Abstract
Alternagin‐C (ALT‐C) is a disintegrin‐like, Cys‐rich protein isolated from Bothrops alternatus snake venom, which has been shown to induce in vivo angiogenesis. Therefore, this protein could be interesting as a new approach for tissue regeneration studies. Here the effects of ALT‐C on fibroblasts and inflammatory cells, collagen type III and type I and TGF‐α expression in a rat wounded skin model were studied. Thirty‐five male Wistar rats (weight 270 ± 20 g) were divided into seven groups with five animals in each of the following groups: a control group which wounded animals received treatment with natrozol® gel only; ALT‐C10, ALT‐C60 and ALT‐C100 groups of wounded animals that were treated with the same amount of gel containing 10, 60 and 100 ng of ALT‐C, respectively. Animals were treated once a day with 20 µl of gel associated or not with ALT‐C for 1, 3, 5 or 7 days. ALT‐C treatment increased the fibroblast density, collagen deposition and accelerated the inflammatory process, mostly in the ALT‐C60 group. These results indicate that ALT‐C improves wound repair process in rat skin. Thus, ALT‐C could be a candidate to the development of a novel therapeutic strategy for wounded skin repair.
Keywords: ALT‐C, Disintegrin, Skin, Wound healing
INTRODUCTION
Wound healing is a dynamic, interactive process among several cell types, involving a cascade of events including inflammation, new tissue formation and tissue remodelling, which finally lead to the reconstruction of the wounded area, which is eventually partial 1, 2. Inflammatory cells invade the wounded tissue and play an important role in the initiation of the inflammatory process. Neutrophils are the first cells to arrive within a few minutes, followed by macrophages and lymphocytes. In addition to the defence functions of inflammatory cells, they are also a key source of growth factors and cytokines, which start the proliferative phase of wound repair (3).
Keratinocytes also migrate and proliferate afterwards at the wound edge and are followed by the proliferation of dermal fibroblasts in the neighbourhood of the wound 2, 3. Concomitantly, fibroblasts synthesise and secrete structural proteins, such as various types of collagens, which deposit in the dermal defect to restore tissue integrity. Collagens, primarily type I along with a small amount of type III, provide tensile strength to the skin. These proteins primarily account for the strength and durability of the leather. Thus, collagen along with the imbedded and newly formed capillaries forms the granulation tissue in open wounds within tissue defects (4). However, the collagen that is deposited into the dermal defect in chronic wounds is disorganised and the resulting scar tissue never achieves the tensile strength of unwounded skin (5).
Wound repair is controlled by a wide variety of different growth factors and cytokines. The epidermal growth factor (EGF) family of mitogens is very important during wound healing. This family comprises several members, including EGF, transforming growth factor‐α (TGF‐α), heparin‐binding EGF, amphiregulin, epiregulin, betacellulin, neuregulins and others 6, 7, 8. TGF‐α is secreted by platelets, keratinocytes, activated macrophages and eosinophils and it works in an autocrine/paracrine manner on keratinocytes and dermal fibroblasts 9, 10. It was shown that TGF‐α has the ability to increase keratinocyte and dermal fibroblast migration (11) and proliferation, and it stimulates and regulates angiogenesis 12, 13. It has a key role in early stimulation and maintenance of wound epithelialisation in partial‐thickness wounds (14). Collectively, TGF‐α plays an important role in the early phase of reepithelialisation 9, 10, 11, 14.
Integrins form a family of cell surface adhesion receptors, mediating both cell–cell and cell–matrix interactions. The α2β1 integrin is a major collagen receptor that plays an essential role in the adhesion of normal and tumour cells to the extracellular matrix (15). A family of small integrin‐binding proteins, named disintegrins, has been described from snake venoms, which strongly inhibit integrin‐mediated cell adhesion and migration (16). These proteins bind to integrins with high affinity therefore inhibiting cell adhesion and migration. Alternagin, a protein purified from the venom of the Brazilian snake Bothrops alternatus, is synthesised as a precursor form belonging to the PIII class of the snake venom metalloproteinases (SVMP) (17). Alternagin‐C (ALT‐C) is a disintegrin‐like, Cys‐rich domain released from alternagin, which is a potent inhibitor of collagen‐induced adhesion by blockage of α2β1 integrin (17).
Previously results from our group have shown that ALT‐C strongly induces human vein endothelial cell (HUVEC) proliferation both in vitro and in vivo by up‐regulating the expression of some growth factors and their receptors, including vascular endothelial growth factor (VEGF) and VEGF receptor 2 (VEGFR‐2) 18, 19. In addition, it was shown that ALT‐C increases myoblast proliferation, modifies gene expression including the pattern of MHC gene expression (20) and modulates matrix metalloprotease (MMP) activity and expression in an in vivo skeletal muscle regeneration model (21).
Recently, we have also shown that ALT‐C and ALT‐C PEP, a peptide derived from its sequence, were able to induce in vivo angiogenesis in wounded rat skin (22). These results suggest a role for α2β1 integrin in the healing process which may be interesting for the development of therapeutic strategies for wounded skin repair or regeneration. Based on our previous results and considering the potential benefits of ALT‐C on the regeneration therapy, particularly on the wounded skin repair, we hypothesised that ALT‐C could improve the cutaneous wound healing. Therefore, the purpose of the present study was to assess the effects of ALT‐C on collagen type I and type III expression, as well as on the fibroblasts and inflammatory cells and TGF‐α expression in a rat wounded skin model.
MATERIALS AND METHODS
Animals
Thirty‐five male Wistar rats weighing 270 ± 20 g were used. The animals were housed in plastic cages in a room with controlled environmental conditions and had free access to water and standard food. The experimental procedures were approved by the Animal Ethics Committee of the Universidade Federal de Alfenas (Process No. 67/2005), and the study was conducted in accordance with national guidelines for the care and use of laboratory animals.
Alternagin‐C
ALT‐C, the processed disintegrin and Cys‐rich domains of alternagin, was isolated from B. alternatus venom as previously described (17) and dissolved in 1·5% natrosol® gel (21).
Skin wound model and treatment
Rats were anaesthetised with ketamine and xylazine (1:1; 0·2 ml/100 g, i.p.) and an excision wound was made as described (22), by excising the skin of the back of each animal, close to the cervical area, with a 4‐mm diameter biopsy punch. After that, animals were randomly divided into seven groups with five animals in each: Control, treatment with natrozol® gel; ALT‐C10, treated with 10 ng of ALT‐C; ALT‐C60, animals treated with 60 ng of ALT‐C; ALT‐C100, animals treated with 100 ng of ALT‐C. Animals were treated topically with 20 µl of gel only or containing ALT‐C in the concentrations stated above, that was applied once a day, into the wound, for 1, 3, 5 or 7 consecutive days (22). After the treatment period animals were euthanised with anaesthesia overdose and the skin was removed including the wounded area plus 2 mm of the edging skin. The cranial portion was used for histological analysis and the caudal portion for protein extraction.
Histology
The wounded skin of the animals from all experimental groups was fixed in 4% buffered p‐formaldehyde, embedded in paraffin, sectioned (5 µm thick) and stained with haematoxylin–eosin or picrosirius red (23). Three digital images per animal, at 400× magnification, were acquired randomly with a Nikon Eclipse E‐400 photomicroscope and used for morphometric quantification of collagen, fibroblasts and inflammatory cells (macrophages, neutrophils and lymphocytes) in a test frame area of 0·0625 mm2. The collagen quantification was used for the determination of collagen type III/type I ratio and the results of cell densities were expressed as the percentage of control value (22).
Immunohistochemical analysis
The sections of rat skin were treated with xylene, hydrated through a graded ethanol series and rinsed in tap water. Antigens were retrieved by boiling the sections in a 10 ml citrate buffer (0·1 M, pH 6·0) for three times (5 minutes each) in a microwave oven. The cooled sections were incubated in 0·3% H2O2 for 15 minutes to block endogenous peroxidase. Non specific binding was blocked by incubating the sections in blocking solution for 1 hour at room temperature. Rabbit primary antibody collagen type III (AB757, Chemicon, Temecula, CA) was diluted in 1% bovine serum albumin (1:50) and incubated with the sections overnight at 4°C. Afterwards, the sections were washed for 15 minutes with TBS‐T [Tris‐buffered saline containing 2% (v/v) Tween 20] and incubated with HRP‐conjugated anti‐rabbit antibody (sc‐3837, Santa Cruz Biotechnology, Santa Cruz, CA) at 1:100 dilution for 2 hours at room temperature. After washing in TBS‐T, peroxidase activity was detected with 3,3‐diaminobenzidine as the chromogenic substrate. Sections were counterstained with Harry's haematoxylin and dehydrated in an increasing ethanol series and xylene, mounted in Entellan (Merck, Darmstadt, Germany) and photographed with a Nikon Eclipse E‐400 photomicroscope. Collagen type III was quantified by densitometry in a test frame area of 0·0625 mm2, using Adobe Photoshop software (22).
Western blotting
Protein content of the wounded skin of rats from all experimental groups was extracted according to Sant’Ana et al. (22). Proteins were quantified, separated (10 µg) by 12% polyacrylamide–SDS electrophoresis and transferred to a PVDF membrane. Blots were blocked with phosphate buffered saline (PBS) 0·01 M, pH 7·3, containing 3% (w/v) bovine serum albumin and 2% milk powder for 1 hour and then washed in PBST [PBS containing 1% (v/v) Tween 20], for 10 minutes. Membranes were incubated for 1 hour with primary antibody anti‐TGFα (sc‐9043, Santa Cruz Biotechnology), followed by 1 hour incubation with the secondary antibody conjugated to alkaline phosphatase (Santa Cruz Biotechnology). Immunoreactive bands were shown with BCIP/NBT colour development substrate (Sigma). The bands were quantified by densitometry, using Kodak 1D 3·0 software and the results were expressed as percentage of increase or decrease from control values. β‐Actin was used for data normalisation.
Statistical analysis
All experiments were made in triplicate. Data are shown as mean ± SEM and comparisons among groups were made by one‐way ANOVA followed by post hoc test of Tukey, when P < 0·05.
RESULTS
Collagen expression
Using the same experimental model, we have previously shown that ALT‐C increased the presence of new blood vessels and the expression of growth factors, mostly VEGF and fibroblast growth factor‐1 (FGF‐1) mainly up to 7 days after injury (22). Now we show here that collagen expression is modulated by ALT‐C treatment (1, 2). ALT‐C treatment increased significantly (P < 0·05) collagen type I expression in the regenerating rat skin. ALT‐C60 was the most effective as it increased collagen type I in all of the times studied. ALT‐C10 and ALT‐C100 were effective only after 3 days of treatment, as compared to the control group (Figure 3A). ALT‐C treatment had less effect on the collagen type III expression in comparison with the control, and only after 7 days of ALT‐C100 treatment a significant (P < 0·05) reduction of the collagen type III expression was observed (Figure 3B). The collagen type III/I ratio was significantly (P < 0·05) reduced by ALT‐C60 treatment, in comparison with the control group. Despite the significant (P < 0·05) ratio reduction observed after 3 days of ALT‐C10 and ALT‐C100 treatment, compared to the control, only ALT‐C100 sustained the significant (P < 0·05) reduction after 7 days of treatment (Figure 3C).
Figure 1.
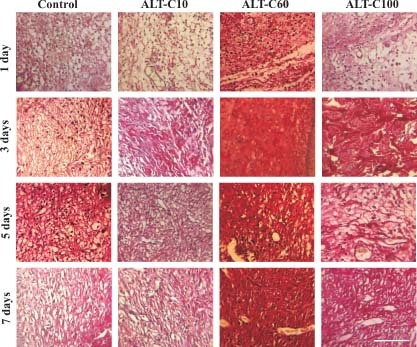
Collagen expression in rat wounded skin of control and treated animals with different concentrations of alternagin‐C (ALT‐C), at 1, 3, 5 and 7 days after injury, stained with picrosirius red. Note that collagen staining increased in the regenerating skin mainly in ALT‐C60 group that indicates increased collagen deposition. Scale bar = 100 µm.
Figure 2.
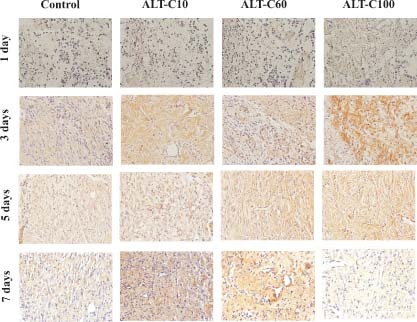
Collagen type III immunohistochemistry of rat wounded skin of control and treated animals with different concentrations of alternagin‐C (ALT‐C), at 1, 3, 5 and 7 days after injury. The colour intensity (chestnut brown) indicates the expression of collagen type III in the regenerating skin. Scale bar = 100 µm.
Figure 3.
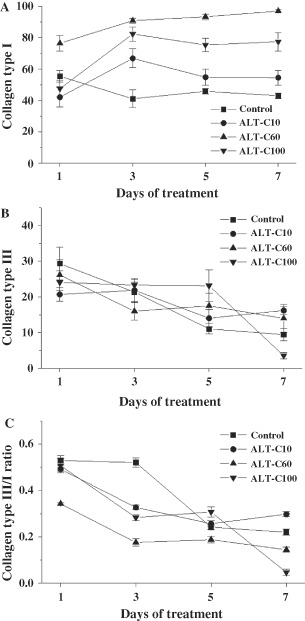
Collagen content in rat wounded skin of control and treated animals with different concentrations of alternagin‐C (ALT‐C), at 1, 3, 5 and 7 days after injury (means ± SEM). Collagen type I (A), collagen type III (B), and collagen type III/type I ratio. Note that the ALT‐C treatment stimulated the collagen type I expression.
Fibroblast and inflammatory cells densities
Fibroblast density increased significantly (P < 0·05) in ALT‐C treated groups, reaching the highest values between 3 and 5 days of treatment and returning to the control level after 7 days of injury (Figure 4A). Inflammatory cells increased mainly at 5 days of treatment, but decreased below the control level at 7 days of treatment (Figure 4B).
Figure 4.
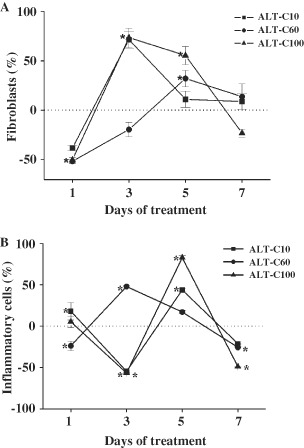
Percentage variation in the densities of fibroblasts (A) and inflammatory cells (B) in rat wounded skin treated with different concentrations of alternagin‐C (ALT‐C), for 1, 3, 5 and 7 days compared to control (means ± SEM). Note that ALT‐C treatment increased the density of fibroblasts and decreased the inflammatory cells.
TGF‐α expression
We next analysed TGF‐α expression as this growth factor is important for skin regeneration. TGF‐α expression was detected only at 1 and 3 days after injury. Interestingly, ALT‐C10 decreased significantly (P < 0·01) TGF‐α expression, in comparison with the control group, while ALT‐C100 increased it (P < 0·001). The effect of ALT‐C60 was similar to the control (Figure 5).
Figure 5.
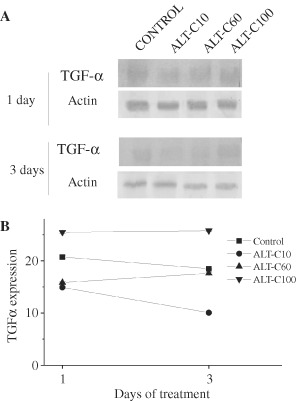
TGF‐α expression in rat wounded skin of control and treated groups with different concentrations of alternagin‐C (ALT‐C), at 1 and 3 days after injury. Western blotting (A) and band densitometry (B).
DISCUSSION
Cutaneous wound repair regenerates skin integrity; however, non healing chronic wounds results in compromised tissue function and increased morbidity being a health problem worldwide. It was shown in this study the influence of a disintegrin‐like, Cys‐rich protein in the regeneration of wounded rat skin. Data analysis showed that ALT‐C treatment after skin lesion increases collagen type I deposition, fibroblast density and accelerates the inflammatory process, observed namely with the ALT‐C60 treatment. Collectively, these results showed that ALT‐C could be beneficial for wound skin regeneration.
Previous studies from our laboratory have shown that ALT‐C enhances VEGF expression in fibroblasts, as well as endothelial cell proliferation (18). ALT‐C also induces in vivo angiogenesis in nude mice and up‐regulates the expression of VEGFR‐2(KDR) in HUVEC cells (19). Recently, we have shown that ALT‐C increases angiogenesis, in a dose‐dependent way, using the same wounded rat skin model of the present work (22). All these results support the hypothesis that ALT‐C possesses a good potential for tissue regeneration studies.
In this study, it was observed that the collagen type I deposition increased with ALT‐C treatment, mainly with ALT‐C 60, but a lesser effect was observed in the collagen type III expression. These findings are interesting, as the collagen III is considered an immature collagen and its deposition did not provide tensile strength to the skin (4). Collagen type I is the major collagen type in skin, where the collagen type III intercalates into the collagen type I fibrils and produces smaller and less organised fibrils (24). The decrease in the collagen type III to collagen type I ratio in our study was thus consistent with a more organised and stronger repaired skin induced by ALT‐C treatment and this occurred because of the increase of collagen type I deposition rather than the decrease of collagen type III. Besides contributing to the skin strength, collagen type I is also important to guide keratinocytes and dermal fibroblasts migration in the wounded area. A human wound‐healing study showed that the proportion of collagen III relative to collagen I increased significantly up to 6 weeks after initial injury and remained elevated up to 6 months (25). Considering this, our results suggested that ALT‐C could be beneficial to wounded skin repair, as this treatment enhanced collagen type I deposition and also fibroblasts density, mainly in the animals treated with ALT‐C60. Fibroblasts are important cells for wound healing as they cover the injured area, secrete the provisional matrix and growth factors that are indispensable for initiating repair and progressing into the healing state 13, 26.
Regarding the inflammatory cells, it is well established that macrophages and neutrophils are predominant during inflammation phase, which is around 3 days after lesion. After this period the density of these cells is reduced and other cells migrate into the wound (26). Our results suggest that ALT‐C could be a good chemical to improve skin regeneration as it accelerated the acute phase of inflammatory process, as seen by the inflammatory cells density increasing between 3 and 5 days after injury. The treatment avoided the chronic inflammatory process, as the inflammatory cells decreased below the control level at 7 days after injury. Persistence of an excess pro‐inflammatory infiltrate at the wound site delays wound closure by the establishment of a hostile, unbalanced microenvironment, dominated by proteolytic rather than protective mechanisms (26). These results seem to be well correlated to the ALT‐C effects on angiogenesis as previously reported 18, 19.
Wound healing process engages several cell types such as fibroblasts, platelets, macrophages, neutrophils, endothelial cells and keratinocytes (26). ALT‐C treatment shows a time‐ and concentration‐dependent action in different cell types, both in vitro and in vivo 18, 19, 21, 22, 23. However, in our study, it was not easy to determine exactly the target cell of ALT‐C. It is possible that ALT‐C affects distinct cell types as they become present at the injured site during the evolution of wound repair. It was previously shown that ALT‐C competitively interacts with the α2β1 integrin and induces integrin‐mediated signalling and chemotaxis in neutrophils (15) as well as in breast tumour cells (27). Recently, the disintegrin and Cys‐rich domains of ADAM9, a mammalian SVMP homologue, were shown to increase keratinocyte migration and MMP‐9 activity in a wound healing in vitro model, an effect mediated by β1 integrin receptors (28). Therefore, more studies, focussing on the interaction between ALT‐C and different cell populations, are necessary to determine the ALT‐C target cells during wound repair. The specificity of ALT‐C in changing densities of different cell types remains to be elucidated.
ALT‐C selectively binds to α 2 β 1 integrin triggering intracellular signalling characteristic of integrin‐activated pathways and up‐regulating the expression of several growth factors including vascular endothelial growth factor (16). Following this line of evidence, the results of the present work suggest that ALT‐C could be also triggering α 2 β 1 integrin‐mediated fibroblast signalling, which in turn modifies gene expression, including the collagen genes that improve skin regeneration and repair.
TGF‐α is important for the wound repair process, mostly for the early stimulation and maintenance of wound epithelialisation 10, 11, 14. In particular, TGF‐α represents the major keratinocyte pro‐motility factor present in human serum that acts during acute wound healing. In this condition, as the basement membrane is lost, keratinocytes initiate their migration to a provisional extracellular matrix with a complex composition, predominantly with collagen type I (11).
In this study, TGF‐α expression in ALT‐C60 group was similar to the control. However, ALT‐C100 treatment increased the TGF‐α expression, and interestingly the highest fibroblast density was observed in this group between 3 and 5 days after injury, probably following the TGF‐α peak. These results indicate a positive effect of ALT‐C treatment on the skin reepithelialisation and are in agreement with previous studies corroborating the important role of TGF‐α in the early phase of skin wound healing 9, 14.
CONCLUSION
In summary, the study indicates that ALT‐C, mainly ALT‐C60, improves wound repair process in rat skin, by increasing the collagen type I deposition and fibroblast density, and accelerating the inflammatory process. Overall, the results suggest that ALT‐C could be a good candidate to the development of a novel therapeutic strategy for wounded skin repair and regeneration.
ACKNOWLEDGEMENTS
This work was supported by grants from FAPESP and CNPq, Brazil. Durigan JL is post doc fellow from FAPESP (process 08/09408‐4).
REFERENCES
- 1. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–46. [DOI] [PubMed] [Google Scholar]
- 2. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835–70. [DOI] [PubMed] [Google Scholar]
- 3. Martin P. Wound healing‐aiming for perfect skin regeneration. Science 1997;276:75–81. [DOI] [PubMed] [Google Scholar]
- 4. Clark RA. Basics of cutaneous wound repair. J Dermatol Surg Oncol 1993;19:693–706. [DOI] [PubMed] [Google Scholar]
- 5. Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 2009;17:153–62. [DOI] [PubMed] [Google Scholar]
- 6. Tsahar E, Moyer JD, Waterman H, Barbacci EG, Bao J, Levkowitz G, Shelly M, Strano S, Pinkas‐Kramarski R, Pierce JH, Andrews GC, Yarden Y. Pathogenic poxviruses reveal viral strategies to exploit the ErbB signaling network. EMBO J 1998;15:5948–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, Roberts AB, Deng C. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF‐beta. EMBO J 1999;18:1280–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Strachan L, Murison JG, Prestidge RL, Sleeman MA, Watson JD, Kumble KD. Cloning and biological activity of epigen, a novel member of the epidermal growth factor superfamily. J Biol Chem 2001;276:18265–71. [DOI] [PubMed] [Google Scholar]
- 9. Hashimoto K. Regulation of keratinocyte function by growth factors. J Dermatol Sci 2000;24:S46–50. [DOI] [PubMed] [Google Scholar]
- 10. Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL. Growth factors in the treatment of diabetic foot ulcers. Br J Surg 2003;90:133–46. [DOI] [PubMed] [Google Scholar]
- 11. Li Y, Fan J, Chen M, Li W, Woodley DT. Transforming growth factor‐alpha: a major human serum factor that promotes human keratinocyte migration. J Invest Dermatol 2006;126:2096–105. [DOI] [PubMed] [Google Scholar]
- 12. Barrandon Y, Green H. Cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor‐alpha and epidermal growth factor. Cell 1987;50:1131–7. [DOI] [PubMed] [Google Scholar]
- 13. Ellis IR, Schor AM, Schor SL. EGF AND TGF‐α motogenic activities are mediated by the EGF receptor via distinct matrix‐dependent mechanisms. Exp Cell Res 2007;313:732–41. [DOI] [PubMed] [Google Scholar]
- 14. Kim I, Mogford JE, Chao JD, Mustoe TA. Wound epithelialization deficits in the transforming growth factor‐α knockout mouse. Wound Repair Regen 2001;9:386–90. [DOI] [PubMed] [Google Scholar]
- 15. Mariano‐Oliveira A, Coelho AL, Terruggi CH, Selistre‐de‐Araújo HS, Barja‐Fidalgo C, De Freitas MS. Alternagin‐C, a non‐RGD‐disintegrin, induces neutrophil migration via integrin signaling. Eur J Biochem 2003;270:4799–808. [DOI] [PubMed] [Google Scholar]
- 16. Selistre‐de‐Araujo HS, Cominetti MR, Terruggi CH, Mariano‐Oliveira A, De Freitas MS, Crepin M, Figueiredo CC, Morandi V. Alternagin‐C, a disintegrin‐like protein from the venom of Bothrops alternatus, modulates α 2 β 1 integrin‐mediated cell adhesion, migration and proliferation. Braz J Med Biol Res 2005;38:1505–11. [DOI] [PubMed] [Google Scholar]
- 17. Souza DH, Iemma MR, Ferreira LL, Faria JP, Oliva ML, Zingali RB, Niewiarowski S, Selistre‐de‐Araujo HS. The disintegrin‐like domain of the snake venom metalloprotease alternagin inhibits α 2 β 1 integrin‐mediated cell adhesion. Arch Biochem Biophys 2000;384:341–50. [DOI] [PubMed] [Google Scholar]
- 18. Cominetti MR, Ribeiro JU, Fox JW, Selistre‐de‐Araujo HS. BaG, a new dimeric metalloproteinase/disintegrin from the Bothrops alternatus snake venom that interacts with α5β1 integrin. Arch Biochem Biophys 2003;416:171–9. [DOI] [PubMed] [Google Scholar]
- 19. Ramos OH, Terruggi CH, Ribeiro JU, Cominetti MR, Figueiredo CC, Bérard M, Crepin M, Morandi V, Selistre‐de‐Araujo HS. Modulation of in vitro and in vivo angiogenesis by alternagin‐C, a disintegrin‐like protein from Bothrops alternatus snake venom and by a peptide derived from its sequence. Arch Biochem Biophys 2007;461:1–6. [DOI] [PubMed] [Google Scholar]
- 20. Mesquita‐Ferrari RA, Moraes CK, Micocci KC, Selistre‐de‐Araujo HS. ALT‐C, a disintegrin‐like Cys‐rich protein from Bothrops alternatus, increases skeletal myoblast viability. J Venom Anim Toxins Incl Trop Disv 2009;15:325–39. [Google Scholar]
- 21. Durigan JL, Peviani SM, Russo TL, Delfino GB, Ribeiro JU, Cominetti MR, Selistre‐de‐Araujo HS, Salvini TF. Effects of alternagin‐C from Bothrops alternatus on gene expression and activity of metalloproteinases in regenerating skeletal muscle. Toxicon 2008;52:687–94. [DOI] [PubMed] [Google Scholar]
- 22. Sant’ana EMC, Gouvêa CMCP, Nakaie CR, Selistre‐de‐Araújo HS. Angiogenesis and growth factor modulation induced by alternagin C, a snake venom disintegrin‐like, cysteine‐rich protein on a rat skin wound model. Arch Biochem Biophys 2008;479:20–7. [DOI] [PubMed] [Google Scholar]
- 23. Junqueira LCU, Cossermely W, Brentani R. Diferential staining of collagens type‐I, Type‐II and Type‐III by picrosirius red and polarization microscopy. Arch Hist Jpn 1978;41:267–74. [DOI] [PubMed] [Google Scholar]
- 24. Lapiere CM, Nusgens B, Pierard GE. Interaction between collagen type I and type III in conditioning bundles organization. Connect Tissue Res 1977;5:21–9. [DOI] [PubMed] [Google Scholar]
- 25. Robins SP, Milne G, Duncan A, Davies C, Butt R, Greiling D, James IT. Increased skin collagen extractability and proportions of collagen type III are not normalized after 6 months healing of human excisional wounds. J Invest Dermatol 2003;121:267–72. [DOI] [PubMed] [Google Scholar]
- 26. Witte M, Barbul A. General principles of wound healing. Surg Clin North Am 1997;77:509–28. [DOI] [PubMed] [Google Scholar]
- 27. Terruggi CHB, Cominetti MR, Bérard M, Selistre‐de‐Araújo HS. The disintegrin alternagin‐C modulates migration and viability of breast tumor cells. Rencontres en Toxinologie. Toxines et cancer: Lavoisier, 2006:201–8. [Google Scholar]
- 28. Zigrino P, Kamiguti AS, Eble J, Drescher C, Nischt R, Fox JW, Mauch C. The reprolysin jararhagin, a snake venom metalloproteinase, functions as a fibrillar collagen agonist involved in fibroblast cell adhesion and signaling. J Biol Chem 2002;277:40528–35. [DOI] [PubMed] [Google Scholar]


