Abstract
Vacuum‐assisted closure (VAC) device is widely used to treat infected wounds in clinical work. Although the effect of VAC with different negative pressure values is well established, whether different negative pressures could result in varying modulation of wound relative cytokines was not clear. We hypothesise that instead of the highest negative pressure value the suitable value for VAC is the one which is the most effective on regulating wound relative cytokines. Infected wounds created on pigs' back were used to investigate the effects of varying negative pressure values of VAC devices. Wounds were treated with VAC of different negative pressure values or moist gauze, which was set as control. The VAC foam, semiocclusive dresses and moist gauze were changed on days 3, 5, 7 and 9 after wounds were created. When changing dressings, tissues from wounds were harvested for bacteria count and histology examination including Masson's trichrome stain and immunohistochemistry for microvessels. Western blot was carried out to test the expression of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF). Results showed that on days 3 and 5 the number of bacteria in wounds treated by VAC with 75, 150, 225 and 300 mmHg was significantly decreased compared with that in wounds treated by gauze and 0 mmHg pressure value. However, there was no difference in wounds treated with negative pressure values of 75 , 150, 225 and 300 mmHg at any time spot. Immunohistochemistry showed that more microvessels were generated in wounds treated by VAC using 75 and 150 mmHg negative pressure comparing with that using 225 and 300 mmHg on days 3 and 5. However this difference vanished on days 7 and 9. Morphological evaluation by Masson's trichrome staining showed increased collagen deposition in VAC of 75 and 150 mmHg compared with that in VAC of 225 and 300 mmHg. Western blot showed that the expression of VEGF and bFGF significantly increased when the wounds treated with 75 and 150 mmHg negative pressure values compared with the wounds treated with 225 and 300 mmHg on day 5. Treatment using VAC with different negative pressure values more than 75 mmHg has similar efficiency on reducing bacteria in the infected wound. VAC with negative pressure values of 75 and 150 mmHg promote wound healing more quickly than other pressure values. Moreover, comparing with vigorous negative pressure, relatively moderate pressures contribute to wound healing via accelerated granulation growth, increased angiogenic factor production and improved collagen fibre deposition. Further study of this model may show other molecular mechanisms.
Keywords: Different negative pressure values; VAC; Vacuum‐assisted closure; Wound healing
Introduction
In recent years, it has been difficult to treat wounds with infection and soft tissue defect resulted from accidents, injuries, trauma, constant pressure or burn injury. Reliable and efficacious treatment should not only be able to control infection but also promote healing of wounds. Traditional therapeutic approaches in the management of these wounds have encountered few successes.
One treatment modality that has been proved to be efficient in treating those complex wounds is the vacuum‐assisted closure (VAC) device 1, 2, 3. This device consists of a vacuum pump, a canister with a connecting tube, an open‐pore foam and a semiocclusive dressing. A tube connecting the dressing to a vacuum source applies sub‐atmospheric pressure to the wound. Argenta and Morykwas (4) and Morykwas et al.(5) first described the beneficial use of the VAC device for wound healing in 1997. VAC has been showed to enhance wound healing by increasing blood flow (6), granulation tissue promotion (7), increased angiogenesis 8, 9 and bacterial clearance (5). Recently, molecular mechanisms of wound healing by VAC therapy were discovered. Scherer et al.(10) showed that VAC therapy induced granular tissue formation, angiogenesis and cell proliferation. Derrick et al.(11) found that the VAC treatment in diabetic rat wounds led to more than 1·6‐fold change in 5072 genes expression. Another in vitro experiment showed that negative pressure could help disassemble the cell junction to enhance epithelial migration and subsequently resulted in quick wound closure (12). Other studies confirmed that VAC therapy can lead to increased expression of cytokines, which plays a critical role in wound healing. Labler et al.(13) showed that neovascularisation, interleukin (IL)‐8 and vascular endothelial growth factor (VEGF) in wound fluids were significantly higher in patients treated by VAC than those treated by gauze. Another study showed that CD31, VEGF, bFGF and collagen fibres were also increased by treating with VAC (14).
However, the effect was changed when using different negative pressure values. One study reported that wounds treated with a negative pressure value of 125 mmHg exhibited a significant increase in granulation tissue formation compared with treatment at 25 or 500 mmHgin a swine model (8). Morykwas et al.(4) showed that when using 125 mmHg, blood flow increased four times but decreased when the pressure value was 500 mmHg (15).
Considering different effects of VAC with varying negative pressure values and modulated cytokines by VAC, we hypothesise that different negative pressures may also alter wound‐healing related cytokines to different extents. This study was done to evaluate the effects of different negative pressures used in VAC therapy on VEGF, bFGF and collagen fibrin as well as the process of wound healing, which has been shown to be modulated by VAC.
Materials and methods
Experimental animal model and gross wound measurement
Six pigs (Guizhou Miniature Pigs, China) weighing 15–25 kg were purchased and housed in an approved animal care facility. Six full‐thickness wounds were created on the back of each pig. They were anaesthetised by intramuscular injection of ketamine (3–5 mg/kg), and then injected with 1% pentobarbital sodium 30 mg/kg. The backs of animals were then shaved and prepped with betadine solution and sterilised by 1% iodine and followed by 75% ethanol. Three rows of 2 cm × 5 cm length wounds were excised down to the deep fascia of the muscles over the spine. All 36 wounds were divided into six groups. All of them were infected with Staphylococcus aureus at the concentration of 106 colony forming units (CFUs) per millilitre. Every wound was inoculated with 2‐ml strains. Three days later infected wounds were counted successfully.
Wounds covered with gauze were taken as a control group. Wounds with negative pressure of 0 mmHg were dressed by VAC foam (VSD Inc, Wuhan, Hubei, China) and occlusive dressings (VSD Inc) without suction. Wounds treated with VAC were dressed with the standard VAC dressing which consists of foam, semiocclusive dressing and drainage pipe connected to the VAC device, and then subjected to continuous negative pressure values of 75, 150, 225, 300 mmHg. There were six groups: VAC device without suction (0 mmHg), VAC device with −75 mmHg negative pressure, VAC device with −150 mmHg negative pressure, VAC device with −225 mmHg negative pressure, VAC device with −300 mmHg negative pressure, and a control group in which the wounds were covered by gauze.
Tissues were collected from the central portion of the wounds, and then bacterial count and histological and protein analyses were taken on days 3, 5, 7 and 9 after surgery. On day 3, dressings were changed and thereafter changed every 48 hours. The pigs were euthanised on day 9.
Bacteria counting of wounds
After removing foam dressings and gauze, cleaning surface exudates with a sterile saline solution, biopsies were taken under aseptic conditions using a scalpel, taking granulation tissue from the centre of the wounds. The biopsies were taken at the beginning of the treatment and continued until the end of the therapy. The samples were immediately weighed, then homogenised and diluted. The diluted samples were placed on normal agar and chocolate agar and incubated at 37°C with 5% CO2 for 48 hours. The total number of CFUs per disk and the number of CFU per species were calculated. Biopsies were processed blinded and evaluated by a medical microbiologist. Results of the tissue biopsies were not revealed to the researchers until the end of the study.
Histology and immunohistochemical analysis
Granulation tissues of wounds were harvested and bisected, with half placed in 4% paraformaldehyde for 3 days, other half snap‐frozen in liquid nitrogen and then stored at −80°C for Western blot. The fixed tissues were embedded in paraffin and cut into 4‐µm sections. Sections were placed on glass slides for routine haematoxylin and eosin (H&E) staining. Masson's trichrome stain for collagen fibres in the wound was detected by performing standard protocol and blindly assessed independently. Images were acquired with 20× objective microscope lens. Then tissue section images were analysed using Image Pro‐Plus 6.0 (Media Cybernetics, Bethesda, MA). Six high‐power fields of 200× non‐overlapping areas were randomly selected at each section. The ratios of blue areas (collagen fibres) in a whole high‐power field were also calculated by the Image Pro‐Plus 6.0 analysis system.
Vasculature in the granulation tissues was assessed by staining for Factor VIII. Tissues were cut at 5 µm thickness. Sections were deparaffinized and treated with 20 mg/ml protenase K (Roche Inc., Basel, Switzerland) for 15 minutes. After washing with phosphate buffered saline (PBS), endogenous peroxidase was blocked for 5 minutes. Then sections were washed again with PBS and treated with blocking reagent. Primary multiclone Rabbit anti‐pig antibody of Factor VIII ((Santa Cruz Biotechology Inc., Santa Cruz, CA; 1:1000) was applied to the sections and incubated at room temperature for 1 hour, rinsed again with PBS repeatedly, and then the sections were incubated with fluoresceine isothiocyanate (FITC)‐ or rhodamine‐conjugated secondary antibody (Santa Biotechnology Inc.) for 30 minutes. Antibodies were visualised by treating with avidin–biotinylated enzyme complex, then with peroxidase substrate solution for 2 minutes. They were counter‐stained with Mayer's haematoxylin for 5 minutes. The stained blood microvessels on each slide were counted in the most vascular area of the section. Briefly, this method involves scanning tissue sections under high magnification to identify the hotspot. Within the hotspot, the number of vessels in a high‐power field of 400× over six non‐overlapping areas was counted.
Western blot analysis
Granulation tissues were homogenised in buffer with an added protease inhibitor cocktail (Roche Inc., Switzerland), made of 50 mM Tris, 10 mM NaCl, 1% NP40 and 0·02% sodium azide. Homogenates were placed into filtered centrifuge tubes and centrifuged at 13 000 rpm for 10 minutes at 4°C. Aliquots were dissolved in Laemmli buffer and boiled at 95°C for 5 minutes, and the proteins separated by 10% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) were transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked for 1–2 hours with 5% milk. The membranes were incubated with a primary antibody at 4°C overnight for either VEGF (Santa Biotechnology Inc., 1:200 dilution), bFGF (Santa Biotechnology Inc., 1:200 dilution) or β‐actin (Santa Biotechnology Inc, 1:10000) as a loading control. Blots were then washed several times with Tris‐buffered saline containing 0·05% Tween‐20 (TBST). Following this, blots were incubated for 1 hour with a horseradish‐peroxidase (HRP)‐coupled secondary antibody, then washed again with TBST. Blots were incubated with a luminescent HRP substrate for 1 minute and positive signals were detected. All primary and secondary antibodies were obtained from Santa Biotechnology Inc..
Densitometric quantification of the immuneblot bands was performed using high‐resolution flatbed scanner (Hewlett‐Packard Company, Palo Alto, CA) and Image J densitometry software. The optical densities of at least three replicates of six group samples were quantified. The densities of the VAC for pressure values of 0, 75, 150, 225, 300 mmHg and wounds treated by control were expressed on the basis of their relative intensities to the control.
Statistical analysis
All results were expressed as the mean ± standard deviation, and statistical comparisons of the means were performed on measured sample using unpaired Student's t‐test. Statistical significance was accepted when P value was less than 0·05.
Results
Bacterial colonies counts
In this study, all 36 wounds were infected by bacteria with density of 7·2 × 109 CFU per gram tissue prior to the operation. Wounds treated by VAC of 75, 150, 225 and 300 mmHg bacterial counts showed significant decrease in number compared to gauze and 0 mmHg (Figure 1). There was no significant difference between wounds treated by VAC device pressures of 75, 150, 225 and 300 mmHg. The bacterial count decreased to less than 105 CFU per gram tissue in wounds treated by VAC with 75, 150, 225 and 300 mmHg on day 7 (Figure 1B,C). Number of bacteria remained above 105CFU per gram of tissue in wounds treated with gauze and VAC of 0 mmHg (Figure 1C,D).
Figure 1.
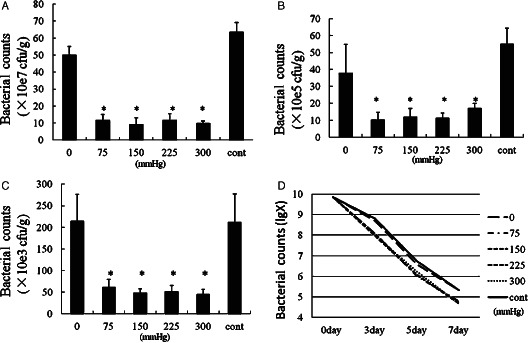
Bacterial growth kinetics in six groups of in vivo wound models. The number of bacteria is counted on days 0, 3, 5 and 7. The symbol * shows that there was no significant difference between vacuum‐assisted closure (VAC) of 75, 150, 225 and 300 mmHg on days 3, 5 and 7 (P > 0·05) and there is notable significant difference between the VAC of 75, 150, 225 and 300 mmHg with the control (P < 0·01).
Density of microvessels
On days 3, 5 and 7, microvessels of wounds were counted by staining for Factor VIII (Figure 2). On days 3 and 5, VAC device of 75, 150 and 225 mmHg effectively resulted in increasing microvessels number as shown in Figure 3A,B. VAC of 150 mmHg was the most effective one. In contrast, VAC of 300 mmHg resulted in decreasing microvessels number comparing with gauze and VAC of 0 mmHg. On day 7, there was no significant difference between VAC of 150 mmHg and control (Figure 3C). Furthermore, the VAC of 75 and 150 mmHg had well developed, larger calibre vessel compared with the control, 0 and 300 mmHg.
Figure 2.
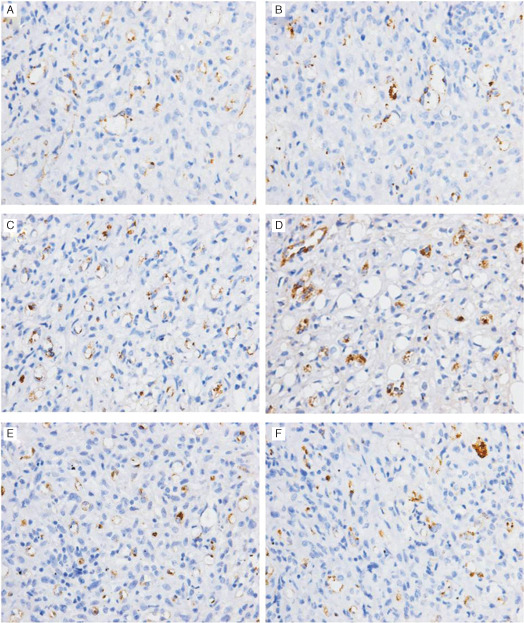
Microvessels in the granulation tissues of wound are shown by staining for Factor VIII on day 3. Wound treated with vacuum‐assisted closure (VAC) of (A) 0 mmHg, (B) 75 mmHg, (C) 150 mmHg, (D) 225 mmHg, (E) 300 mmHg and (F) wound treated with gauze (control). Microvessels are shown in brown colour.
Figure 3.
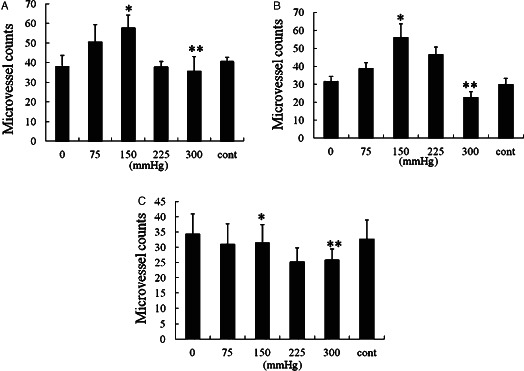
Microvessel counts of granulation tissues. On days 3 (A) and 5 (B), * shows that there are significantly higher microvessels in vacuum‐assisted closure (VAC) of 150 mmHg than in VAC of 300 mmHg (P < 0·05) and control (P < 0·01). The symbol ** shows that there is no significant difference between VAC of 300 mmHg and control (P > 0·05). On day 7 (C), * shows that there is no significant difference between VAC of 0 mmHg and control, and VAC of 150 mmHg is notably higher than that of 300 mmHg (P < 0·05). The symbol ** shows that VAC of 300 mmHg is significantly less than control (P < 0·05).
Collagen fibrin
Collagen fibrin formation was enhanced by the VAC of 75, 150, 225 and 300 mmHg compared with wounds treated with gauze and 0 mmHg on day 5 by histological analysis (Figure 4). The collagen fibrin deposition of wounds treated by VAC of 75 and 150 mmHg was significantly higher than that treated by VAC of 300 and gauze. There were no statistical difference between VAC of 300, 0 and gauze (Figure 5).
Figure 4.
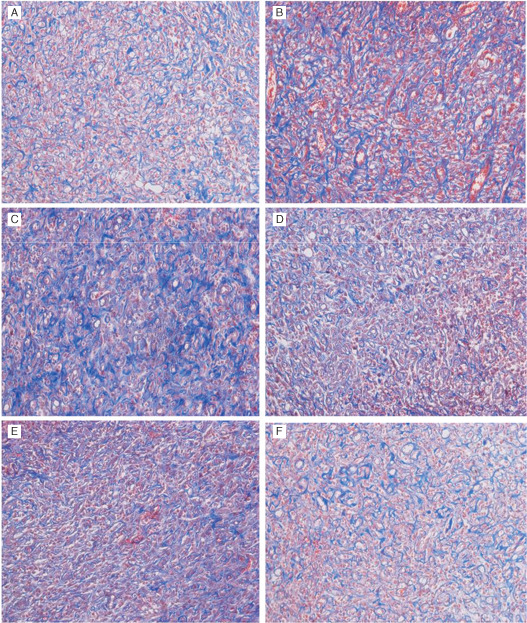
Collagen fibrins are shown by Masson's trichrome stain on day 5. Collagen fibrins are shown in blue colour. (A) Wound treated with vacuum‐assisted closure (VAC) of (A) 0 mmHg, (B)75 mmHg, (C) 150 mmHg, (D) 225 mmHg, (E) 300 mmHg and (F) wound treated with gauze (control).
Figure 5.
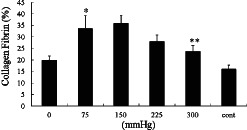
On day 5, * shows that there is no significant difference between vacuum‐assisted closure (VAC) of 150 and 75 mmHg (P > 0·05), but notably higher than VAC of 300 mmHg and control (P < 0·05). ** shows that VAC of 300 mmHg is higher than control (P < 0·05).
Express of VEGF and FGF‐2 protein
VEGF and bFGF were well verified to promote angiogenesis in the wound healing cascade 16, 17. To test the effect of VAC with different negative pressures on wound healing Western blot for VEGF and bFGF was performed. The expression of VEGF and bFGF in wounds treated with different methods was assayed. On day 5, the expression of VEGF and bFGF of VAC of 75 and 150 mmHg was significantly higher than VAC of 300 mmHg and gauze (Figure 6). Specially, the expression of VEGF increased four manifolds in the VAC of 75 and 300 mmHg compared to the wound treated by the control. Expression of bFGF in wounds treated with VAC of 75 mmHg was less than VAC of 0 mmHg. This result suggested that VAC of 150 mmHg was most effective in regulating VEGF and bFGF.
Figure 6.
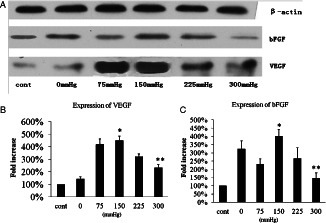
Western blot for vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) on day 5 (A). (B) Expression of VEGF. The symbol * shows that the expression of VEGF in vacuum‐assisted closure (VAC) of 150 mmHg is significantly higher than VAC of 300 mmHg and control (P < 0·05); ** shows that the expression of VEGF in VAC of 300 mmHg is less than 225 mmHg (P < 0·05), but notably higher than control (P < 0·05). (C) The expression of bFGF; * shows that there is significant difference between VAC of 150 and 300 mmHg and control; ** shows that there is no significant difference between VAC of 300 mmHg and control. But the expression of bFGF in VAC of 300 mmHg is less than in VAC of 225 mmHg.
Discussion
Wound healing is a complex series of biochemical and cellular events, including haemostasis, inflammation, proliferation and remodelling. Successful healing is contingent on continuous processes of biochemical signal pathways from a wide range of cell types throughout the antibody. Lack of wound progression is relative to a number of factors, including increased protease production, infection, vascular disease and nutritional state. There are a variety of cellular, biochemical and mechanical methods that affect the normal wound healing sequences 18, 19, 20. An effective treatment that improves wound healing is considered to involve more than one of these components. The VAC device, proven to be efficient by clinical application, can provide a new insight at molecular events of wound healing (10). Previous clinical and experimental studies have shown that the VAC therapy can better promote wound healing when the negative pressure value is set at 125 mmHg 21, 22 compared with others. Furthermore, some studies of VAC device have shown that it can improve removal of oedema fluid (23), increase blood flow, formation of granulation tissue and bacterial clearance.
It is important to diminish bacteria in infected wounds, which hinders wound healing. It has been debated whether VAC therapy is useful to reducing bacteria of infected wounds. Some clinical studies have shown that VAC therapy can decrease the bacterial number in infected wound (3). On the contrary, other clinical studies proved that there was no difference in bacterial count between the VAC therapy groups and non‐VAC therapy groups (24). Deva Boone (25) showed that the bacterial burden in all wounds continued to increase and broadened to include local skin flora, which had been absent immediately after wounding. This study showed that VAC device of negative pressure promoted reduction of bacteria, but there was no significant difference in VAC of different negative pressure. This result suggested that increasing negative pressure value did not facilitate bacterial clearance.
Tissue adaptation to changing physical stresses is a basic requirement for growth and survival of living systems (26). Wounds treated by VAC of negative pressure can result in cellular strain and microdeformations at the foam–wound interface (27). Microdeformations induce cellular proliferation and angiogenesis on the wound in vivo study (10). Ilizarov et al. applied mechanical stress to stimulate mitosis in wound tissues and new vessels were formed (28). Other histologic studies showed that when changing VAC device after application, small blood vessels of granulation tissue increased 29, 30. One study showed statistically significant increase of microvessel density in VAC‐treated wounds as compared with gauze on day 3 when the negative pressure value was set at 125 mmHg (14). This study shows that wounds treated by VAC with suction displayed increasing microvessel density. Meanwhile, for VAC with negative pressure values of 75 and 150 mmHg, the microvessel density of wounds was more than VAC of 300 mmHg. This result may suggest that it was harmful to angiogenesis when wounds were treated by VAC pressure that was too high.
VEGF and bFGF are involved in vasculogenesis and angiogenesis of wounds. Cells were subjected to biaxial cyclical stretch, following which the expression of VEGF and bFGF increased. It was shown that mechanical stretch regulates VEGF and bFGF gene expression in cultured pulmonary artery smooth muscle cells (31). Sharone Jacobs et al.(14) designed an animal model experiment which has shown that VAC therapy led to increased expression of VEGF and bFGF, in the first 5 days. This study also shows that the expression of VEGF and bFGF notably increased in wounds treated by VAC of 75 and 150 mmHg. However, there was no difference in the expression of VEGF and bFGF between wounds treated by VAC of 300 mmHg and gauze. It maybe suggested that high mechanical force did not stimulate the tissue proliferation and angiogenesis.
One study showed that collagen deposition of wounds increased when negative pressure value was set at 125 mmHg compared with the gauze. Greene et al.(32) showed that dramatic differences in collagen response and decreases in the matrix metalloproteinase (MMP)‐9 and MMP‐2 were recorded in the wounds treated by VAC compared with those treated by non‐VAC. A consecutive study of chronic wounds showed a decrease in MMP‐1 and MMP‐13 (33). This study shows that organisation of collagen fibres increased in wounds treated by VAC of 75 and 150 mmHg and also shows that VAC with suitable negative pressure can better promote wound healing.
The major limitation of this study was that it failed to measure the volume of granulation precisely and that we did not compare the growth speed of wounds in each group. In addition, the number of wounds in each group was small, and the duration of observation at 9 days was too short to effectively evaluate infected wound healing. The other possible mechanisms may be involved in regulating gene expressions which were related to growth of granulation and angiogenesis.
Acknowledgements
The authors would like to thank Chen Wei‐ming for his instruction on this trial, and thank VSD company (Wuhan, China) for supplying vacuum pump, open‐pore foam and semiocclusive dressing. This study was financed by the National Natural Science Funds of China. Project code: 81171713.
References
- 1. Baharestani MM, Gabriel A. Use of negative pressure wound therapy in the management of infected abdominal wounds containing mesh: an analysis of outcomes. Int Wound J 2011;8:118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vuerstaek JD, Vainas T, Wuite J. State‐of‐the‐art treatment of chronic leg ulcers: a randomized controlled trial comparing vacuum‐assisted closure (VAC) with modern wound dressings. J Vasc Surg 2006;44:1029–38. [DOI] [PubMed] [Google Scholar]
- 3. Gabriel A, Shores J, Berntein B, de Leon J, Kamepalli R, Wolvos T, Baharestani MM, Gupta S. A clinical review of infected wound treatment with vacuum assisted closure (R)(VAC (R)) therapy: experience and case series. Int Wound J 2009;6:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76. [PubMed] [Google Scholar]
- 5. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment:animal studieds and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 6. Borgquist O, Ingemansson R, Malmsjö M. The effect of intermittent and variable negative pressure wound therapy on wound edge microvascular blood flow. Ostomy Wound Manage 2010;56:60–5. [PubMed] [Google Scholar]
- 7. Fabian TS, Kaufman HJ, Lett ED, Thomas JB, Rawl DK, Lewis PL, Summitt JB, Merryman JI, Schadffer TD, Sargent LA, Burns RP. The evaluation of subatmospheric pressure and hyperbaric oxygen in ischemic full‐thickness wound healing. Am Surg 2000;66:1136–43. [PubMed] [Google Scholar]
- 8. Morykwas MJ, Faler BJ, Pearce DJ. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg 2001;47:547–51. [DOI] [PubMed] [Google Scholar]
- 9. Joseph E, Hamori C, Bergman S, Roaf E, Swann NF, Anastasi GW. A prospective randomized trial of vacuum assisted closure versus standard therapy of chronic nonhealing wounds. Wounds 2000;12: 60–7. [Google Scholar]
- 10. Scherer SS, Pietramaggiori G, Mathews JC. The mechanism of action of the vacuum‐assisted closure device. Plast Reconstr Surg 2008;122:786–97. [DOI] [PubMed] [Google Scholar]
- 11. Derrick KL, Norbury K, Kieswetter K. Comparative analysis of global gene expression profiles between diabetic rat wounds treated with vacuum‐assisted closure therapy, moist wound healing or gauze under suction. Int wound J 2008;5:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsu CC, Tsai WC, Chen CP. Effects of negative pressures on epithelial tight junctions and migration in wound healing. Am J Physiol Cell Physiol 2010;299:528–34. [DOI] [PubMed] [Google Scholar]
- 13. Labler L, Rancan M, Mica L. Vacuum‐assisted closure therapy increases local interleukin‐8 and vascular endothelial growth factor levels in traumatic wounds. J Trauma 2009;3:749–57. [DOI] [PubMed] [Google Scholar]
- 14. Jacobs S, Simhaee DA, Marsano A. Efficacy and mechanisms of vacuum‐assisted closure (VAC) therapy in promoting wound healing:a rodent model. J Plast Reconstr Aesthet Surg 2009;62:1331–8. [DOI] [PubMed] [Google Scholar]
- 15. Ichioka S, Watanabe H, Sekiva N. A technique to visualize wound bed microcirculation and the acute effect of negative pressure. Wound Repair Regen 2008;16:460–5. [DOI] [PubMed] [Google Scholar]
- 16. Bao P, Kodra A, Marjana TC. The role of vascular endothelial growth factor in wound healing. J Surg Res 2009;153:347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kondo T, Ishida Y. Molecular pathology of wound healing. Fore Sci Int 2010;203:93–8. [DOI] [PubMed] [Google Scholar]
- 18. Nauta A, Gurtner GC, Longaker MT. Wound healing and regenerative strategies. Oral Dis 2011;17:541–9. [DOI] [PubMed] [Google Scholar]
- 19. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83 835–70. [DOI] [PubMed] [Google Scholar]
- 20. Vallas MD, Cronstein BN, Montesinos MA. Adenosine receptor agonists for promotion of dermal wound healing. Biochem pharmacol 2009;77:1117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mouës CM, Heule F, Hovius SER. A review of topical negative pressure therapy in wound healing:sufficient evidence? Am J Surg 2011;201:544–56. [DOI] [PubMed] [Google Scholar]
- 22. Ubbink DT, Westerbos SJ, Nelson EA. A systematic review of topical negative pressure therapy for acute and chronic wounds. Br J Surg 2008;95:685–92. [DOI] [PubMed] [Google Scholar]
- 23. Orgill DP, Manders EK, Sumpio BE. The mechanisms of action of vacuumassisted closure: more to learn. Surgery 2009;146:40–51. [DOI] [PubMed] [Google Scholar]
- 24. Assadian O, Assadian A, Stadler M, Diab‐Elschahawi M, Kramer A. Bacterial growth kinetic without the influence of the immune system using vacuum‐assisted closure dressing with and without negative pressure in an in vitro wound model. Int Wound J 2010;7:283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boone D, Braitman E, Gentics C. Bacterial burden and wound outcomes as influenced by negative pressure wound therapy. Wounds 2010;22:32–7. [PubMed] [Google Scholar]
- 26. McLeod KJ, Lee RC, Ehrlich HP. Frequency dependence of electrical field modulation of fibroblast protein synthesis. Science 1987;236:1465–9. [DOI] [PubMed] [Google Scholar]
- 27. Wilkes R, Zhao Y, Kieswetter K. Effects of dressing type on 3D tissue microdeformations during negative pressure wound therapy: a computational study. J Biomech Eng 2009;131:031012. [DOI] [PubMed] [Google Scholar]
- 28. Ilizarov GA. The tension‐stress effect on the genesis and growth of tissues: part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res 1989;239:263–85. [PubMed] [Google Scholar]
- 29. Saxena V, Hwang CW, Huang S, Eichbaum Q, Inghber D, Orgill DP. Vacuum‐assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004;114:1086–96. [DOI] [PubMed] [Google Scholar]
- 30. Chen SZ, Li J, Li XR. Effects of vacuum‐assisted closure on wound microcirculation: an experimental study. Asian J Surg 2005;28: 211–7. [DOI] [PubMed] [Google Scholar]
- 31. Quinn TP, Schlueter M, Soifer SJ. Cyclic mechanical stretch induces VEGF and FGF‐2expression in pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2002;282:897–903. [DOI] [PubMed] [Google Scholar]
- 32. Greene AK, Puder M, Roy R, Arsenault D, Kwei S, Moses MA, Orgill DP. Microdeformational wound therapy: effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients. Ann Plast Surg 2006;56:418–22. [DOI] [PubMed] [Google Scholar]
- 33. Shi B, Chen SZ, Zhang P, Li JQ. Effects of vacuum‐assisted closure (VAC) on the expressions of MMP‐1, 2, 13 in human granulation wound [in Chinese]. Zhonghua Zheng Xing Wai Ke Za Zhi 2003;19:279–81. [PubMed] [Google Scholar]


