Abstract
The topical application of the antiseptics octenidine and polyhexanide on wounds seems to improve microcirculation. These two antiseptics were tested in combination with neuronal inhibition and sympathethic receptor blockade to verify these findings, explore the influence of β blockers on these microcirculative effects, and find out the principle of operation. Investigations were carried out on a standardised cremaster muscle model in rats (n = 66). The tested antiseptics, octenidine and polyhexanide were investigated alone (n = 12) and in combination with bupivacaine (n = 12), metoprolol (n = 12), phentolamine (n = 12) and surgical denervation (n = 12). Physiological saline was used for control (n = 6). The arteriolar diameter and functional capillary density (FCD) were investigated via trans‐illumination microscopy before, as well as 60 and 120 minutes after application. Polyhexanide caused a significant increase in arteriolar diameter (86·5 ± 3·8 µm versus 100·0 ± 3·6 µm) and, like octenidine (7·2 ± 0·7 n/0·22 mm2 versus 11·6 ± 0·6 n/0·22 mm2), in FCD (9·2 ± 0·5 versus 12·6 ± 0·9) as well. When the antiseptics are used in combination with bupivacaine, metoprolol, phentolamine or surgical sympathectomy, these effects were eliminated or inverted. Assessing the results of the different blockades in combination with polyhexanide, we surmise that the antiseptic polyhexanide acts on the microcirculation mainly by blocking α receptors. This study shows that polyhexanide and octenidine improve muscular perfusion. Interestingly, the benefit of polyhexanide and octenidine on muscular perfusion is eliminated when the antiseptics are combined with other vasoactive agents, especially β blockers.
Keywords: Antiseptics, Microcirculation, Octenidine, Polyhexanide
INTRODUCTION
If the microcirculation is impaired, for example, by diabetes mellitus, radiation therapy, bacterial infection or peripheral arterial occlusive disease, wound healing is compromised (1). It is therefore crucial that microcirculation not be inhibited additionally by any locally or systemically applied agent. Especially in the case of chronic wounds, it would be beneficial if antiseptics improved microcirculation and epithelialisation. Polyhexanide and, in part, octenidine, seem to fulfil these functions 2, 3, 4, 5, 6.
The reasons for the arteriolar dilation and increase of capillary density are unknown, as is the effect of using these antiseptics in combination with other vasoactive agents.
As a result of the increasing rate of cardiovascular diseases, β blockers are one of the most widely used drugs (7). The β receptors have a decisive function in the adrenergic‐mediated dilation of the arterioles. By blocking this system, the microcirculation could worsen and the wound healing could be delayed or inhibited in patients with already poor health.
To validate the previously shown positive effects of these antiseptics on microcirculation, discover whether they still have a positive influence when combined with widely prescribed β blockers and better understand the functional mechanisms underlying the arteriolar dilation, parts of the vasomodulating system were neutralised. The vaso receptors were inhibited either by α or β blockers and the neuronal influences by local anaesthetics or surgical sympathectomy.
METHODS
Animals
The cremaster muscle of male Wistar rats [n = 66, 188 g (range: 166–201 g), Harlan & Winkelmann, Borchen, Germany] was the subject of this study. The experiments were conducted in accordance with the German laws for the protection of animals. The guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources was followed (NIH Publication No. 86‐23, Revised 1985).
Anaesthesia
Animals were anaesthetised by intraperitoneally injected sodium pentobarbital (Narcoren®, 0·3–0·7 g/kg body weight; Merial GmbH, Hallbergmoos, Germany). To maintain the anaesthesia, one third of the initial dose was injected hourly. To maintain a stable body temperature, the animals were placed on a heated observation platform (Effenberger, Pfaffing, Germany).
After shaving the throat, the left carotid artery was prepared and a probe inserted to monitor the blood pressure (Intramedic Polyehylene Tubing, PE‐50, Becton Dickson, Parippany, NY; blood pressure meter: Monitor BP‐1, World Precision Instruments, Berlin). To stabilise blood pressure, saline solution was injected through the same catheter. A tracheotomy was performed to keep the airway open by tubing (Venofix®, Luer Lock 21G, 0·8 × 2·0 mm, Braun, Melsungen, Germany).
Cremaster preparation
The model was first described by Baez et al. in 1973 (8). After isolating the cremaster muscle, it was stretched using seven loops on a specially designed observation platform (5‐0 Prolene®, Ethicon, Norderstedt, Germany), where the temperature was monitored (9).
Study groups
Octenidine dihydrochloride (n = 6; Octenisept®; bispyridinamine, 0·1%, supplemented by 2% phenoxythanol, Schuelke & Mary GmbH, Norderstedt, Germany) and polyhexanide (n = 6; Lavasept®, biguanide, 0·2%, B. Braun Melsungen AG, Melsungen, Germany) were the tested antiseptic solutions.
The blockade of the α receptors was achieved through topical application of phentolamine hydrochloride (n = 12; Sigma Chemicals, Seelze, Germany), which was dissolved in aqua purificata (20 mg/ml), resulting in a concentration of 5 mg/cm2 at the cremaster muscle 10, 11. The blockade of the β receptors was caused by metoprolol tartrate (n = 12; 3 mg, Beloc®, Astra, Wedel, Germany).
The suppression of the sympathetic fibres was achieved through topical application of bupivacaine (n = 12; 0·5%, Carbostesin®, Astra, Wedel, Germany), resulting in 15 mg bupivacaine hydrochloride. The denervation (n = 12) was performed at the proximal cremaster muscle, where the peri‐arteriolar tissue, including the nerves, was dissected (12).
Octenidine and polyhexanide were used alone (n = 12) and in combination with each of the following treatments: metoprolol (n = 12), phentolamine (n = 12), bupivacaine (n = 12) and surgical denervation (n = 12).
Physiological saline was used for control (n = 6; saline solution; Natriumchlorid, B. Braun Melsungen AG, Melsungen, Germany)
After recording the baseline values, 3 ml of the single (control and denervation group) or combined solutions were applied to the surface of the muscle for 30 minutes and covered with a piece of self‐adherent polyurethane dressing (OpSite™ Dressing Film, Smith and Nephew Wound Management, Largo, FL) to prevent the muscle from drying out. After 30 minutes, the solutions were rinsed out with saline solution. The investigations were repeated after 60 and 120 minutes.
Exclusion criteria
In addition to the arterial blood pressure (80–120 mmHg), the core body temperature (34·5–37·5°C) and the temperature of the cremaster muscle surface (31·0–34·0°C) were measured continuously. Animals whose recorded measurements exceeded or dropped below the determined values were excluded from this study.
Microscopy and recordings
For the trans‐illumination microscopy (Axiotech vario, Carl Zeiss, Oberkochen, Germany) and recording, a charge‐coupled video camera was used (Model Nr. MC‐3309, AVT‐Horn, Aalen, Germany) and the recordings stored on super Video Home System videotapes (Panasonic AG‐7350).
Microscopic observations were performed before application of the agents, as well as 60 and 120 minutes after.
We used a tenfold water immersion objective (Achroplan 4×/20×, Zeiss), resulting in a total magnification of about 100‐fold. Photographs were taken to ensure accurate relocation of the areas throughout this study.
Altogether, nine visual fields with a dimension of 0·55 to 0·40 mm (0·22 mm2) were investigated. The diameter of the first‐ (A1), second‐ (A2) and third‐order (A3) arterioles in micrometre, as well as the perfused capillaries per area, was measured.
Statistics
The commercially available computer program SPSS version 18 (SPSS GmbH, Munich, Germany) was used for statistical analysis of the data. The data collection of the recordings was blinded; the researcher did not know the interval of time between recordings or the agent being tested. Out of the single data, the mean value, the standard error and the standard error of the mean of each animal were calculated.
To compare the different groups with each other, a variance analysis for repeated measurements was used, followed by a post hoc analysis (Tukey). The comparisons of the different points in time within the same group were performed with the t test. The mean value of the significant data was compared with the t test for paired samples. A P < 0·05 was considered statistically significant.
RESULTS
All animals maintained the required core and muscle temperature and their blood pressure stayed within the determined limits.
For the control group, no alterations in arteriolar diameter or functional capillary density (FCD) could be found (1, 2, 3, 4 and Table 1).
Figure 1.
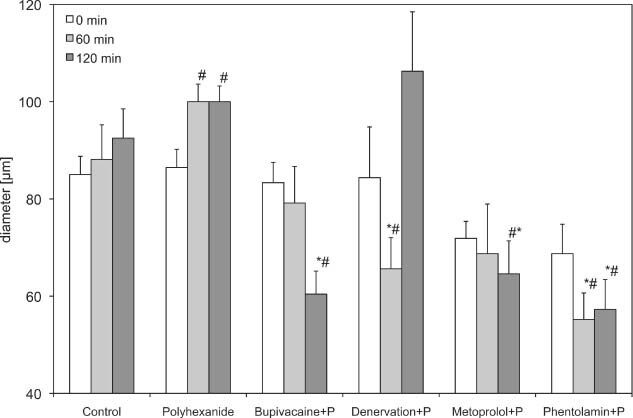
Diameter of first order arterioles (A1) of the polyhexanide group. Recordings were performed before and 60 and 120 minutes after drug application or denervation. Data are given as mean and standard error of mean (each group consists of six rats; *P < 0.05 versus control; # P < 0.05 versus polyhexanide). 256 × 156 mm (600 × 600 DPI).
Figure 2.
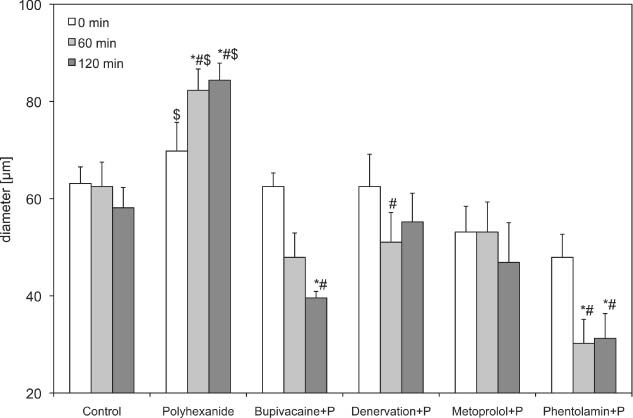
Diameter of second order arterioles (A2) of the polyhexanide groups. Recordings were performed before and 60 and 120 minutes after drug application or denervation. Data are given as mean and standard error of mean (each group consists of six rats; *P < 0.05 versus control; # P < 0.05 versus polyhexanide; $ P < 0.05 versus basic values of polyhexanide). 256 × 156 mm (600 × 600 DPI).
Figure 3.
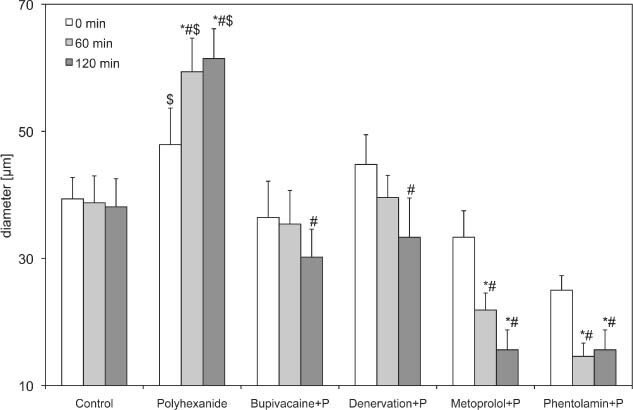
Diameter of third order arterioles (A3) of the polyhexanide groups. Recordings were performed before and 60 and 120 minutes after drug application or denervation. Data are given as mean and standard error of mean (each group consists of six rats; *P < 0.05 versus control; # P < 0.05 versus polyhexanide; $ P < 0.05 versus basic values of polyhexanide). 256 × 156 mm (600 × 600 DPI).
Figure 4.
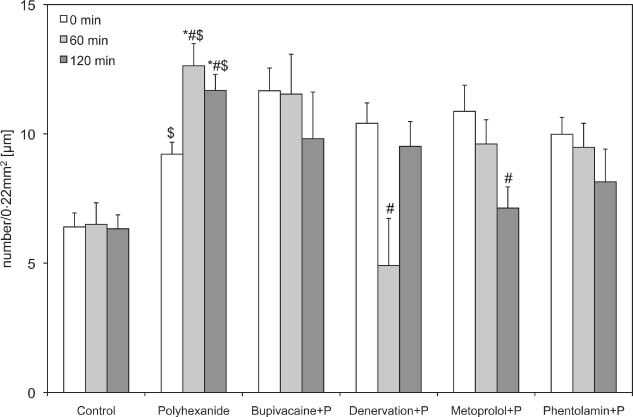
Functional capillary density of the polyhexanide test groups. Recordings were performed before (0) and 60 and 120 minutes after drug application or denervation. Data are given as mean and standard error of mean (each group consists of six rats; *P < 0.05 versus control; # P < 0.05 versus polyhexanide; $ P < 0.05 versus polyhexanide basic values). 256 × 1 56 mm (600 × 600 DPI).
Table 1.
Changes in diameter and FCD of the ten treatment groups †
| Parameter | Time (minutes) | Control | Polyhexanide | Bupivacaine + P | Denervation + P | Metoprolol + P | Phentolamine + P |
|---|---|---|---|---|---|---|---|
| Diameter A1 (µm) | 0 | 85·00 ± 3·75 | 86·46 ± 3·76 | 83·33 ± 4·17 | 84·38 ± 10·43 | 71·88 ± 3·52 | 68·75 ± 6·04 |
| 60 | 88·13 ± 7·12 * | 99·97 ± 3·61 ** | 79·17 ± 7·51 | 65·63 ± 6·40 [Link] , [Link] | 68·75 ± 10·21 | 55·21 ± 5·45 [Link] , [Link] | |
| 120 | 92·50 ± 6·02 * | 99·99 ± 3·23 ** | 60·42 ± 4·75 [Link] , [Link] | 106·25 ± 12·23 | 64·58 ± 6·78 [Link] , [Link] | 57·29 ± 6·13 [Link] , [Link] | |
| Diameter A2 (µm) | 0 | 63·13 ± 3·42 | 69·79 ± 5·91 *** | 62·50 ± 2·80 | 62·50 ± 6·65 | 53·13 ± 5·29 | 47·92 ± 4·75 |
| 60 | 62·50 ± 5·02 * | 82·29 ± 4·39 [Link] , [Link] , [Link] | 47·92 ± 5·02 | 51·04 ± 6·13 ** | 53·12 ± 6·20 | 30·21 ± 4·95 [Link] , [Link] | |
| 120 | 58·13 ± 4·17 * | 84·38 ± 3·52 [Link] , [Link] , [Link] | 39·58 ± 1·32 [Link] , [Link] | 55·21 ± 5·91 | 46·88 ± 8·19 | 31·25 ± 5·10 [Link] , [Link] | |
| Diameter A3 (µm) | 0 | 39·38 ± 3·37 | 47·92 ± 5·74 *** | 36·46 ± 5·69 | 44·79 ± 4·68 | 33·33 ± 4·17 | 25·00 ± 2·28 |
| 60 | 38·75 ± 4·25 [Link] , [Link] | 59·38 ± 5·29 [Link] , [Link] , [Link] | 35·42 ± 5·27 | 39·58 ± 3·49 | 21·88 ± 2·68 [Link] , [Link] | 14·58 ± 2·08 [Link] , [Link] | |
| 120 | 38·13 ± 4·41 [Link] , [Link] | 61·46 ± 4·68 [Link] , [Link] , [Link] | 30·21 ± 4·39 ** | 33·33 ± 6·18 ** | 15·63 ± 3·13 [Link] , [Link] | 15·63 ± 3·13 [Link] , [Link] | |
| FCD (n/0·22 mm2) | 0 | 6·40 ± 0·54 | 9·22 ± 0·46 *** | 11·67 ± 0·88 | 10·41 ± 0·79 | 10·87 ± 1·01 | 9·98 ± 0·66 |
| 60 | 6·50 ± 0·83 | 12·63 ± 0·86 [Link] , [Link] , [Link] | 11·54 ± 1·54 | 4·91 ± 1·82 ** | 9·61 ± 0·93 | 9·48 ± 0·92 | |
| 120 | 6·33 ± 0·54 | 11·68 ± 0·62 [Link] , [Link] , [Link] | 9·81 ± 1·80 | 9·52 ± 0·96 | 7·13 ± 0·82 ** | 8·15 ± 1·26 |
| Parameter | Time | Control | Octenidine | Bupivacaine + O | Denervation + O | Metoprolol + O | Phentolamine + O |
|---|---|---|---|---|---|---|---|
| Diameter A1 (µm) | 0 | 85·00 ± 3·75 | 70·17 ± 2·64 | 96·88 ± 5·99 | 91·66 ± 6·79 | 86·46 ± 4·09 | 84·38 ± 12·16 |
| 60 | 88·13 ± 7·12 | 85·42 ± 1·32 | 68·75 ± 11·64 | 89·59 ± 7·34 | 88·54 ± 5·21 | 93·75 ± 7·03 | |
| 120 | 92·50 ± 6·02 | 92·50 ± 6·02 | 80·21 ± 11·00 | 72·33 ± 7·70 | 87·50 ± 4·56 | 82·29 ± 9·47 | |
| Diameter A2 (µm) | 0 | 63·13 ± 3·42 | 55·21 ± 2·51 | 69·79 ± 6·54 | 63·54 ± 5·69 | 76·04 ± 13·54 | 54·17 ± 6·97 |
| 60 | 62·50 ± 5·02 | 61·46 ± 4·09 | 62·50 ± 7·79 | 61·46 ± 7·98 | 69·79 ± 12·12 | 59·38 ± 4·77 | |
| 120 | 58·13 ± 4·17 | 56·25 ± 2·28 | 67·71 ± 6·93 | 51·04 ± 9·19 | 69·62 ± 12·09 | 54·17 ± 5·02 | |
| Diameter A3 (µm) | 0 | 39·38 ± 3·37 | 32·29 ± 1·92 | 34·38 ± 4·78 | 36·46 ± 6·34 | 38·54 ± 7·81 | 34·38 ± 4·77 |
| 60 | 38·75 ± 4·25 | 37·50 ± 2·28 | 31·25 ± 4·28 | 44·79 ± 8·45 | 36·46 ± 6·93 | 31·25 ± 4·27 | |
| 120 | 38·13 ± 4·41 | 35·42 ± 3·09 | 29·16 ± 3·84 | 35·41 ± 6·59 | 39·58 ± 7·68 | 29·17 ± 3·84 | |
| FCD (n/0·22 mm2) | 0 | 6·40 ± 0·54 | 7·18 ± 0·67 *** | 11·59 ± 0·97 | 8·63 ± 0·74 | 9·17 ± 1·01 | 12·46 ± 1·80 |
| 60 | 6·50 ± 0·83 * | 11·58 ± 0·64 [Link] , [Link] , [Link] | 4·46 ± 1·38 ** | 7·33 ± 1·73 | 3·33 ± 1·41 [Link] , [Link] | 13·54 ± 1·99 | |
| 120 | 6·33 ± 0·54 * | 11·20 ± 0·44 [Link] , [Link] , [Link] | 3·13 ± 1·19 ** | 6·85 ± 1·85 | 2·72 ± 1·34 [Link] , [Link] | 14·00 ± 2·32 |
A1, first‐order arterioles; A2, second‐order arterioles; A3, third‐order arterioles; FCD, functional capillary density.
†Each group consists of six rats.
* P < 0·05 vs. control. ** P < 0·05 vs. polyhexanide respectively octenidine, *** P < 0·05 vs. basic values of polyhexanide respectively octenidine.
After application of polyhexanide, the arteriolar diameter in the second‐ and third‐order arterioles increased significantly 60 (99·97 ± 3·61 µm) and 120 minutes (99·99 ± 3·23 µm) after the initial application time, compared with the baseline values (86·46 ± 3·76 µm) and control (Table 1 and 2, 3).
Polyhexanide in combination with bupivacaine led to a significant decrease of the A1‐arteriole (60·42 ± 4·75) and A2‐arteriole diameter (39·58 ± 1·32) after 120 minutes, compared with the control (92·50 ± 6·02 and 58·13 ± 4·17) and isolated polyhexanide groups (99·99 ± 3·23 and 84·38 ± 3·52). The A3 arterioles showed the same tendency, but without significant differences. The FCD decreased slightly, but without significant differences.
After denervation and application of polyhexanide (84·38 ± 10·43), a significant decrease in arteriolar diameter was observed after 60 minutes, compared with the isolated polyhexanide group (1, 2, 3). In the A1 arterioles, an increase could be observed after 120 minutes, but without significant differences. The FCD decreased significantly 1 hour after denervation and polyhexanide application, and increased after 2 hours to the original baseline value.
The blockade of β receptors by metoprolol used in combination with polyhexanide caused a decrease in first and second arterioles and a significant decrease of third‐order arterioles, compared with polyhexanide alone (21·88 ± 2·68 µm versus 59·38 ± 5·29 µm after 60 minutes and 15·63 ± 3·13 µm versus 61·46 ± 4·68 µm after 120 minutes) and control (38·75 ± 4·25 and 38·13 ± 4·41). The FCD also decreased significantly compared with the polyhexanide alone (7·13 ± 0·82 versus 11·68 ± 0·62; Figure 4).
The use of phentolamine in combination with polyhexanide caused a significant decrease in first‐, second‐ and third‐order arterioles compared with the isolated polyhexanide and the control group (1, 2, 3 and Table 1). The FCD decreased also, but without significant differences.
While the use of octenidine caused no alteration in the arterioles, the FCD increased significantly (11·58 ± 0·64 and 11·20 ± 0·44) compared with the baseline value (7·18 ± 0·67) and with the control group (6·50 ± 0·83 and 6·33 ± 0·54).
The combination of octenidine and bupivacaine caused a decrease in the arteriolar diameter, but the level of decrease was insignificant. The FCD, however, decreased significantly compared with isolated octenidine (4·46 ± 1·38 and 3·13 ± 1·19 versus 11·58 ± 0·64 and 11·20 ± 0·44; Table 1 and Figure 5).
Figure 5.
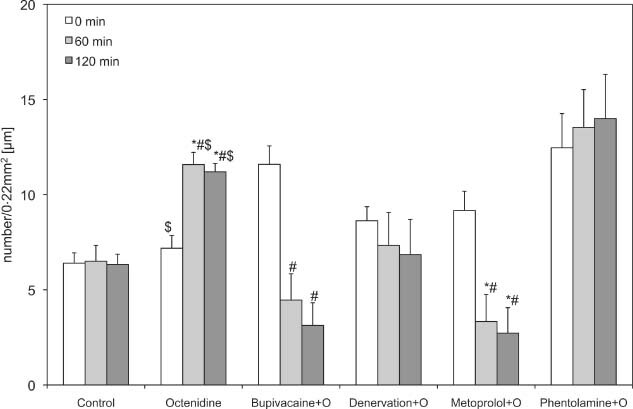
Functional capillary density of the octenidine groups. Recordings were performed before (0) and 60 and 120 minutes after drug application or denervation. Data are given as mean and standard error of mean (each group consists of six rats; *P < 0.05 versus control; # P < 0.05 versus polyhexanide; $ P < 0.05 versus octenidine basic values). 256 × 156 mm (600 × 600 DPI).
Octenidine in combination with the denervated vessels caused no alteration in the arteriolar diameter. The FCD was lower than that of isolated octenidine, but not significantly (Table 1).
The β‐blocker metoprolol used in combination with octenidine seems to have no influence on arterioles, but it decreased the FCD significantly (3·33 ± 1·41 and 2·72 ± 1·34) compared with isolated octenidine (11·58 ± 0·64 and 11·20 ± 0·44) and control (6·50 ± 0·83 and 6·33 ± 0·54; Figure 5).
Phentolamine, an α‐receptor blocker, used in combination with octenidine caused no alteration in first‐ and second‐arteriolar diameter, and a slight decrease in third‐order arterioles. The FCD, which increased in the isolated octenidine group, stayed at a constant level in the phentalomine combination group (Figure 5).
DISCUSSION
We assessed the influence of octenidine and polyhexanide on microcirculation because they are used daily in hospitals, and their excellent antiseptic ability 13, 14, 15 and superior tissue tolerance have been previously shown 2, 3, 4, 5, 6, 14, 16.
The aim of this study was to validate the positive influences of octenidine and polyhexanide on microcirculation, to discover whether the widely used β blockers could influence these effects and to learn more about the mode of action 4, 5, 6. The model we used allows for the direct visualisation of the muscular vasculature and therefore, for direct investigations of the influence of topically applied agents on arteriolar diameter and FCD (8).
The control group showed no alterations in arteriolar diameter and FCD compared with the baseline values. This result could be expected, because previous experiments have shown no change in blood flow after preparation of the cremaster muscle (12).
In this study, polyhexanide, a biguanide with a strongly cationic charge, improved muscular perfusion by increasing the arteriolar diameter and functional vessel density over at least a period of 2 hours. The observed arteriolar dilatative effect confirms results from previous experiments on the skin of mice (5).
Directly after the surgical sectioning of the sympathetic fibres, an arteriolar vasodilation could be observed, which lasted approximately 30 minutes. At the 60‐minute measurement, the denervation in combination with polyhexanide showed a decrease of arterioles, with a recovery after 2 hours; the same reaction could be observed in the FCD. After denervation of the sympathetic fibres, the influences of the central nervous system on the vasomotion are interrupted (17). Long‐term follow‐up studies have shown that the FCD reduces to 10% of its original value after denervation, which could be interpreted as an appropriate reaction to the reduced physiological requirements (18).
The combination of polyhexanide with the β‐blocker metoprolol influenced the microcirculation negatively. The arteriolar diameter and FCD decreased continually, and the positive effects of isolated polyhexanide were reversed. The positive‐dilatative effect of polyhexanide is voided by the β‐receptor blockade (metoprolol), and the combination, consequently, leads to vasoconstriction.
Further studies must prove these results, but a possible consequence of these findings could be a more censorious use of β blockers in patients with critically perfused wounds.
The combination of polyhexanide with the α‐blocker phentolamine caused significant vasoconstriction in all arterioles, but no alteration in the FCD. The α receptors were probably already blocked by phentolamine, and the additional indirect potential sympathomimetic activity of polyhexanide, mediated by noradrenaline, then caused vasoconstriction (19).
For octenidine, we found a significant increase of the FCD, but no significant influence on arteriolar diameter, even when the first‐order arterioles increased over the course of the investigation. Previous experiments on the effects of octenidine in mice showed a significant increase in dermal arteriolar diameter (5).
The increase of FCD, which could be observed during surgery, occurs when octenidine is used for disinfection, causing minor disseminated bleedings.
We observed only significant negative effects on the FCD when octenidine was combined with bupivacaine and metoprolol.
The described positive impact of polyhexanide on arteriolar diameter and – like octenidine – on FCD, is eliminated, or even inverted, by local anaesthetics, denervation and α and β blockers. Polyhexanide could influence the vasomotion sympathomimetically and by α‐receptor blockade. However, further research is needed to understand the exact mechanisms of these reactions.
The β blockers are widely used in patients with artery occlusive diseases. Because these types of patients often also have wound healing complications, further research focussing on the effects of β blockers on wound healing is needed. The increasing life expectancies in developed countries and the subsequent increase in cardiovascular diseases make this issue even more important.
REFERENCES
- 1. Suh DY, Hunt TK. Time line of wound healing. Clin Podiatr Med Surg 1998;15:1–9. [PubMed] [Google Scholar]
- 2. Goertz O, Abels C, Knie U, May T, Hirsch T, Daigeler A, Steinau HU, Langer S. Clinical safety and efficacy of a novel thermoreversible polyhexanide‐preserved wound covering gel. Eur Surg Res 2010;44:96–101. [DOI] [PubMed] [Google Scholar]
- 3. Kramer A, Roth B, Muller G, Rudolph P, Klocker N. Influence of the antiseptic agents polyhexanide and octenidine on FL cells and on healing of experimental superficial aseptic wounds in piglets. A double‐blind, randomised, stratified, controlled, parallel‐group study. Skin Pharmacol Physiol 2004;17:141–6. [DOI] [PubMed] [Google Scholar]
- 4. Goertz O, Ring A, Knie U, Abels C, Daigeler A, Steinau HU, Steinstraesser L, Langer S. Evaluation of a novel polihexanide‐preserved wound covering gel on dermal wound healing. Eur Surg Res 2010;44:23–9. [DOI] [PubMed] [Google Scholar]
- 5. Langer S, Sedigh Salakdeh M, Goertz O, Steinau HU, Steinstraesser L, Homann HH. The impact of topical antiseptics on skin microcirculation. Eur J Med Res 2004;9:449–54. [PubMed] [Google Scholar]
- 6. Daeschlein G, Assadian O, Bruck JC, Meinl C, Kramer A, Koch S. Feasibility and clinical applicability of polihexanide for treatment of second‐degree burn wounds. Skin Pharmacol Physiol 2007;20:292–6. [DOI] [PubMed] [Google Scholar]
- 7. Merino J, Planas A, Elosua R, de Moner A, Gasol A, Contreras C, Vidal‐Barraquer F, Clara A. Incidence and risk factors of peripheral arterial occlusive disease in a prospective cohort of 700 adult elderly men followed for 5 years. World J Surg 2010;34:1975–9. [DOI] [PubMed] [Google Scholar]
- 8. Baez S. An open cremaster muscle preparation for the study of blood vessels by in vivo microscopy. Microvasc Res 1973;5:384–94. [DOI] [PubMed] [Google Scholar]
- 9. Peter FW, Li‐Peuser H, Vogt PM, Muehlberger T, Homann HH, Steinau HU. The effect of wound ointments on tissue microcirculation and leucocyte behaviour. Clin Exp Dermatol 2002;27: 51–5. [DOI] [PubMed] [Google Scholar]
- 10. Leech CJ, Faber JE. Different alpha‐adrenoceptor subtypes mediate constriction of arterioles and venules. Am J Physiol 1996;270:H710–22. [DOI] [PubMed] [Google Scholar]
- 11. Sakanashi M, Hiraki I. Effects of vasodilators on microcirculation of the rat cremaster muscle: a microscopic method for screening drugs. Jpn J Pharmacol 1979;29:125–31. [DOI] [PubMed] [Google Scholar]
- 12. Lohman R, Siemionow M, Lister G. Advantages of sharp adventitial dissection for microvascular anastomoses. Ann Plast Surg 1998;40:577–85. [DOI] [PubMed] [Google Scholar]
- 13. Rietkotter J, Korber A, Grabbe S, Dissemond J. Eradication of methicillin‐resistant Staphylococcus aureus in a chronic wound by a new polyhexanide hydrogel. J Eur Acad Dermatol Venereol 2007;21:1416–7. [DOI] [PubMed] [Google Scholar]
- 14. Koburger T, Hubner NO, Braun M, Siebert J, Kramer A. Standardized comparison of antiseptic efficacy of triclosan, PVP‐iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. J Antimicrob Chemother 2010;65:1712–9. [DOI] [PubMed] [Google Scholar]
- 15. Rohrer N, Widmer AF, Waltimo T, Kulik EM, Weiger R, Filipuzzi‐Jenny E, Walter C. Antimicrobial efficacy of 3 oral antiseptics containing octenidine, polyhexamethylene biguanide, or citroxx: can chlorhexidine be replaced? Infect Control Hosp Epidemiol 2010;31:733–9. [DOI] [PubMed] [Google Scholar]
- 16. Hubner NO, Siebert J, Kramer A. Octenidine dihydrochloride, a modern antiseptic for skin, mucous membranes and wounds. Skin Pharmacol Physiol 2010;23:244–58. [DOI] [PubMed] [Google Scholar]
- 17. Fleming BP, Barron KW, Howes TW, Smith JK. Response of the microcirculation in rat cremaster muscle to peripheral and central sympathetic stimulation. Circ Res 1987;61(5 pt 2):26–31. [PubMed] [Google Scholar]
- 18. Borisov AB, Huang SK, Carlson BM. Remodeling of the vascular bed and progressive loss of capillaries in denervated skeletal muscle. Anat Rec 2000;258:292–304. [DOI] [PubMed] [Google Scholar]
- 19. Peuler JD. Opposing adrenergic actions of intravenous metformin on arterial pressure in female spontaneously hypertensive rats. Cardiovasc Res 1999;43:237–47. [DOI] [PubMed] [Google Scholar]


