Abstract
The objective of this study was to describe demographic and clinical characteristics of hospitalised US veterans with nosocomial pressure ulcer (NPU) referred to a certified Wound, Ostomy & Continence Nurse (WOCN). We conducted a retrospective review of electronic records at a Veterans Affairs Medical Center in the northwestern USA. Records of veterans with NPU referred to a WOCN (n = 29) from May 2005 to June 2006 were reviewed. Location and stage of pressure ulcer(s), Braden score on admission and when the ulcer was first noted, day of hospital stay when the ulcer was first noted, medical diagnoses and clinical conditions and events such as surgery, hypoxemia, hypoalbuminemia and hypotension were recorded. Mean age of the patients was 69·8. The most common location was the sacrum/coccyx. Most ulcers were stage 1 when identified. Braden score during admission classified half of the sample at risk, but 81% of Braden scores at ulcer occurrence were <18. Ninety percent of the sample had three or more comorbidities. Over half had died in the 1–14 months after the reviewed hospitalisation. Hospitalised veterans referred for WOCN consultation had multiple risk factors and comorbid conditions, including hypoxemia, serum albumin depletion, anaemia and hypotension. Veterans cared for in Veterans Affairs Medical Centers are known to have multiple health problems, and those in this sample not only had nosocomial pressure ulcer, but also other physiological derangements that may shorten survival.
Keywords: Complications, Hospital‐acquired, Nosocomial, Pressure ulcer, Veterans
Prevention of nosocomial pressure ulcers (NPUs) in hospitals has long been a concern of health care providers and regulatory agencies because of the suffering, disability and health care costs associated with them. NPU incidence in acute care is estimated at 7–9%, costing $2·2–6·4 billion annually in the USA, with higher incidence in intensive care units (ICUs) 1, 2. This study's purpose was to identify characteristics of US veterans who developed NPU during hospitalisation, which have not been described previously in the literature. Data were obtained from referrals to a certified Wound, Ostomy & Continence Nurse (WOCN). Describing the characteristics of this population may facilitate early identification of hospitalised veterans at high risk of developing a NPU.
INTRODUCTION
Nosocomial pressure ulcers
Pressure ulcers (PUs) are thought to be caused by local tissue ischaemia, interstitial and lymphatic blockage, reperfusion injury related to free radical damage and mechanical deformation of cells by compressive forces 3, 4. One conceptual scheme proposes that tissue tolerance of pressure and oxygen deprivation mediates the effect of compressive and shearing forces in ultimately determining NPU occurrence 5, 6. Tissue tolerance is affected by clinical and demographic variables that will be reviewed in this section.
Mean ages of hospitalised patients with NPU fall between 59 and 73 years 7, 8, 9, 10, 11, 12, 13, 14. Patients with NPUs tend to be older than those without, but, in some studies, age is non significant 9, 15, 16, 17, 18 and in others age becomes non significant when analysed with age‐related conditions such as decreased weight, sensory deficit, incontinence, altered mental status, malnutrition, immobility and low serum albumin 9, 14, 15, 19.
The largest percentage of PUs occur on the sacrum, followed by the heels 1, 7, 9, 19, 20. Some authors classify locations in the pelvic girdle such as the sacrum, coccyx, ischial tuberosities and the buttocks together and report this as the most common location 11, 19. In one ICU study (21) incidence was higher on the heels than on the sacrum.
Incidence studies report NPUs as largely stage 1 and 2 7, 11, 13, 22. Some do not report stage 1 ulcers, and find the majority of breakdown to be partial thickness 10, 12. Some studies 9, 11, 20 found stage 1 to be more common than stage 2, while others 7, 22 found the opposite. These differences may be the result of different measurement intervals, populations and length of follow up or difficulty in detecting stage 1 PU (1).
Literature on ICU stays and overall length of stay (LOS) varies. Although many sources cite higher PU incidence in ICUs versus acute care, the actual reported incidence in ICUs varies widely, from 1% to 56% (23), and studies are not consistent with regard to an ICU stay as a risk factor (13). In a study of surgical ICU patients (8), 96% of NPUs occurred in patients whose LOS was greater than 7 days. Among all patients whose LOS was at least 7 days, those who developed NPUs had a total stay twice as long as those who did not. However, LOS was not significant in multivariate analysis when age, emergent admission, days without nutrition and days in bed were included in a predictive model. Lindgren (24) found LOS to be significant in predicting NPU incidence and Scott (25) found LOS to be significantly longer in those with NPUs compared to those without (mean difference = 6·7 days). Although associations between longer LOS and NPU appear valid, no causal association can be inferred from these studies.
Most studies show that NPUs occur relatively early in the hospital stay (1). Baumgarten (22) found 6·2% NPU incidence within 2 days of admission in hospitalised older adults. Whittington (20) found a 7–9% incidence after 5 days of hospitalisation. In an ICU study, stages 1, 2 and 3 NPUs developed within an average of 5–6, 12–17·5 and 17–20 days of admission, respectively (21). An ICU study of mechanically ventilated patients reported that stages 1, 2 and 3 NPUs developed within 13, 16·1 and 19 days of admission (26). In a third ICU study, 68·7% of patients acquired their NPUs within 7 days (13). Schultz et al. (11) found 21·5% incidence of NPU within 6 days in a surgical population.
The Braden scale is the most extensively studied PU risk assessment scale. Total scores, ranging from 6 to 23, are obtained by summing six subscale scores: sensory perception, moisture, nutrition, activity, mobility and friction/shear. A lower score indicates higher risk. Most studies find that lower Braden scores are significantly associated with PU occurrence 7, 11, 13, 21, 27, 28. Pender and Frazier (26) found that the lowest Braden score was not predictive in an ICU where all the study participants were at risk. In a study of the predictive validity of the Braden scale in tertiary care, Veterans Affairs Medical Centers (VAMCs) and skilled nursing facilities, Bergstrom et al. (29) recommended an overall cutoff score of 18, although the cutoff that maximised sensitivity and specificity in the VAMCs was 19. The study also showed that in VAMCs, Braden scores on admission and 48–72 hours after admission were predictive of NPU development, but the score at the time of NPU occurrence was the most predictive. Keller et al. (23) noted that scales and cutoff scores with acceptable sensitivity generally lack specificity, but ultimately concluded that the Braden was preferable to other scales for use in predicting NPUs in ICUs.
Relationships between medical diagnosis and NPU are not clear. Lindgren et al. (24) found that cardiovascular diseases were the most common medical diagnoses among patients with NPUs. Whittington and Brionnes (20) found that 18–22% and 20–23% of acute care patients with NPUs had primary cardiovascular and respiratory diagnoses, respectively. Bergstrom et al. (7) found that cardiovascular diseases were the only diagnoses associated with NPU occurrence, but this was not significant after controlling for Braden score. In an ICU (21), cardiovascular disease was the most common reason for admission (50·7%), but people with cardiovascular disease had the lowest NPU prevalence. Despite the predominance of cardiovascular disease in some studies, it is not clear whether the percentage of people developing a NPU who have cardiovascular disease is merely reflective of the prevalence of cardiovascular disease or whether there is actually an association between cardiovascular disease and NPU development.
Individual cardiovascular diagnoses, such as coronary artery disease, stroke, hypertension and congestive heart failure have been studied with mixed results 7, 10, 13, 15, 27. Endothelial cell dysfunction may link hypertension and NPUs (30). Endothelial dysfunction is associated with diabetes, coagulopathy and peripheral vascular disease, which have been studied for their association with NPU development, albeit with inconsistent results 7, 11, 15, 23, 25, 27, 31. The use of ankle‐brachial pressure index has been advocated to identify arterial insufficiency as a risk factor for heel PUs (32).
Infection and sepsis have been examined in relation to NPU development in multiple studies. Reed et al. (10) found that neither pneumonia nor white blood cell count >20 000/ml was associated with NPU development. Bergstrom et al. (7) found no link between sepsis and NPU. Others have found that most NPUs occurred in people with a diagnosis of infection or sepsis 8, 12, 21, 25. In a study of acute care patients with HIV, CD4 count <100 cells/µl was a predictor of NPU development and 12 of 44 NPUs occurred in people with bacteremia, half originating from the NPU (33). Redelings (34) showed that septicemia was much more prevalent in persons who died with a pressure ulcer than those who did not. Blood flow irregularities in sepsis include higher skin blood flow and lower peak hyperemic response (35), phenomena that may be associated with susceptibility to NPU.
The role of nutritional supplementation, body mass index (BMI) and indicators of nutritional status is unclear (36). In three studies of surgical patients, serum albumin was not significant in multivariate analysis 9, 11, 31. Schultz et al. (11) found no difference in serum albumin between surgical patients with and without NPUs, although lower BMI, which affects the intensity of pressure over bony prominences, was associated with NPU occurrence. Others 15, 36 have reported relationships between low BMI and NPU, and between decreased serum albumin and NPUs 12, 20. A very large prevalence study (37) showed higher prevalence of stage 1 NPU in those with low BMI and higher prevalence of stage 2 NPU in people with very high BMI. It is not clear if these findings are related to the difficulty in identifying stage 1 NPU in the very obese patient, or a true effect of extremes of body habitus.
Although, in some studies, serum albumin was not a NPU predictor 9, 11, 22, 31, serum albumin depletion has been associated with NPUs in many other studies, including a study of 2771 VAMC patients (10). Both Theaker et al. (31) and Baumgarten et al. (22) found that although serum albumin was not significant, reduced nutritional intake was associated with NPU occurrence, likely due to the time required for decreased nutrition to be manifested in serum albumin decrements. In a hierarchical model including the Braden subscales, Fisher et al. (14) found that the Braden moisture, mobility and sensory perception subscales were more predictive than nutrition. Nutritional intake may not be closely related to nutritional state when illness causes hypermetabolism and cachexia. Four of five studies reviewed by Reddy et al. (38) found that nutritional supplementation had no significant effect on pressure ulcer occurrence. A Cochrane review of nutritional interventions (39) identified only one study of acceptable quality; that study found that nutritional supplements reduced the number of new NPUs in nutritionally depleted critically ill elders (40).
Theaker et al. (31) found anaemia increased incidence of NPUs threefold, but haematocrit <30% was not related to the development of NPUs in another study (10). A review of risk factors for cardiac surgical patients (27) noted that preoperative haematocrit was a significant NPU predictor in two studies, and haemoglobin in one study.
Vasopressors and hypotensive episodes potentially affect skin integrity by decreasing peripheral blood flow. A number of studies have found vasopressors to be a significant factor in NPU development in the ICU 20, 25, 26, 31. In one study, 14% of people with NPUs were on vasopressors, and 5% of those without NPUs were on vasopressors (26), although no statistical analysis was provided. Another study found that use of intravenous norepinephrine for more than 60% of a patient's LOS increased incidence of NPU eight times when compared to patients receiving intravenous norepinephrine for 0–40% of the ICU stay (31).
NPUs appear to be associated with mortality. Allman et al. (41), found that 4 of 6 (67%) patients who developed NPUs died in the hospital, compared to 11 of 72 (15%) at‐risk patients who did not develop NPU. Critically ill surgical patients with NPU had (8) mortality rates between 33% and 61% in one 5‐year study. Keller et al. (23) cite a study of 638 ICU patients in which 63% of patients with NPUs died, compared to 15% of patients without NPUs. A study of patients with HIV infection found a mortality rate of 50% for those with NPUs, and 7·2% for those without (33). A longitudinal study (42) found that more patients with NPUs died (59·5%) within 1 year of discharge than those without (38·2%), but NPUs were not significant in predicting death in multivariate analysis when other indicators of prognosis and illness severity were included. Reed et al. (10) found that having a do‐not‐resuscitate (DNR) order was a significant predictor of NPU, perhaps because illness severe enough to necessitate a DNR order links NPU occurrence to the end of life.
Patients may have concurrent illnesses that increase risk of NPU and bring about consideration of end of life choices. Although it is possible that DNR status may cause neglect of NPU prevention, there is evidence that seriously ill patients develop NPUs despite preventive care 10, 43, 44, 45. PUs may be part of a syndrome of progressive organ failure, and the term ‘skin failure’ has been proposed to describe this phenomenon (43). The National Pressure Ulcer Advisory Panel issued a press release endorsing the existence of skin failure and stating that certain NPU, such as those in haemodynamically unstable patients, are not always preventable (45).
In summary, our review of the literature identified a number of potential risk factors for NPU that we chose to examine in this study. It remains clear that risk factors vary considerably and that more study is needed to define a robust set of variables influencing tissue tolerance and NPU formation.
The US veteran population
The health of the veteran population differs substantially from that of the general US population. In the 4‐year Veterans Health Study (N = 2425) (46), 22% of participants were more than 50% disabled, and 46% had combat experience. The median age was 65 years and the mean number of medical diagnoses was six. In a nationwide sample, Nelson (47) showed a higher prevalence of obesity among veterans receiving care through the US Veterans Health Administration (VHA) compared to other veterans and civilians (27·7, 23·9 and 22·8% respectively.) There are two explanations for the relatively poor health status of US veterans as compared to US civilians. Veterans qualify for care through the VHA either because they have a disability related to their military service or because their annual income is below an established threshold (46). Veterans seeking care through the VHA thus tend to be more disabled and poorer than others. Additionally, military service exposed veterans to unique health risks both physically, such as Agent Orange used in the Vietnam War, and psychologically, such as combat, to which the civilian population is not exposed.
The diminished health status of US Veterans seeking care through the VHA may increase risk of NPU when these people are hospitalised. Disability may impede mobility, and chronic illnesses may impair tissue tolerance. It is thus important to describe this population and begin to understand its characteristics in order to improve prevention of NPU.
METHODS
A retrospective chart review was done to identify the veterans who developed NPU and received WOCN consultation. A data collection instrument that listed pressure ulcer risk factors was developed from the literature review and consultation with Veterans Affairs (VA) practitioners. Demographic and clinical factors were included. A list of all the study variables and their operational definitions is given in Table 1. The data collection form was reviewed by a WOCN at the VAMC where the study was done for completeness, appropriateness to the population and consistency with the literature.
Table 1.
Study variable definitions
| Variables | Definitions |
|---|---|
| Age | Age in years |
| Gender | Male or female |
| Total LOS | Number of days in hospital |
| ICU admission | Whether in ICU at any time during hospital stay |
| Surgery | Whether had any surgery during hospital stay |
| Length of surgery | In hours to the nearest half hour |
| Died | No longer alive at the time of data collection in July to August 2006 |
| Admission Braden | First recorded Braden score during hospital stay |
| Days before NPU noted | Days between admission and first recording of NPU |
| Braden when NPU noted | Braden score recorded nearest in time to the first recording of NPU |
| Lowest Braden | Lowest Braden during hospital stay |
| Location of NPU #1 | Sacrum/coccyx, heel, occiput, scapulae, or other; pertains to NPU identified earlier, if two |
| Stage when NPU #1 noted | NPUAP‐defined stage of NPU #1 as recorded by WOCN |
| Highest stage of NPU #1 | Highest NPUAP‐defined stage of NPU #1 as recorded by WOCN |
| Location of NPU #2 | If two NPU, location of NPU identified later |
| Serum albumin | Mg/dL albumin; value closest in time to the first recording of NPU |
| Serum prealbumin | Mg/dL prealbumin closest in time to the first recording of PU |
| Hypotension | Positive if any two systolic blood pressures under 90 mm Hg |
| Hypoxemia | Positive if any two oxygenation saturation values under 90% |
| Haematocrit | As recorded by hospital laboratory in %; value closest in time to the first recording of NPU |
| Vasopressor therapy | Positive if any infusion of dopamine, norepinephrine, phenylephrine, vasopressin or epinephrine |
| Without nutrition | Positive if 48 hours or more without oral, enteral or parenteral nutrition |
| Medical diagnoses | As recorded in electronic health record at time of death or discharge |
LOS, length of stay.
WOCN consultation notes were obtained for the 13‐month period from May 2005 to June 2006. A retrospective review of all WOCN‐authored consultation notes, and corresponding electronic health records (EHRs), identified 29 patients with NPU. PUs caused by devices such as braces and prostheses were excluded. The two researchers extracted data from the EHRs on the relevant variables and recorded the data on a study form. A subset of five records was cross‐checked to confirm the accuracy of data recording. In cross‐checking, the researchers accessed EHRs independently to confirm the accuracy of the other's data abstraction. Disagreements were discussed and mutually resolved.
Medical diagnoses collected were systemic diseases known to occur frequently in the VA population (46). These were coronary artery disease, congestive heart failure, atrial fibrillation, peripheral vascular disease, hypertension, chronic obstructive pulmonary disease, diabetes mellitus, renal failure, liver failure, sepsis and depression. Additional systemic illnesses were classified as ‘other’. Non systemic illnesses such as glaucoma and peptic ulcer were excluded. Medical diagnoses with systemic implications, such as cardiac, pulmonary, renal and liver disease, were recorded, but common illnesses without systemic effects, such as glaucoma and haemorrhoids, were not. Institutional Review Board approval was obtained from the VAMC. Consent was waived because the study entailed only a retrospective chart review, and all recorded data were de‐identified.
RESULTS
Data were analysed using IBM (Armonk, NY, USA) SPSS versions 19 and 20. The sample (n = 29) of veterans had a mean age of 70; most (97%) were men (Table 2). All subjects had at least two comorbid illnesses, as shown in Figure 1. Ninety percent of the sample had at least three comorbid illnesses. The mean number of comorbidities was 4·1 and the median was 4. Figure 2 shows the number of specific comorbidities in the sample. Serum albumin, prealbumin and haematocrit values were below normal. Serum albumin was subnormal in 20 of 25 subjects (80%) (M = 2·9, SD = 0·7). Serum prealbumin concentrations were available on 13 subjects, and ranged from 4·4 to 28 mg/dL (reference range 18–36 mg/DL). Eight (62%) serum prealbumin values were subnormal. Haematocrit was subnormal in 25 of 29 veterans (86%) (M = 30·6, SD = 6·5).
Table 2.
Demographic and clinical characteristics
| Variables | Findings |
|---|---|
| Age | X = 70, SD = 9·5 |
| Gender | Male = 97% |
| Total LOS | X = 23, R = 3–80 |
| ICU admission | Yes = 69% |
| Surgery | Yes = 55% |
| Length of surgery | X = 5·2, SD = 2·8, R = 1–9·5 |
| Died during or since hospitalisation | Yes = 51% |
| Admission Braden scale score | M = 19, R = 9–23 |
| Number of days hospitalised before NPU noted | M = 4·0, R = 1–20 |
| Braden Scale Score when NPU noted | M = 15, R = 9–21 |
| Lowest Braden | M = 13, R = 8–22 |
| Location of NPU #1 | Sacrum/coccyx = 62%, heels = 7%, elbow = 3%, other = 28% |
| Stage when NPU #1 noted | One = 55%, two = 35%, unstageable/closed = 10% |
| Location of NPU #2 | Sacrum/coccyx = 66%, heels = 34% |
LOS, length of stay; NPU, nosocomial pressure ulcers; X, mean; M, median; SD, standard deviation; R, range.
Figure 1.
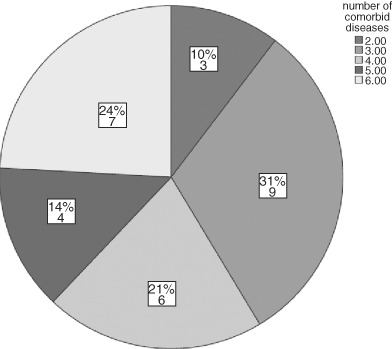
Number of comorbidities in the sample (N and percentage).
Figure 2.
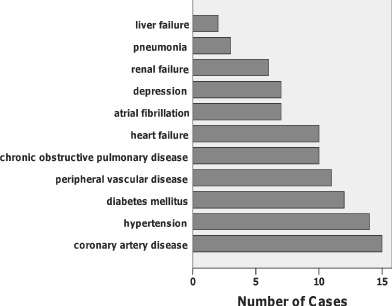
Specific medical diagnoses in the sample.
Certain clinical events possibly associated with NPU occurred in over 30% of the sample. Hypotension occurred in 70%, surgery in 55%, hypoxemia in 41%, lack of nutritional intake in 41% and vasopressor infusion in 30%. More than two thirds of veterans (N = 20) were admitted to the ICU during the hospital stay, and ICU LOS (mean = 10·9 days) was nearly as long as ward LOS (mean = 11·9 days). More than half of the patients in the sample had died by the time the data were collected, which took place from 1 to 14 months after the hospital stay in which the NPU occurred.
Thirty‐three percent of NPU were documented within 3 days of hospitalisation, 62% within 1 week and 86% within 2 weeks. Fifty‐five percent of NPUs were stage 1 and 34·5% were stage 2 when first noted. Median NPU stage at the time of detection was 1, and median highest stage was 2. Three NPUs were documented as closed, but unstageable, with characteristics indicative of deep tissue damage upon detection. Six patients had more than one pressure ulcer.
The sacrum or coccyx was the most common location, followed by heels and elbows. Other NPU locations were the gluteus, scrotum, thoracic spine and earlobe. The type of mattress in use was recorded in only 12 cases; of these, 8 were the hospital's standard foam mattresses.
Non parametric tests were used to determine whether those who had died versus survived and those whose highest PU stage was 2 versus 1 differed with respect to number of comorbid illnesses, serum albumin levels, occurrence of any surgery, length of surgery, length of ICU stay, length of ward stay, overall LOS, whether they went 48 hours or more without nutrition, occurrence of hypoxia, occurrence of hypotension, any use of vasopressors, serum haematocrit, and age. Only older age differentiated those who died from those who did not (Mann‐Whitney U = 50, exact P value = 0·029), with more aged veterans dying. Only length of surgery differentiated those with stage 2 as the highest stage of PU versus stage 1 (Mann‐Whitney U = 3·5, exact P value = 0·031), with those who had longer surgeries incurring more stage 1 PU.
LIMITATIONS
This study does not compare risk factors in this group with a population norm or to a group of patients without NPUs, nor does it analyse the relative importance of risk factors in NPU development. Because this was a retrospective chart review, the researchers relied on prior documentation and there were some incomplete data. The investigators identified charts to review by referrals made to the WOCNs, so hospitalised veterans with NPUs who did not receive WOCN referrals were excluded. The demographic and clinical characteristics of those with NPU but without WOCN referrals are not known. Although including only patients seen by a WOCN did limit the sample size, the expertise of these certified nurses imparted credibility to the data regarding NPU staging and differentiation of NPU from other types of wounds such as moisture‐associated skin damage and incontinence‐related dermatitis.
DISCUSSION
In previous studies 24, 31, longer LOS was associated with NPU development, but the LOS in those studies was shorter than in this study, which may be indicative of the overall illness severity of the hospitalised veterans who received WOCN consultation. We cannot tell whether LOS increases because of NPU or if illness severity increases both LOS and NPU occurrence. Research into these potential causal relationships would shed light on the pathogenesis of NPU.
The median lowest Braden recorded during each patients' stay (13) was lower than the median on admission (18·5), indicating variability in patient condition over the course of hospitalisation, increasing NPU risk and need for continuous assessment over the course of their stay. The current results were consistent with literature on the Braden in VAMCs (29), showing higher sensitivity with subsequent assessments compared to admission assessments.
Cardiovascular diseases were common in this sample. This group of patients with NPUs had approximately twice the incidence of diabetes (41%) as patients with NPUs from other studies (23·7, 21·5, 19%) 20, 35, 41 and the incidence of hypertension in this sample (48%) was much higher than that in the general population. These rates are, in part, reflective of the veteran population. The hypertension incidence is comparable to that found in the Veterans Health Study (46), which was 49·2% in the 65–91‐year‐old age group, but the diabetes incidence in this sample remains considerably higher than the 20·3% rate in that study.
Thirty‐four percent of the patients in this sample had chronic obstructive pulmonary disease and 41% experienced hypoxic episodes during their stay. Hypoxemia decreases oxygen available to tissues, impairing the tissue's ability to withstand pressure‐related ischaemia. Shear may also cause NPUs in patients with respiratory compromise, as these patients often sit upright, a position that contributes to shear forces (30).
More than half of our sample had undergone a surgical procedure. Many studies have shown that surgical patients are at increased risk of NPU 9, 23, 24, 28, 48. Reported rates vary widely, however, from 4% to 66% 11, 30, likely because length of surgery, operating room support surfaces and patient characteristics vary. Length of time without repositioning on an operating room support surface would lead to the expectation that longer surgeries would be associated with stage 2 versus stage 1 PU, but we found the opposite. This may be related to the differing aetiologies of stage I and stage II PU and the concept that PU do not proceed through the stages in a linear fashion, as hypothesised by some prominent investigators (49).
Severity of illness has been shown to be a NPU indicator in multiple studies. Disease severity indices such as the Apache II (Acute Physiology and Chronic Health Evaluation II) (50), SAPS II (Simplified Acute Physiology Score II) (48), ASA (American Society of Anesthesiology) score and NYHA (New York Heart Association) class have been shown to be NPU predictors 9, 21, 25, 31, 33.
These indicators appear more predictive of NPU than medical diagnoses 10, 25, 32. The high incidence of hypotension, use of vasopressors, and depressed haematocrit and albumin levels in this sample are consistent with the literature. Mean albumin was lower than in previous studies 9, 11. The finding that over half of the patients (51·2%) in the current study died within 14 months of developing a NPU is consistent with the literature and lends weight to the argument that NPUs are associated with more serious illness 43, 51.
This study shows that caregivers should prioritize skin care early in the hospital stay. Nurses should use PU risk assessment tools throughout the hospital stay, as later assessments may be more predictive than admission assessments. Although validated PU risk assessment scales have widespread support (52), it is also important to consider overall illness severity, treatment variables such as surgery, ICU stay, nutritional status, hypoxemia, hypotension and vasopressor support as well as comorbidities when assessing NPU risk. Skin failure should be considered when clinicians make decisions about palliative versus aggressive care, as it may be a part of the multi‐system alterations that occur at end of life.
This sample of acutely ill US veterans experienced many factors associated with NPU that were consistent with published evidence. While expert nursing care is crucial in preventing NPUs in hospitalised people, many other factors must be considered in NPU causation. Clinicians should consider broadening NPU risk evaluation from the Braden and other standard scales to include other cooccurring factors. Including other influences on NPU formation may enhance the validity of NPU risk assessment and encourage earlier use of prevention techniques, reducing NPU incidence. Further research is needed in this and other severely ill populations to determine the effectiveness of such an approach.
ACKNOWLEDGEMENTS
Funded in part by Sam Medical, Inc., Newport, Oregon, USA. The authors would like to acknowledge Cathryn Vogeley, MSN,WOCN and Mark Deffebach, MD, for their assistance with the study.
REFERENCES
- 1. Berlowitz, D. Pressure ulcers: staging; epidemiology; pathogenesis; clinical manifestations; and staging. 2011. http://www.uptodate.com/contents/pressure‐ulcers‐epidemiology‐pathogenesis‐clinical‐manifestations‐and‐staging?source=search_result&search=pressure+ulcer&selectedTitle=296 [accessedon 12 August 2011].
- 2. Wound, Ostomy and Continence Nurses Society. Overview white paper. N.D. http://www.wocn.org/resource/resmgr/advocacy_policy_white_papers/white_papers.pdf [accessed on 12 August 2011].
- 3. Bouten CV, Oomens CW, Baaijens FP, Bader DL. The etiology of pressure ulcers: skin deep or muscle bound? Arch Phys Med Rehabil 2003;84:616–9. [DOI] [PubMed] [Google Scholar]
- 4. Thompson D. A critical review of the literature on pressure ulcer aetiology. J Wound Care 2005;14: 87–90. [DOI] [PubMed] [Google Scholar]
- 5. Braden B, Bergstrom N. A conceptual schema for the study of the etiology of pressure sores. Rehabil Nurs 1987;12:8. [DOI] [PubMed] [Google Scholar]
- 6. Defloor T. The risk of pressure sores: a conceptual scheme. J Clin Nurs 1999;8:206–16. [DOI] [PubMed] [Google Scholar]
- 7. Bergstrom N, Braden B, Kemp M, Champagne M, Ruby E. Multi‐site study of incidence of pressure ulcers and the relationship between risk level, demographic characteristics, diagnoses, and prescription of preventative interventions. J Am Geriatr Soc 1996;44:22. [DOI] [PubMed] [Google Scholar]
- 8. Eachempati SR, Hydo LJ, Barie PS. Factors influencing the development of decubitus ulcers in critically ill surgical patients. Crit Care Med 2001;29:1678–82. [DOI] [PubMed] [Google Scholar]
- 9. Lindgren M, Unosson M, Krantz AM, Ek AC. Pressure ulcer risk factors in patients undergoing surgery. J Adv Nurs 2005;50:605–12. [DOI] [PubMed] [Google Scholar]
- 10. Reed RL, Hepburn K, Adelson R, Center B, McKnight P. Low serum albumin levels, confusion, and fecal incontinence: are these risk factors for pressure ulcers in mobility‐impaired hospitalized adults? Gerontology 2003;49:255–9. [DOI] [PubMed] [Google Scholar]
- 11. Schultz A, Bien M, Dumond K, Brown K, Myers A. Etiology and incidence of pressure ulcers in surgical patients. AORN J 1999;70:434–49. [DOI] [PubMed] [Google Scholar]
- 12. Fogerty MD, Abumrad NN, Nanney L, Arbogast PG, Poulose B, Barbul A. Risk factors for pressure ulcers in acute care hospitals. Wound Rep Regen 2008;16,11–18. [DOI] [PubMed] [Google Scholar]
- 13. Fife C, Otto G, Capsuto EG, Brandt K, Lyssy K, Murphy K, Short C. Incidence of pressure ulcers in a neurologic intensive care unit. Crit Care Med 2001;29:283–90. [DOI] [PubMed] [Google Scholar]
- 14. Fisher, AR , Wells G, Harrison MB. Factors associated with pressure ulcers in adults in acute care hospitals. Adv Skin Wound Care 2004;17:80–90. [DOI] [PubMed] [Google Scholar]
- 15. Allman RM, Goode PS, Patrick MM, Burst N, Bartolucci AA. Pressure ulcer risk factors among hospitalized patients with activity limitation. JAMA 1995;273:865–70. [PubMed] [Google Scholar]
- 16. Compton F, Hoffmann F, Hortig T, Strauss M, Frey J, Zidek W, Schäfer JH. Pressure ulcer predictors in ICU patients: nursing skin assessment versus objective parameters. J Wound Care 2008;17:417 [DOI] [PubMed] [Google Scholar]
- 17. Hengstermann S, Fischer A, Steinhagen‐Thiessen E, Schulz RJ. Nutrition status and pressure ulcer: what we need for nutrition screening. JPEN J Parenter Enteral Nutr 2007;31:288–94. [DOI] [PubMed] [Google Scholar]
- 18. Walsh JS, Plonczynski DJ. Evaluation of a protocol for prevention of facility‐acquired heel pressure ulcers. J WOCN 2007;34:178–83. [DOI] [PubMed] [Google Scholar]
- 19. Maklebust J, Magnan MA. Risk factors associated with having a pressure ulcer: a secondary data analysis. Adv Wound Care 1994;7:25–42. [PubMed] [Google Scholar]
- 20. Whittington KT, Briones R. National prevalence and incidence study: 6 year sequential acute care data. Adv Skin Wound Care 2004;17: 490–4. [DOI] [PubMed] [Google Scholar]
- 21. Bours GJJ, De Laat E, Halfens RJG, Lubbers M. Prevalence, risk factors and prevention of pressure ulcers in Dutch intensive care units. Results of a cross‐sectional survey. Intensive Care Med 2001;27:1599–605. [DOI] [PubMed] [Google Scholar]
- 22. Baumgarten M, Margolis DJ, Localio AR, Kagan SH, Lowe RA, Kinosian B, Holmes JH, Abbuhl SB, Kavesh W, Ruffin A. Pressure ulcers among elderly patients early in the hospital stay. J Gerontol A Biol Sci Med Sci 2006;61:749–754. [DOI] [PubMed] [Google Scholar]
- 23. Keller BPJA, Wille J, van Ramshorst B, van der Werken C. Pressure ulcers in intensive care patients: a review of risks and prevention. Intensive Care Med 2002;28:1379–88. [DOI] [PubMed] [Google Scholar]
- 24. Lindgren M, Unosson M, Fredrikson M. Ek AC: immobility – a major risk factor for development of pressure sores among adult hospitalized patients: a prospective study. Scand J Caring Sci 2004;18:57–64. [DOI] [PubMed] [Google Scholar]
- 25. Scott JR, Gibran NS, Engrav LH, Mack CD, Rivera FP. Incidence and characteristics of hospitalized patients with pressure ulcers: State of Washington, 1987 to 2000. J Am Soc Plast Surg 2006;117:630–4. [DOI] [PubMed] [Google Scholar]
- 26. Pender LR, Frazier SK. The relationship between dermal pressure ulcers, oxygenation and perfusion in mechanically ventilated patients. Intensive Crit Care Nurs 2005;21:29–38. [DOI] [PubMed] [Google Scholar]
- 27. Feuchtinger J, Halfens RJG, Dassen T. Pressure ulcer risk factors in cardiac surgery; a review of the literature. Heart Lung 2005;34:375–85. [DOI] [PubMed] [Google Scholar]
- 28. Wolverton CL, Hobbs LA, Benjamin M, Forbes C, Kieninger M, Luebbehusen M, White S. Nosocomial pressure ulcer rates in critical care: performance improvement project. J Nurs Care Q 2004;19:56–62. [DOI] [PubMed] [Google Scholar]
- 29. Bergstrom NJ, Braden N, Kemp M, Champagne M, Ruby E. Predicting pressure ulcer risk: a multi‐site study of the predictive validity of the Braden scale. Nurs Res 1998;47:261–9. [DOI] [PubMed] [Google Scholar]
- 30. Price MC, Whitney JD, King CA. Development of a risk assessment tool for intraoperative pressure ulcers. J Wound Ostomy Continence Nurs 2005;32:19–30. [DOI] [PubMed] [Google Scholar]
- 31. Theaker C, Mannan M, Ives N, Soni N. Risk factors for pressure sores in the critically ill. Anaesthesia 2000;55:221–4. [DOI] [PubMed] [Google Scholar]
- 32. Graham J. Heel pressure ulcers and ankle brachial pressure index. Nurs Times 2005;101:47–8. [PubMed] [Google Scholar]
- 33. Nicastri E, Vaile P, Lyder CH, Cristini F, Martini l, Preziosi G, Dodi F, Irato L, Angelo P, Petrosillo N. Incidence and risk factors associated with pressure ulcers in among patients with HIV infection. Adv Skin Wound Care 2003;17:226–31. [DOI] [PubMed] [Google Scholar]
- 34. Redelings MD, Lee NE, Sorvillo F. Pressure ulcers: more lethal than we thought? Adv Skin Wound Care 2005;18:367–72. [DOI] [PubMed] [Google Scholar]
- 35. Young JD, Cameron EM. Dynamics of skin blood flow in human sepsis. Intensive Care Med 1995;21:669–74. [DOI] [PubMed] [Google Scholar]
- 36. Shahin ESM, Meijers JMM, Schols JMG, Tannen A, Halfens RJG, Dassen T. The relationship between malnutrition parameters and pressure ulcers in hospitals and nursing homes. Nutrition 2010;26: 886–9. [DOI] [PubMed] [Google Scholar]
- 37. VanGilder C, MacFarlane G, Meyer S, Lachenbruch C. Body mass index, weight, and pressure ulcer prevalence: an analysis of the 2006‐2007 International Pressure Ulcer Prevalence Surveys. J Nurs Care Qual 2009;24:127–35. [DOI] [PubMed] [Google Scholar]
- 38. Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA 2006; 296:974–84. [DOI] [PubMed] [Google Scholar]
- 39. Langer G, Knerr A, Kuss O, Behrens J, Schlomer GJ. Nutritional interventions for preventing and treating pressure ulcers. Cochrane Database Syst Rev 2009;2. [DOI] [PubMed] [Google Scholar]
- 40. Bourdel‐Marchasson I, Barateau M, Rondeau V, Dequae‐Merchadou L, Salles‐Montaudon N, Emeriau JP, Manciet G, Dartigues JF. A multi‐center trial of the effects of oral nutritional supplementation in critically ill older inpatients. GAGE Group. Groupe Aquitain Geriatrique d’Evaluation. Nutrition 2000;16:1–5. [DOI] [PubMed] [Google Scholar]
- 41. Allman RM, Laprade CA, Noel LB, Walker JM, Moorer CA, Dear MR, Smith CR. Pressure sores among hospitalized patients. Ann Int Med 1986;105:337–42. [DOI] [PubMed] [Google Scholar]
- 42. Thomas DR, Goode PS, Tarquine PH, Allman RM. Hospital‐acquired pressure ulcers and risk of death. J Am Geriatr Soc 1996;44:1435–40. [DOI] [PubMed] [Google Scholar]
- 43. Langemo DK, Brown G. Clinical concepts. Skin fails too: acute, chronic, and end‐stage skin failure. Adv Skin Wound Care 2006;19:206–11. [DOI] [PubMed] [Google Scholar]
- 44. Witkowski JA, Parish LC. Skin failure and the pressure ulcer. Decubitus 1993;6:4. [PubMed] [Google Scholar]
- 45. National Pressure Ulcer Advisory Panel. Not all pressure ulcers are avoidable. 2010. http://www.npuap.org/A_UA%20Press%20Release.pdf [accessed on 12 August 2011].
- 46. Kazis LE, Ren XS, Lee A, Skinner K, Rogers W, Clark J, Miller DR. Health status in VA patients: results from the Veterans Health Study. Am Med Qual 1999;14:28–38. [DOI] [PubMed] [Google Scholar]
- 47. Nelson KM. The burden of obesity among a national probability sample of veterans. J Gen Intern Med 2006;21:915–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Le Gall J, Loirat P, Alperovitch A. A simplified acute physiology score for ICU patients. Crit Care Med 1984;12:975–7. [DOI] [PubMed] [Google Scholar]
- 49. Sibbald RG, Krasner DL, Woo K. Pressure ulcer staging revisited: superficial skin changes & deep pressure ulcer framework(C). Adv Skin Wound Care 2011;24:571–80. [DOI] [PubMed] [Google Scholar]
- 50. Knaus WA, Wagner DP, Draper EA, Zimmerman JE. APACHE II final form and national validation results of a severity of disease classification system. Crit Care Med 1984;12:213. [PubMed] [Google Scholar]
- 51. Shoonhoven L, Grobbee DE, Donders ART, Algra A, Grypdonck MH, Bousema MT, Schrijvers AJP, Buskens E. Prediction of pressure ulcer development in hospitalized patients: a tool for risk assessment. Qual Saf Health Care 2006;15:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. National Pressure Ulcer Advisory Panel and European Pressure Ulcer Advisory Panel. Prevention and treatment of pressure ulcers: clinical practice guideline. Washington, DC: National Pressure Ulcer Advisory Panel, 2009.


