Abstract
Diabetic patients are at high risk of foot ulcerations that may lead to limb amputations with important socio‐economic impact. Peripheral vascular disease may be frequently associated in diabetes mellitus type II with its main symptom, intermittent claudication. Many studies reported the known efficacy of cilostazol in treating vascular claudication. Metalloproteinase‐9 (MMP‐9) seems to be a biochemical marker implicated in chronic wounds and in particular in diabetic foot ulcers. Cilostazol appears to have a lowering effect on MMP‐9 levels and this may suggest a beneficial effect in order to prevent or retard the onset of foot ulcer in diabetic patients. In our study, two groups of diabetic patients with peripheral vascular disease were divided into two groups according to the presence of claudication in order to receive cilostazol. Group A (31 patients without claudication) were not eligible to receive cilostazol whereas Group B (47 patients with claudication) received cilostazol administration for 24 weeks (100 mg orally twice daily). Median follow up was of 16 months. During the follow up, 4·25% of patients of Group B and 35·48% of patients of Group A (P < 0·01) showed onset of foot ulceration. Although further randomised and controlled studies are required cilostazol seems to show beneficial effects for primary prevention of diabetic foot ulcers.
Keywords: Cilostazol, Diabetic foot, Intermittent claudication, Metalloproteinase‐9, Peripheral vascular disease
INTRODUCTION
Diabetes mellitus type II (DMII) may be frequently associated with peripheral vascular disease (PVD) which tends to be multisegmental and often with the involvement of the crural arteries 1. The prevalence of PVD in diabetic subjects is about 10%, whereas it is 2·6% in non‐diabetic patients. About 35·3% of patients with diabetic foot ulceration have PVD and ischaemia, which may contribute (together with the coexistence of neuropathy) to the progression of trophic lesions in the foot 2. Although the majority of diabetic ulcers are neuropathic, almost 60% have an ischaemic component, and 10% have only an ischaemic disturbance 3. Therefore, diabetic people have a greater risk of gangrene and amputation, which requires prolonged and costly hospital stay. Moreover amputation affects the quality of life, the physiological welfare and represents a premortal event; in this light, the prevention of foot ulceration plays an important role 2, 4, 5.
Cilostazol is the most clinically effective pharmacological option for intermittent claudication (IC), the most common symptom in patients with PVD 6.
Some studies have focused on the potential beneficial effect of cilostazol on peripheral neuropathy (PN) in diabetic patients with PVD 7, 8.
Moreover cilostazol seems to inhibit metalloproteinase‐9 (MMP‐9) expression, a biochemical marker recently identified in the development and in the maintaining stages of chronic wounds of lower limbs 9, 10 and, in particular, in diabetic foot ulcers 11, 12.
Aim of this study is to evaluate the effects of cilostazol in diabetic patients with PVD and IC.
METHODS
We performed an open label, parallel group, double clinical centre study, conducted between January 2010 and June 2012 with prior approval from the Institutional Review Board, in accordance with the Declaration of Helsinki and the Guideline for Good Clinical Practice. Before beginning the study, all participants provided written informed consent.
Patients
We enrolled patients with PVD as confirmed by either a resting ankle/brachial index of ≤0·90 and a ≥10 mmHg decrease in ankle pressure measured 1 minute after walking to maximal distance and a positive ultrasound evaluation of lower limb arteries with the evidence of atherosclerotic occlusive or stenotic lesions in the peripheral circulation and a baseline pain‐free walking distance of at least 50 m. Patients with Buerger's disease, critical limb ischaemia or lower extremity reconstruction (surgical or endovascular) or who underwent sympathectomy were excluded from this study.
Experimental protocol
The enrolled patients were divided into two groups according to the presence of symptoms related to IC:
Group A did not have symptoms related to IC and therefore did not receive cilostazol.
Group B with symptoms (moderate to severe) of IC, present for at least 6 months but without substantial change during the previous 3 months, were assigned to 24 weeks of treatment with cilostazol (100 mg orally, twice daily).
End point
The endpoint was to evaluate the effect of cilostazol in order to prevent foot ulcerations.
Evaluation of MMP‐9 plasma levels
In agreement with our recent papers 9, 10, an enzyme‐linked immunosorbent assay (ELISA) kit was used to determine the concentration of MMP‐9 in plasma at T0 (admission), T1 (3 months later), at T2 (6 months after the beginning of the treatment).
RESULTS
Patients eligible for the study were 78 subjects of both sexes, 31 in Group A and 47 in Group B (see Table 1). During cilostazol treatment, 12 patients (25·53%) developed moderate adverse events (e.g. headache, diarrhoea and weakness, see also Table 2) and 5 of these (10·63%) dismissed the treatment. No patients enrolled in Group A dismissed the study.
Table 1.
Demographics
| Group B | Group A | Total | |
|---|---|---|---|
| Sex | |||
| Male | 27 (57·45%) | 17 (54·83%) | 44 (56·41%) |
| Female | 20 (42·55%) | 14 (45·16%) | 34 (43·59%) |
| Total | 47 (100%) | 31 (100%) | 78 (100%) |
| Age | |||
| Range | 57–78 | 56–75 | 56–78 |
| Median | 65 | 66 | 65·5 |
| Overweight (BMI 25–29·9 kg/m2) | 23 (48·93%) | 14 (45·16%) | 37 (47·43%) |
| Obesity (BMI ≥ 30 kg/m2) | 11 (23·40%) | 9 (29·03%) | 20 (25·64%) |
| Smoking | |||
| Never | 8 (17·02%) | 6 (19·35%) | 14 (17·94%) |
| Previous | 27 (57·44%) | 16 (51·61%) | 43 (55·12%) |
| Current | 12 (25·53%) | 7 (22·58%) | 19 (24·35%) |
| Hypertension | 32 (68·08%) | 21 (67·74%) | 53 (67·95%) |
| Hypercholesterolemia | 29 (61·70%) | 18 (58·06%) | 47 (60·25%) |
| Baseline resting ABI | |||
| Affected limb* | 0·65 ± 0·21 | 0·65 ± 0·19 | 0·65 ± 0·20 |
| Opposite limb | 0·85 ± 0·20 | 0·81 ± 0·22 | 0·85 ± 0·21 |
ABI, ankle brachial index; BMI, body mass index.
The affected limb was defined as the limb that developed IC. If the patient reported IC as equal, the limb with the lower resting ABI was defined as ‘affected’.
Table 2.
Most commonly reported adverse events with cilostazol treatment in Group B
| Event | Number |
|---|---|
| Headache | 7 (14·89%) |
| Nausea | 1 (2·12%) |
| Diarrhoea | 10 (21·27%) |
| Tachycardia | 4 (8·51%) |
| Weakness | 12 (25·53) |
| Leg cramps | 5 (10·64%) |
| Stuffy nose | 4 (8·51%) |
| Numbness or tingling | 6 (12·76%) |
| Patients with at least one event | 12 (25·53%) |
| Patients with more than one event | 7 (14·89%) |
Patients of both groups were followed up for a minimum period of 6 months and a maximum of 24 months. Median follow up was of 16 months.
During the follow up period 2 patients in Group B (4·25%) and 11 patients (35·48%) in Group A (P < 0·01) showed onset of foot ulceration.
ELISA test revealed that cilostazol was able to reduce significantly the plasma levels of MMP‐9 with a time‐dependent pattern (Figure 1).
Figure 1.
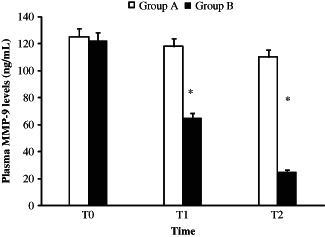
MMP‐9 plasma levels in cilostazole treated (Group B) and untreated (Group A) patients, measured through ELISA test. The MMP‐9 levels was measured at the time of admission (T = 0) and 3 (T1) and 6 months later (T2). *P < 0·01.
DISCUSSION
Cilostazol, a selective inhibitor of type 3 phosphodiesterase that increases cAMP levels in platelet and vascular cells, is a potent antiatherosclerotic agent and has been found to be effective in the treatment of IC, improving the distance that patients are able to walk before the onset of leg pain 11, 13. Furthermore it may also have a likely beneficial effect on nerve functions in diabetic patients 7, 8.
The prevalence of PVD in diabetic patients is quite frequent and although IC is the most common clinical manifestation of PVD, in diabetes, pain perception may be blunted by the presence of PN. This allowed us to identify two groups of patients among diabetic subjects with PVD.
The coexistence of neuropathy, ischaemia and leukocyte function disorders in diabetic patients encourages the development of severe and extensive infections in the lower limbs, which may lead to amputations and death 2.
Metalloproteinases (MMPs) are the main proteases involved in remodelling of extracellular matrix in several pathological processes characterised by disorganisation of extracellular matrix such as in chronic wounds. Chronic wounds are probably caused by an exaggeration of their inflammatory phase 12, probably mediated by anomalous leukocyte function with elevated production of MMPs and in particular of MMP‐9, as previously reported 9, 10.
In addition, diabetic neuropathy seems to be caused by an impaired inflammatory response to microvascular disfunction 3.
Previously it has been reported that several drugs (such as statins and antimicrobials) induce additive effects (e.g. antinflammatory effects) other than their primary effects 14, 15, 16
In this article, we documented that cilostazol significantly reduced the plasma MMP‐9 levels showing that this drug may be able to induce an antinflammatory effect, even if we are not able to measure other inflammatory markers.
In conclusion, we postulated a role for cilostazol in the prevention of foot ulcers in diabetic patients. However, a large‐scale prospective trial is needed to establish the use of cilostazol for primary prevention of diabetic foot ulcers.
Acknowledgements
The authors declare that they have no competing interests. This work received no funding.
References
- 1. Mackaay AJ, Beks PJ, Dur AH, Bischoff M, Scholma J, Heine RJ, Rauwerda JA. The distribution of peripheral vascular disease in a Dutch Caucasian population: comparison of type II diabetic and non‐diabetic subjects. Eur J Vasc Endovasc Surg 1995;9:170–5. [DOI] [PubMed] [Google Scholar]
- 2. Zubair M, Malik A, Ahmad J. Incidence, risk factors for amputation among patients with diabetic foot ulcer in a North Indian tertiary care hospital. Foot (Edinb) 2012;22:24–30. [DOI] [PubMed] [Google Scholar]
- 3. Heikkinen M, Salmenperä M, Lepäntalo A, Lepäntalo M. Diabetes care for patients with peripheral arterial disease. Eur J Vasc Endovasc Surg 2007;33:583–91. [DOI] [PubMed] [Google Scholar]
- 4. Redekop WK, Stolk EA, Kok E, Lovas K, Kalo Z, Busschbach JJV. Diabetic foot ulcers and amputations: estimates of health utility for use in cost‐effectiveness analyses of new treatments. Diabetes Metab 2004;30:549–56. [DOI] [PubMed] [Google Scholar]
- 5. Serra R, Buffone G, Dominijanni A, Molinari V, Montemurro R, de Franciscis S. Applicationif platelet‐rich gel to enhance healing of transmetatarsal amputaions in diabetic dysvascular patients. Int Wound J 2013. DOI: 10.1111/iwj.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hiatt WR. The US experience with cilostazol in treating intermittent claudication. Atheroscler Suppl 2005;6:21–31. [DOI] [PubMed] [Google Scholar]
- 7. O'Donnell ME, Badger SA, Sharif MA, Makar RR, Young IS, Lee B, Soong CV. The effects of cilostazol on peripheral neuropathy in diabetic patients with peripheral arterial disease. Angiology 2008;59:695–704. [DOI] [PubMed] [Google Scholar]
- 8. Papanas N, Maltezos E. Cilostazol in diabetic neuropathy: premature farewell or new beginning? Angiology 2011;62:605–8. [DOI] [PubMed] [Google Scholar]
- 9. Serra R, Buffone G, Falcone D, Molinari V, Scaramuzzino M, Gallelli L, de Franciscis S. Chronic venous leg ulcers are associated with high levels of metalloproteinases‐9 and neutrophil gelatinase‐associated lipocalin. Wound Rep Reg 2013. DOI: 10.1111/wrr.12035. [DOI] [PubMed] [Google Scholar]
- 10. Serra R, Gallelli L, Buffone G, Molinari V, Stillitano DM, Palmieri C, de Franciscis S. Doxycycline speeds up healing of chronic venous ulcers. Int Wound J 2013. DOI: 10.1111/iwj.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chuang SY, Yang SH, Chen TY, Pang JH. Cilostazol inhibits matrix invasion and modulates the gene expressions of MMP‐9 and TIMP‐1 in PMA‐differentiated THP‐1 cells. Eur J Pharmacol 2011;670:419–26. [DOI] [PubMed] [Google Scholar]
- 12. Li Z, Guo S, Yao F, Zhang Y, Li T. Increased ratio of serum matrix metalloproteinase‐9 against TIMP‐1 predicts poor wound healing in diabetic foot ulcers. J Diabetes Complications 2013. DOI: 10.1016/j.jdiacomp.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 13. Biscetti F, Pecorini G, Straface G, Arena V, Stigliano E, Rutella S, Locatelli F, Angelini F, Ghirlanda G, Flex A. Cilostazol promotes angiogenesis after peripheral ischemia through a VEGF‐dependent mechanism. Int J Cardiol 2012. DOI: 10.1016/j.ijcard.2012.03.103. [DOI] [PubMed] [Google Scholar]
- 14. Russo E, Donato di Paola E, Gareri P, Siniscalchi A, Labate A, Gallelli L, Citraro R, De Sarro G. Pharmacodynamic potentiation of antiepileptic drugs' effects by some HMG‐CoA reductase inhibitors against audiogenic seizures in DBA/2 mice. Pharmacol Res 2012;70:1–12. [DOI] [PubMed] [Google Scholar]
- 15. Pelaia G, Gallelli L, Renda T, Fratto D, Falcone D, Caraglia M, Busceti MT, Terracciano R, Vatrella A, Maselli R, Savino R. Effects of statins and farnesyl transferase inhibitors on ERK phosphorylation, apoptosis and cell viability in non‐small lung cancer cells. Cell Prolif 2012. Dec;45:557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gallelli L, Gioffrè V, Vero G, Gallelli A, Roccia F, Naty S, Pelaia G, Capano A, Loiacono A, De Sarro G, Maselli R. Clarithromycin in the treatment of Legionella pneumophila pneumonia associated with multiorgan failure in a previously healthy patient. Clin Drug Investig 2005;25:485–90. [DOI] [PubMed] [Google Scholar]


