:325–30. [DOI] [PubMed] [Google Scholar]
- 8. Witso E, Ronningen H. Lower limb amputations: registration of all lower limb amputations performed at the University Hospital of Trondheim, Norway, 1994–1997. Prosthet Orthot Int 2001;25:181–5. [DOI] [PubMed] [Google Scholar]
- 9. Global Lower Extremity Amputation Study Group . Epidemiology of lower extremity amputation in centres in Europe, North America and East Asia. The Global Lower Extremity Amputation Study Group. Br J Surg 2000;87:328–37. [DOI] [PubMed] [Google Scholar]
- 10. Spichler ER, Spichler D, Lessa I, Costa e Forti A, Franco LJ, LaPorte RE. Capture‐recapture method to estimate lower extremity amputation rates in Rio de Janeiro, Brazil. Rev Panam Salud Publica 2001;10:334–40. [DOI] [PubMed] [Google Scholar]
- 11. Wrobel JS, Mayfield JA, Reiber GE. Geographic variation of lower‐extremity major amputation in individuals with and without diabetes in the Medicare population. Diabetes Care 2001;24:860–4. [DOI] [PubMed] [Google Scholar]
- 12. van Houtum WH, Lavery LA. Regional variation in the incidence of diabetes‐related amputations in The Netherlands. Diabetes Res Clin Pract 1996;31:125–32. [DOI] [PubMed] [Google Scholar]
- 13. Chaturvedi N, Abbott CA, Whalley A, Widdows P, Leggetter SY, Boulton AJ. Risk of diabetes‐related amputation in South Asians vs. Europeans in the UK. Diabet Med 2002;19:99–104. [DOI] [PubMed] [Google Scholar]
- 14. Kumar S, Ashe HA, Parnell LN, Fernando DJ, Tsigos C, Young RJ, Ward JD, Boulton AJ. The prevalence of foot ulceration and its correlates in type 2 diabetic patients: a population‐based study. Diabet Med 1994;11:480–4. [DOI] [PubMed] [Google Scholar]
- 15. Walters DP, Gatling W, Mullee MA, Hill RD. The distribution and severity of diabetic foot disease: a community study with comparison to a non‐diabetic group. Diabet Med 1992;9:354–8. [DOI] [PubMed] [Google Scholar]
- 16. Larsson J, Apelqvist J, Agardh CD, Stenstrom A. Decreasing incidence of major amputation in diabetic patients: a consequence of a multidisciplinary foot care team approach? Diabet Med 1995;12:770–6. [DOI] [PubMed] [Google Scholar]
- 17. Faglia E, Dalla Paola L, Clerici G, Clerissi J, Graziani L, Fusaro M, Gabrielli L, Losa S, Stella A, Gargiulo M, Mantero M, Caminiti M, Ninkovic S, Curci V, Morabito A. Peripheral angioplasty as the first‐choice revascularization procedure in diabetic patients with critical limb ischemia: prospective study of 993 consecutive patients hospitalized and followed between 1999 and 2003. Eur J Vasc Endovasc Surg 2005;29:620–7. [DOI] [PubMed] [Google Scholar]
- 18. Holstein P, Ellitsgaard N, Olsen BB, Ellitsgaard V. Decreasing incidence of major amputations in people with diabetes. Diabetologia 2000;43:844–7. [DOI] [PubMed] [Google Scholar]
- 19. Faglia E, Favales F, Aldeghi A, Calia P, Quarantiello A, Barbano P, Puttini M, Palmieri B, Brambilla G, Rampoldi A, Mazzola E, Valenti L, Fattori G, Rega V, Cristalli A, Oriani G, Michael M, Morabito A. Change in major amputation rate in a center dedicated to diabetic foot care during the 1980s: prognostic determinants for major amputation. J Diabetes Complications 1998;12:96–102. [DOI] [PubMed] [Google Scholar]
- 20. Faglia E, Mantero M, Caminiti M, Caravaggi C, De Giglio R, Pritelli C, Clerici G, Fratino P, De Cata P, Dalla Paola L, Mariani G, Poli M, Settembrini PG, Sciangula L, Morabito A, Graziani L. Extensive use of peripheral angioplasty, particularly infrapopliteal, in the treatment of ischaemic diabetic foot ulcers: clinical results of a multicentric study of 221 consecutive diabetic subjects. J Intern Med 2002;252:225–32. [DOI] [PubMed] [Google Scholar]
- 21. Edmonds ME, Foster AV. Reduction of major amputations in the diabetic ischaemic foot: a strategy to “take control” with conservative care as well as revascularisation. Vasa 2001;30(Suppl 58):6–14. [Google Scholar]
- 22. Holstein PE, Sorensen S. Limb salvage experience in a multidisciplinary diabetic foot unit. Diabetes Care 1999;22(Suppl 2):B97–103. [PubMed] [Google Scholar]
- 23. Rosenblum BI, Pomposelli FB Jr, Giurini JM, Gibbons GW, Freeman DV, Chrzan JS, Campbell DR, Habershaw GM, LoGerfo FW. Maximizing foot salvage by a combined approach to foot ischemia and neuropathic ulceration in patients with diabetes. A 5‐year experience. Diabetes Care 1994;17:983–7. [DOI] [PubMed] [Google Scholar]
- 24. Lipsky BA. Infectious problems of the foot in diabetic patients. In: Bowker JH, Pfeifer MA, editors. Levin and O'Neal's the diabetic foot, 6th edn. St. Louis: Mosby, Inc., 2001:467–80. [Google Scholar]
- 25. Grayson ML, Gibbons GW, Balogh K, Levin E, Karchmer AW. Probing to bone in infected pedal ulcers. A clinical sign of underlying osteomyelitis in diabetic patients. JAMA 1995;273:721–3. [PubMed] [Google Scholar]
- 26. Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet‐derived growth factor‐BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo‐controlled double‐blind study. Diabetes Care 1998;21:822–7. [DOI] [PubMed] [Google Scholar]
- 27. Caravaggi C, De Giglio R, Pritelli C, Sommaria M, Dalla Noce S, Faglia E, Mantero M, Clerici G, Fratino P, Dalla Paola L, Mariani G, Mingardi R, Morabito A. HYAFF 11‐based autologous dermal and epidermal grafts in the treatment of noninfected diabetic plantar and dorsal foot ulcers: a prospective, multicenter, controlled, randomized clinical trial. Diabetes Care 2003;26:2853–9. [DOI] [PubMed] [Google Scholar]
- 28. Veves A, Falanga V, Armstrong DG, Sabolinski ML, Apligraf Diabetic Foot Ulcer Study . Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers a prospective randomized multicenter clinical trial. Diabetes Care 2001;24:290–5. [DOI] [PubMed] [Google Scholar]
- 29. Pollack RA, Edington H, Jensen JL, Kroeker RO, Gentzkow GD. A human dermal replacement for the treatment of diabetic foot ulcers. Wounds 1997;9:175–83. [Google Scholar]
- 30. Lookingbill DP, Miller SH, Knowles RC. Bacteriology of chronic leg ulcers. Arch Dermatol 1978;114:1765–8. [PubMed] [Google Scholar]
- 31. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 32. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76. [PubMed] [Google Scholar]
- 33. Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum‐assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004;114:1086–96. [DOI] [PubMed] [Google Scholar]
- 34. Joseph E, Hamori CA, Bergman S, Roaf E, Swann NF, Anastasi GW. A prospective, randomized trial of vacuum‐assisted closure versus standard therapy of chronic nonhealing wounds. Wounds 2000;12:60–7. [Google Scholar]
- 35. McCallon SK, Knight CA, Valiulus JP, Cunningham MW, McCulloch JM, Farinas LP. Vacuum‐assisted closure versus saline‐moistened gauze in the healing of postoperative diabetic foot wounds. Ostomy Wound Manage 2000;46:28–34. [PubMed] [Google Scholar]
- 36. Armstrong DG, Attinger CE, Boulton AJ, Frykberg RG, Kirsner RS, Lavery LA, Mills JL. Guidelines regarding negative pressure wound therapy (NPWT) in the diabetic foot. Ostomy Wound Manage 2004;50(4 Suppl):3S–27S. [PubMed] [Google Scholar]
- 37. Armstrong DG, Lavery LA, Diabetic Foot Study Consortium . Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704–10. [DOI] [PubMed] [Google Scholar]
- 38. Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase‐9 to tissue inhibitor of matrix metalloproteinase‐1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen 2002;10:26–37. [DOI] [PubMed] [Google Scholar]
- 39. Katz MH, Alvarez AF, Kirsner RS, Eaglstein WH, Falanga V. Human wound fluid from acute wounds stimulates fibroblast and endothelial cell growth. J Am Acad Dermatol 1991;25(6 pt 1):1054–8. [DOI] [PubMed] [Google Scholar]
- 40. Scherer LA, Shiver S, Chang M, Meredith JW, Owings JT. The vacuum assisted closure device: a method of securing skin grafts and improving graft survival. Arch Surg 2002;137:930–4. [DOI] [PubMed] [Google Scholar]
- 41. Blackburn JH, Boemi L, Hall WW, Jeffords K, Hauck RM, Banducci DR, Graham WP. Negative‐pressure dressings as a bolster for skin grafts. Ann Plast Surg 1998;40:453–7. [DOI] [PubMed] [Google Scholar]
- 42. Raffetto JD, Mendez MV, Marien BJ, Byers HR, Phillips TJ, Park HY, Menzoian JO. Changes in cellular motility and cytoskeletal actin in fibroblasts from patients with chronic venous insufficiency and in neonatal fibroblasts in the presence of chronic wound fluid. J Vasc Surg 2001;33:1233–41. [DOI] [PubMed] [Google Scholar]
- 43. Nakayama Y, Iino T, Soeda S. A new method for the dressing of free skin grafts. Plast Reconstr Surg 1990;86:1216–9. [DOI] [PubMed] [Google Scholar]
- 44. Chang KP, Tsai CC, Lin TM, Lai CS, Lin SD. An alternative dressing for skin graft immobilization: negative pressure dressing. Burns 2001;27:839–42. [DOI] [PubMed] [Google Scholar]
- 45. Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State‐of‐the‐art treatment of chronic leg ulcers: a randomized controlled trial comparing vacuum‐assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg 2006;44:1029–38. [DOI] [PubMed] [Google Scholar]
- 46. Moisidis E, Heath T, Boorer C, Ho K, Deva AK. A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg 2004;114:917–22. [DOI] [PubMed] [Google Scholar]
- 47. Jeschke MG, Rose C, Angele P, Fuchtmeier B, Nerlich MN, Bolder U. Development of new reconstructive techniques: use of Integra in combination with fibrin glue and negative‐pressure therapy for reconstruction of acute and chronic wounds. Plast Reconstr Surg 2004;113:525–30. [DOI] [PubMed] [Google Scholar]
- 48. Svedman P. Irrigation treatment of leg ulcers. Lancet 1983;2(8349):532–4. [DOI] [PubMed] [Google Scholar]
- 49. Gabriel A, Shores J, Heinrich C, Baqai W, Kalina S, Sogioka N, Gupta S. Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J 2008;5:399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fleischmann W, Russ M, Westhauser A, Stampehl M. [Vacuum‐sealing‐technique used as drug release system for topical treatment of wound infections]. Unfallchirurg 1998;101:649–54. [DOI] [PubMed] [Google Scholar]
- 51. Jerome D. Advances in negative pressure wound therapy: the VAC Instill. J Wound Ostomy Continence Nurs 2007;34:191–4. [DOI] [PubMed] [Google Scholar]
- 52. Lehner B, Bernd L. [V.A.C. Instill therapy in periprosthetic infection of hip and knee arthroplasty]. Zentralbl Chir 2007;131(Suppl 1):S160–4. [DOI] [PubMed] [Google Scholar]
- 53. Moch D, Fleischmann W, Westhauser A. [Instillation vacuum sealing – report of initial experiences]. Langenbecks Arch Chir Suppl Kongressbd 1998;115:1197–9. [PubMed] [Google Scholar]
- 54. Fleischmann W, Lang E, Kinzl L. [Vacuum assisted wound closure after dermatofasciotomy of the lower extremity]. Unfallchirurg 1996;99:283–7. [PubMed] [Google Scholar]
- 55. Moch D, Fleischmann W, Russ M. [The BMW (biosurgical mechanical wound treatment) in diabetic foot]. Zentralbl Chir 1999;124(1 Suppl):69–72. [PubMed] [Google Scholar]
- 56. European Wound Management Association (EWMA) . Position document: wound bed preparation in practice. European Wound Management Association, London, UK, 2004. [Google Scholar]
- 57. Ennis WJ, Valdes W, Salzman S, Fishman D, Meneses P. Trauma and wound care. In: Morison Moya, Ovington Liza G., Wilkie Kay. Chronic wound care: a problem‐based learning approach. Mosby, London, UK, 2004:291–307. [Google Scholar]
- 58. Kaehn K, Eberlein T. In‐vitro test for comparing the efficacy of wound rinsing solutions. Br J Nurs 2009;18:S4–6. [DOI] [PubMed] [Google Scholar]
- 59. Schintler MV, Prandl EC, Kreuzwirt G, Grohmann MR, Spendel S, Scharnagl E. The impact of V.A.C. Instill in severe soft tissue infections and necrotizing fasciitis. Infection 2009;37(Suppl 1):31–2. [Google Scholar]
- 60. Timmers MS, Graafland N, Bernards AT, Nelissen RG, van Dissel JT, Jukema GN. Negative pressure wound treatment with polyvinyl alcohol foam and polyhexanide antiseptic solution instillation in posttraumatic osteomyelitis. Wound Repair Regen 2009;17:278–86. [DOI] [PubMed] [Google Scholar]
- 61. Lehner B, Fleischmann W, Becker R, Jukema GN. First experiences with negative pressure wound therapy and instillation in the treatment of infected orthopaedic implants: a clinical observational study. Int Orthop 2011;35:1415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bernstein BH, Tam H. Combination of subatmospheric pressure dressing and gravity feed antibiotic instillation in the treatment of post‐surgical diabetic foot wounds: a case series. Wounds 2005;17:37–48. [Google Scholar]
- 63. Wolvos T. The use of negative pressure wound therapy with an automated, volumetric fluid administration: an advancement in wound care. Wounds 2013;25:75–83. [PubMed] [Google Scholar]


