Abstract
The use of lavage was compared to negative pressure wound therapy (NPWT) with instillation (NPWTi) to assess extent of soft tissue damage, debris removal and environmental cross‐contamination susceptibility in three distinct models. Scanning electron microscopy in an ex vivo model showed increased visible tissue trauma from lavage treatment at low and high pressures versus NPWTi, with the degree of trauma relative to the pressure of the irrigant. These results were corroborated in granulating full‐thickness excisional swine wounds coated with dextran solution to simulate wound debris. Both low‐pressure lavage and NPWTi demonstrated effective cleansing in this model, reducing debris by >90%. However, using three‐dimensional photography to evaluate tissue damage by measuring immediate tissue swelling (changes in wound volume and depth) showed significantly greater (P < 0.05) swelling in low‐pressure lavage‐treated wounds compared with NPWTi‐treated wounds. Lastly, bench top wound models were inoculated with fluorescent bacterial particles to assess environmental cross‐contamination potential and collected at measured distances after treatment with low‐pressure lavage and NPWTi. No evidence of cross‐contamination was found with NPWTi, whereas one‐half of the particles became ‘aerosolised’ during low‐pressure lavage (P < 0.05). Collectively, these studies demonstrate the effective wound cleansing capabilities of NPWTi without the tissue damage and environmental contamination associated with lavage.
Keywords: Environmental cross‐contamination; Lavage; NPWTi; Tissue trauma; Wound cleansing
Introduction
Wound cleansing, a crucial step in wound management and healing, uses fluid to remove loosely adherent cellular debris and necrotic tissue from the wound bed (1). There is also a general consensus that cleansing helps reduce bacterial counts 2, 3. In essence, wound cleansing removes impediments to the healing process in order to create an optimal wound healing environment. Successful management of a wound also involves removing contaminants while inflicting minimal injury to the tissue.
Currently, wound cleansing is often achieved through lavage, a procedure in which the wound is irrigated under pressure with one of several widely used solutions. Isotonic (normal) saline solution is physiologically compatible and non‐toxic and, thus, can maintain cell viability. Normal saline does not damage tissue, cause allergies or sensitisation or alter the normal flora of the skin; therefore, it does not interfere with the normal healing process (4).
Various irrigation methods that are mainly categorised as either low‐pressure lavage (4–15 psi) or high‐pressure lavage (35–70 psi) exist; examples include the bulb syringe method, gravity flow, manual pump irrigation and mechanical/pulsed lavage. The Agency for Health Care Policy and Research (AHCPR) has suggested that cleansing with low‐pressure lavage is sufficient to remove surface pathogens and debris without causing wound trauma or bacterial spreading. AHCPR has also suggested that pressures above 15 psi may cause tissue damage (5). Studies have shown that lavage, specifically high‐pressure lavage, can cause considerable trauma to bone tissue 6, 7. In 2002, Adili et al. illustrated that this macroscopic damage had an adverse effect on early fracture healing in rat femurs (7). Lavage has also been reported to cause considerable disruption to soft tissue in both in vivo and in vitro studies (8). Furthermore, healthy tissue traumatised by lavage cleansing may have impaired capabilities in resisting infection 8, 9. In addition, high‐pressure lavage has been shown to increase the depth of contaminant and bacterial penetration into soft tissue 9, 10. High‐pressure lavage can therefore lead to complications and/or delays in the wound healing process and may even be deleterious to the wound healing progression.
During wound lavage, the potential for spreading pathogens, especially resistant organisms, in the hospital environment is also a cause for concern. For instance, the rates of detection for methicillin‐resistant Staphylococcus aureus or vancomycin‐resistant enterococci on the protective gowns and/or gloves of health care workers have been reported to be as low as 18% and as high as 67% 11, 12, 13. Reports of emerging health‐care‐associated pathogens that are relatively resistant to the most common surface disinfectants and alcohol‐based antiseptics, such as Clostridium difficile, are also disconcerting (14). Therefore, given that the pressure delivered to the wound by lavage must be high enough to remove the adhered microbes from the surface of the wound bed, it is reasonable to express concern regarding the possibility of bacterial cross‐contamination due to splatter. During lavage, bacteria can become ‘aerosolised’ and particulate matter disseminated. In fact, Maragakis et al. (15) investigated and reported the first recognised hospital‐acquired outbreak associated with pulsed lavage wound care as a novel mode of transmission for Acinetobacter baumannii. Also, the US Food and Drug Administration (FDA) issued a medical device safety article warning of the infection risks associated with pulsed lavage (16). The use of lavage can, therefore, be potentially hazardous for the patient and the health care provider by contributing to the spread of hospital‐acquired infections (HAIs) in both patients and visitors.
Negative pressure wound therapy (NPWT) is widely recognised as a powerful tool in wound care. NPWT in conjunction with instillation (NPWTi) has the advantage of timed, automated and controlled delivery of the chosen irrigant uniformly in the wound bed followed by a programmed ‘soak’ time and the subsequent removal of solution through application of NPWT (17). Studies have shown NPWTi to be effective in instilling various wound treatment solutions, such as cleansers, antiseptics and analgesics (18).
NPWTi delivered by V.A.C. Instill® Wound Therapy (KCI USA, Inc., San Antonio, TX) was first introduced to clinicians in 2003 (19). Since then, other clinical benefits have been reported including efficacy in treating wounds with high levels of exudate, debris and slough content, and use in pain management 19, 20. In a recent porcine study, use of NPWTi with saline as the instillant increased collagen deposition in granulation tissue for accelerated wound fill (21).
Three independent studies (ex vivo, in vivo and bench top) were conducted to evaluate the effect of NPWTi in comparison to lavage on tissue trauma, cleansing and environmental cross‐contamination. The ex vivo model was used to qualitatively evaluate the effect of NPWTi and both high‐ and low‐pressure lavage on tissue trauma. On the basis of the results from the ex vivo study, the in vivo porcine study quantitatively compared the effect of NPWTi and low‐pressure lavage on tissue trauma and cleansing. The bench top study using synthetic polymeric wound models quantitatively evaluated the environmental effects of NPWTi and low‐pressure lavage.
Materials and methods
Soft tissue damage in the ex vivo wound model
Experimental setup
Raw porcine meat was purchased from a local butcher shop, stored at 4°C until ready for use and sliced into approximately 4.0 cm sections. These sections were then immediately processed for wound creation. A 4.5 cm × 4.5 cm stainless steel block of 1/3 cm thickness was placed underneath the centre of the pork loin section (Figure 1A). A stainless steel template with a 5 cm × 5 cm opening in the centre was placed over the pork loin such that the stainless steel block beneath caused the tissue to protrude through the opening in the template (Figure 1B). A 15 cm Padgett Dermatome (Integra, Plainsboro, NJ), set at the highest thickness (0.076 cm), was passed over the template five times to create an approximately 5 cm × 5 cm wound of uniform thickness via three‐dimensional (3D) camera photographs and software analysis (Figure 1C). Two hundred and fifty microlitres of simulated wound fluid (0.14% bovine serum albumin and 0.01 M phosphate‐buffered saline, pH 7.4–8) were then applied to the pork loin wound and allowed to adhere to the tissue at room temperature for 2 hours before cleansing treatment.
Figure 1.
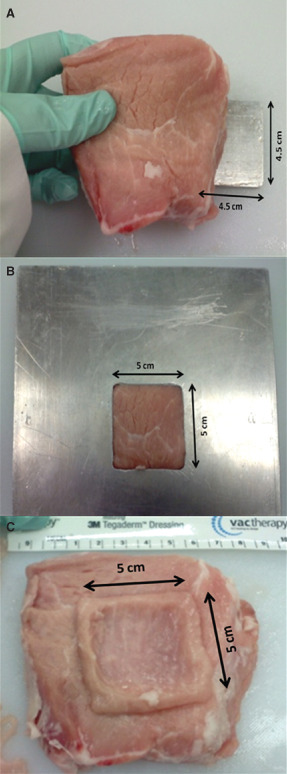
Process of ex vivo wound model generation. (A) 4.5 cm × 4.5 cm stainless steel block placed underneath the pork loin. (B) Stainless steel template with a 5 cm × 5 cm opening placed over the pork loin and the block underneath it so that this portion rises through the opening. (C) Wound created utilising the wound template.
Cleansing therapy: NPWTi
Following simulated wound fluid application to the ex vivo wound model, the wound was dressed using the new reticulated open cell foam (ROCF) V.A.C. VeraFlo™ Dressing (ROCF‐V; V.A.C. VeraFlo™ Medium Dressing; KCI USA, Inc.). The dressing was cut into 5 cm × 5 cm pieces, which were placed into the simulated wound bed. A semi‐occlusive adhesive drape covered the wound and dressing. NPWTi (V.A.C. VeraFlo™ Therapy; KCI USA, Inc.), which was delivered using a prototype of the V.A.C.ULTA™ Therapy System (KCI USA, Inc.), consisted of six complete cycles of negative pressure at −125 mmHg for 5 minutes and instillation of normal saline followed by a 10 seconds soak time.
Cleansing therapy: low‐ and high‐pressure lavage
Following simulated wound fluid application to the ex vivo wound model, the entire wound bed was irrigated with 200 ml of normal saline with either the low‐ (12.7 psi) or high‐pressure (45 psi) pulsed lavage devices. A commercially available low‐pressure lavage device with a soft‐splash shield tip was used per manufacturer's instructions. For the high‐pressure pulsed lavage device, the low setting of a commercially available jet‐tipped water flosser used in medical applications was utilized at a distance of 5 cm from the wound bed.
Tissue damage evaluation: scanning electron microscopy
After treatment, a 10 mm biopsy punch sample was cored from the wound and digitally photographed before placement on ice for 5 minutes with the treated side facing up. The cooling of the sample was a necessary step to make the slicing of the sample more manageable. The top layer of the cored sample was sliced in one continuous, smooth motion for a thickness of 2 ± 0.2 mm. The slice diameter was not to exceed 10 mm. The sample slice was stored on ice until used for environmental scanning electron microscopy (SEM) imaging. SEM images were acquired using a Zeiss EVO LS10 (Carl Zeiss Microscopy; LLC, Thornwood, NY) with the backscatter electron detector. Samples were cooled using a Peltier cooling stage (Deben UK Ltd, Suffolk, UK). Variable pressure mode was used with a pressure range from 400 to 1000 Pa and an accelerating voltage of 25 keV with a probe current of 2 nA. Samples were not pre‐treated or sputter coated.
Debris cleansing and tissue trauma in porcine experimental wounds
Animals
The domestic swine Sus scrofa scrofa used in this study were obtained from Gentiporc, Inc. (Alexandria, MN), housed at an Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC)‐accredited facility and maintained on a twice‐daily constant‐nutrient diet with water received adlibitum. The protocol and its amendments were reviewed by the Institutional Animal Care and Use Committee (IACUC) for compliance with regulations prior to study initiation or implementation of amended activities. All animals received humane care.
Wound creation and experimental setup
Three female swine (61.5–64.7 kg) were used for the study. Each animal was anaesthetised with a mixture of Tiletamine (Fort Dodge Veterinaria S.A., Fort Dodge, IA), Zolazepam (Fort Dodge Veterinaria S.A.) and Xylazine (Lloyd Laboratories, Shenadoah, IO) to create dorsal full‐thickness excisional wounds. Four wounds of 5 cm diameter were created on each side of the spine for a total of eight wounds. The day of wound creation was termed day 0. In order to form a light bed of granulation tissue, all wounds were dressed with ROCF (V.A.C.® GranuFoam™ Round Dressing; KCI USA, Inc.) that was cut to size and placed in each wound cavity for direct contact with the wound bed. Semi‐occlusive adhesive drape (V.A.C.® Drape; KCI USA, Inc.) was used to secure the dressings on the wounds. The wounds were bridged in pairs with a T.R.A.C.™ Pad (KCI USA, Inc.) connected to an NPWT unit (V.A.C. ATS®; KCI USA, Inc.) via T.R.A.C.™ tubing (KCI USA, Inc.). The wounds received continuous NPWT of −125 mmHg for a 4‐day period to establish a lightly granulated wound bed. On day 4, the animals were anaesthetised and the dressings removed, showing a thin base layer of granulation tissue at the wound bed. The wounds were gently wiped with saline‐soaked gauze prior to application of the simulated wound debris. A 10 mg/ml stock of fluorescein isothiocyanate (FITC)‐conjugated dextran solution (average molecular weight 59 000–77 000 kDa; Sigma Aldrich, St Louis, MO) was prepared in dd H2O and 0.45 ml of this solution applied to each wound prior to cleansing. The cleansing treatments were evaluated in contralateral wounds.
Cleansing therapy: NPWTi
Four wounds per pig received NPWTi. These wounds were dressed with ROCF‐V and semi‐occlusive adhesive drape connected to the therapy system according to its instructions for use. The system was programmed to deliver NPWTi with the following settings: negative pressure at −125 mmHg for 5 minutes and instillation of sterile normal saline followed by a 5‐minute soak time. The duration of the negative pressure phase was reduced to 5 minutes to simulate 24 hours of typical use (ten therapy cycles) into a shorter 2‐hour period.
Cleansing therapy: low‐pressure lavage
Four wounds per pig received low‐pressure lavage therapy utilising a commercially available low‐pressure lavage device. Low‐pressure lavage therapy was performed according to its instructions for use delivering 1000 ml of sterile normal saline in less than 2 minutes. Care was taken to ensure that the entire surface of the wound bed was lavaged.
Wound examination
Gross observations of the wounds included a visual inspection of the wound bed before and after cleansing treatment. The wound colour, visual presence of peripheral swelling and the amount and colour of wound exudates were amongst the observations noted.
Cleansing evaluation: digital fluorescent imaging and analysis
The cleansing abilities of the two systems were evaluated using digital fluorescence imaging of the FITC‐dextran. A fluorescence camera system was assembled using a digital camera (Canon Rebel T1i; Canon U.S.A. Inc., Melville, NY), an ultraviolet light source and an opaque hood which covered the wound. Images of the wounds were collected at three time points: before application of the FITC‐dextran (baseline), after application of the FITC‐dextran (pre‐cleansing) and after cleansing treatment (post‐cleansing). Images were loaded into ImageJ (Version 1.44p; U. S. National Institutes of Health, Bethesda, MD) and the green channel isolated for analysis. A threshold value for background fluorescence was determined in each baseline image. The wound edges were outlined, and the total number of pixels and the number of green pixels above the threshold were counted within each wound.
Tissue damage/swelling evaluation: 3D imaging and wound dimension measurements
Prior to and post‐cleansing, 3D images of the wounds were collected with the 60 cm focal length 3D LifeViz™ camera (Quantificare S.A., Sophia Antipolis Cedex, France) to assess tissue damage. Images were imported into the DermaPix® analysis software (Quantificare) for a 3D reconstruction of each wound, allowing for accurate determination of wound depth and wound volume. Changes in wound depth and wound volume were indicative of increases or decreases in wound bed swelling. The swelling ratio was calculated by dividing the pre‐cleansing wound depth or volume by the post‐cleansing wound depth or volume. The swelling ratio is interpreted as follows: ratio = 1 indicates no swelling; ratio > 1 indicates increased swelling and ratio < 1 indicates reduced swelling.
Oedema evaluation: histological analyses
After cleansing, a 12 mm full‐thickness punch biopsy was sampled from the centre of each wound and fixed in 10% neutral buffered formalin for paraffin embedding. Tissue samples were sectioned and stained with haematoxylin and eosin (H&E) and Masson's trichrome for evaluation by a board‐certified pathologist. Oedema in the wound surface and subjacent subcutaneous adipose tissue was scored as follows: 0 = none, 1 = minimal, 2 = moderate, 3 = marked and 4 = severe.
Environmental cross‐contamination using a bench top polymeric sacral pressure ulcer model
Experimental setup
The wound model used in this study (Seymour II – 0910 Wound Care Model; VATA Inc., Canby, OR) simulated a deep stage IV sacral pressure ulcer with exposed bones, undermining, tunnelling, subcutaneous fat, eschar and slough. The model was set on a surface at a height representing that of a patient bed. To collect any droplets/splashing or ‘aerosolisation’ resulting from the wound cleansing methods, 96‐well microplates were arranged around the wound in two concentric levels (Figure 2). One set of 96‐well microplates was placed such that the outer edges of the wound were 7.5 cm from the centre of each plate. The second concentric set of microplates was arranged similarly, with the centre of each plate 15 cm from the outer edges of the wound of the model. The simulated sacral pressure ulcer of the model was then inoculated with 6 × 107 heat‐killed, fluorescently‐labelled bacterial particles (3 × 107 Escherichia coli and 3 × 107 Staphylococcus aureus particles) purchased from Invitrogen (Carlsbad, CA) in a 250 µl total volume of bacterial particles and simulated wound fluid.
Figure 2.
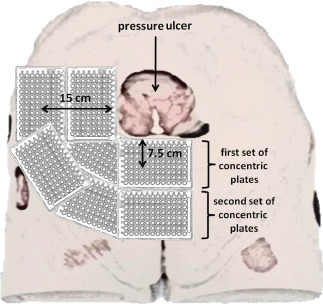
Experimental setup for environmental cross‐contamination assessment illustrating the placement of concentric microplates to surround the wound at specified distances from the wound edge on a sacral pressure ulcer model. (Note: For purposes of clarity, the figure depicts only one corner of the actual experimental setup.)
Cleansing therapy: NPWTi
After inoculation with bacterial particles, NPWTi was applied to the model's simulated sacral pressure ulcer. Two NPWTi dressings were evaluated: ROCF‐V and ROCF‐VC (V.A.C. VeraFlo Cleanse™ Dressing; KCI USA, Inc.). The experiment consisted of five cycles with each cycle comprised of 20 minutes of negative pressure at −125 mmHg followed by instillation of normal saline solution and a 60 seconds soak time. Three replicates of the experiment were performed for each experimental (dressing) condition. The fluid collected in the NPWTi canisters was also quantified for bacterial particles.
Cleansing therapy: low‐pressure lavage
After inoculation with bacterial particles, the simulated sacral pressure ulcer was irrigated with commonly used, commercially available wound cleansers or the low‐pressure pulsed lavage device. The cleansers used were: normal saline solution and antiseptic wound cleansers as per manufacturer's instructions for use. The wound cleansers deliver pressure in the optimal range of 4–15 psi depending on how the cleansers are packaged (pressurised can for the normal saline solution and spray bottles for two different antiseptic wound cleansers). Each wound cleanser was applied to independent simulated wound beds for approximately 1 minute. Normal saline (1000 ml) was used as the irrigant for the pulsed lavage device. Three replicates of the experiment were performed for each of the four low‐pressure lavage treatments.
Bacterial particle aerosolisation evaluation: fluorescence analysis
After the cleansing treatments, the amount of fluorescent bacterial particles collected on the microplates from bacterial aerosolisation or splatter was quantified. The collector microplates were analysed with the Beckman Coulter DTX‐880 Multimode Detector spectrophotometer (Fullerton, CA) using the Beckman Coulter Multimode Analysis Software. The sensitivity of the multimode detector used was 20 fmol fluorescein/200 µl of liquid sample. A standard curve was used to convert the fluorescence signal to number of particles in each plate. The cumulative number of particles in the inner and outer plates, respectively, was each normalised to the initial quantity of particulate inoculated in the simulated wound prior to treatment initiation.
Statistics
The data from the ex vivo study were qualitative in nature and, hence, were not statistically evaluated. Paired statistical analyses were used to analyse the data from the in vivo study. Statistical analyses were performed using JMP 7.0 software (SAS, Cary, NC). A distribution test was first performed to confirm that the data followed a normal distribution followed by the parametric paired t‐test.
The data from the cross‐contamination study were determined to have non‐normal distribution. Hence, non‐parametric analysis of variance (Kruskal–Wallis) was used to test for differences in bacterial particle recovery among the five groups. If differences were detected, the Tukey test was used to compare means between treatments. For all statistical analyses, α was set at 0.05.
Results
Soft tissue damage in ex vivo wound model
Digital photography and SEM images provided a qualitative evaluation of the occurrence of soft tissue trauma caused by the cleansing treatments investigated in this study. A visual assessment of the wound surface before and after treatment showed no observable blanching with NPWTi treatment (Figure 3), whereas blanching was illustrated via digital images in both low‐ and high‐pressure lavage treatments (Figure 3B and C). Compared with tissue that was not treated and NPWTi‐treated samples (Figure 4A and B), SEM images showed obvious disruption of the soft tissue causing the formation of cavities or grooves in both low‐ and high‐pressure lavage‐treated samples (Figure 4C and D). In addition, tissue shredding or tearing was also observed after cleansing with high‐pressure lavage alongside the cavities formed (Figure 4D).
Figure 3.
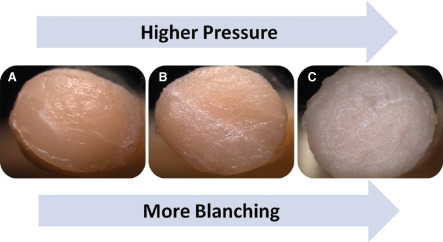
Ex vivo wound model punch biopsy samples illustrating blanching after cleansing treatments. Note the visible increase in degree in maceration or blanching as the pressure of the cleansing treatment increases. (A) Negative pressure wound therapy with instillation (NPWTi). (B) Low‐pressure lavage. (C) High‐pressure lavage.
Figure 4.
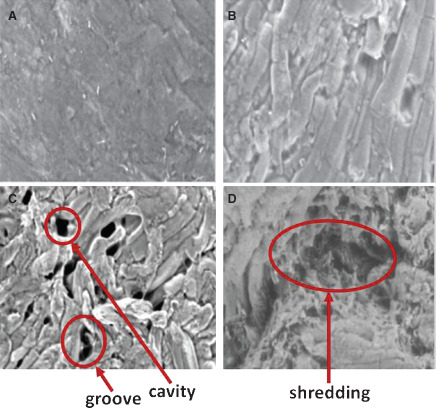
Scanning electron microscope images of ex vivo model wound surfaces after cleansing treatments. Areas of trauma and disruption are indicated by arrows. (A) No treatment at 500× magnification. (B) Negative pressure wound therapy with instillation (NPWTi) at 402× magnification. (C) Low‐pressure lavage at 332× magnification. (D) High‐pressure lavage at 395× magnification.
Debris cleansing and tissue trauma with porcine animal model
Wound examination – visual
During the course of establishing a lightly granulated wound bed, from day 0 to day 4, the wounds healed with normal progression. Mild erythema was observed only in few wounds after inoculation with dextran. Also, compared with before treatment (Figure 5A and C), no observable blanching of the wound bed was observed in NPWTi‐treated samples (Figure 5B), whereas 10 of the 12 wounds that received low‐pressure lavage treatment showed evidence of blanching (Figure 5D). No other differences were noted.
Figure 5.
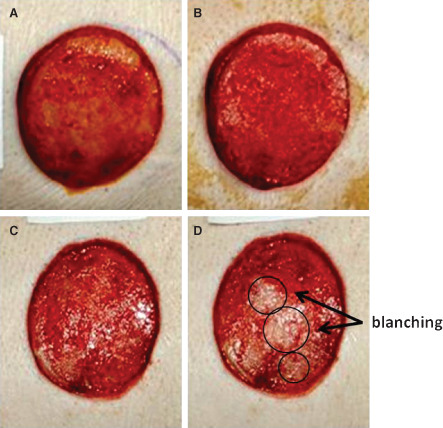
Observed blanching of the porcine wound bed. (A) Before negative pressure wound therapy with instillation (NPWTi) treatment. (B) Post NPWTi treatment. (C) Before low‐pressure lavage treatment. (D) Post low‐pressure lavage treatment with blanched areas circled.
Cleansing evaluation
For the estimation of cleansing efficacy, the FITC‐dextran signal was measured in fluorescence images collected of the wounds before and after cleansing (Figure 6). There was no statistically significant difference in fluorescence signal between treatment groups before introduction of the FITC‐dextran (P = 0.4357) or between treatment groups after introduction of the FITC‐dextran and before cleansing therapy (P = 0.5679). Both therapies were effective at cleansing FITC‐dextran from the wounds, significantly reducing the fluorescence signal by 99.2% (NPWTi, P < 0.0001) and 95.2% (low‐pressure lavage, P < 0.0001) (Figure 7). The percent reduction in fluorescence of low‐pressure lavage‐treated wounds was less than that of NPWTi‐treated wounds (P = 0.0009) (Table 1); however, these results must be interpreted with the caveat that lavage‐induced blanching of the wound may cause an artificial increase in fluorescent signal unrelated to the amount of FITC‐dextran present.
Figure 6.
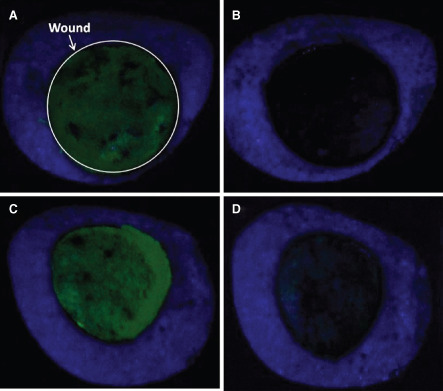
Fluorescence signal of porcine wound bed images before and after cleansing treatments. (A) Before negative pressure wound therapy with instillation (NPWTi) treatment with the wound circled for clarification. (B) Post NPWTi. (C) Before low‐pressure lavage. (D) Post low‐pressure lavage.
Figure 7.
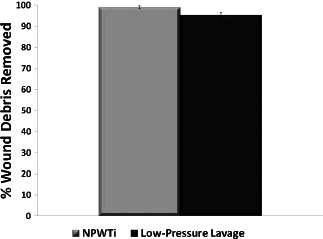
Percentage of debris cleansed on porcine wound bed after cleansing treatments in comparison to before cleansing treatment for negative pressure wound therapy with instillation (NPWTi; P < 0.0001) and low‐pressure lavage (P < 0.0001); n = 12; data shown as mean ± SEM.
Table 1.
Percent fluorescence on porcine wound bed before and after cleansing treatments
| Cleansing treatment | Pre‐cleansing | Post‐cleansing |
|---|---|---|
| NPWTi | 65.6 ± 5.6 | 0.9 ± 0.3 |
| LPL | 60.7 ± 6.3 | 4.9 ± 0.9 |
LPL, low‐pressure lavage, data shown as mean ± SEM.
Tissue damage/swelling evaluation
Tissue damage was evaluated by measuring immediate tissue swelling via changes in wound volume and depth after cleansing treatments. Three‐dimensional imaging analyses suggest that NPWTi‐treated wounds exhibited a decrease in swelling (swelling ratio < 1), whereas low‐pressure lavage‐treated wounds exhibited an increase in swelling (swelling ratio > 1) (Figure 8). The difference in swelling between NPWTi and low‐pressure lavage was significant when using either wound volume (P = 0.0086) or wound depth (P = 0.0006) as the basis for the determination of swelling.
Figure 8.
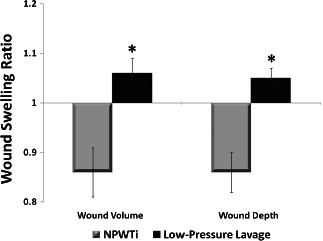
Determination of wound swelling via wound volume and wound depth ratio. Ratio = 1 indicates no swelling; ratio > 1 indicates increased swelling; ratio <1 indicates reduced swelling; * significantly different from negative pressure wound therapy with instillation (NPWTi) for wound volume (P = 0.0086) and wound depth (P = 0.0006); n = 12; data shown as mean ± SEM.
Oedema evaluation
The punch biopsies collected for histology were used to qualitatively evaluate tissue damage, such as oedema, caused by the cleansing techniques. Histological scoring indicates minimal to moderate oedema of the wound resulting from either technique, but there was no significant difference between cleansing regimens (data not shown). However, it should be noted that determination of oedema via histology can be challenging because histological processing can induce artifacts that mask oedema.
Environmental cross‐contamination
Fluorescence spectrometry quantified bacterial crosscontamination levels at two distances from the simulated wound: 7.5 and 15 cm from the edges of the wound on the sacral pressure ulcer model. There was a statistically significant difference between NPWTi treatments and wound cleanser lavage products (P < 0.05). There were no bacterial particles detected with NPWTi using ROCF‐V or ROCF‐VC dressings at either distance tested. In contrast, all three of the wound cleanser lavage products resulted in bacterial cross‐contamination: up to 29% of the bacterial particles were recovered at 7.5 cm away and up to 34% at 15 cm (Table 2). The total quantity of recovered particles (sum of those recovered from both distances) ranged from 50% to 60% for each inoculated bacterial strain (Figure 9). Cleansing with the pulsed lavage device resulted in visible splatter a great deal further than the 7.5 and 15 cm collection plates – no bacterial particles were detected or quantified in the collection plates placed at these distances. The total number of particles transferred from the model to the NPWTi canister was also quantified (Table 2). At least 95% of the bacterial particles were captured in the canister during the five cycles of NPWTi.
Table 2.
Percent detected bacterial particles recovered from concentric microplates after the simulated sacral pressure wound cleansing
| Method/Products | 7.5 cm | 15 cm | ||
|---|---|---|---|---|
| E. coli | S. aureus | E. coli | S. aureus | |
| NPWTi w/ROCF‐V | 0 | 0 | 0 | 0 |
| NPWTi w/ROCF‐VC | 0 | 0 | 0 | 0 |
| LPL w/Pressurised can | 20.8 ± 7.3 | 25.8 ± 10.6 | 20.1 ± 9.8 | 32.1 ± 1.4 |
| LPL w/Spray bottle #1 | 28.3 ± 3.2 | 29.8 ± 6.2 | 16.5 ± 1.4 | 27.9 ± 4.2 |
| LPL w/Spray bottle #2 | 22.5 ± 9.0 | 22.8 ± 7.2 | 34.0 ± 8.6 | 34.3 ± 5.8 |
LPL, low‐pressure lavage; ROCF, reticulated open cell foam; data shown as means ± SEM.
Figure 9.
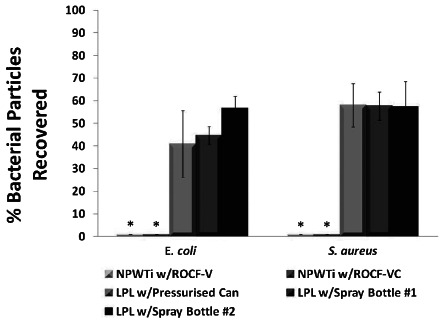
Bacterial particles detected after cleansing treatments. * Significantly different from LPL w/Pressurised Can, LPL w/Spray Bottle #1 and LPL w/Spray Bottle #2, within the bacterial strain group (P < 0.05); LPL, low‐pressure lavage; ROCF, reticulated open cell foam; N/A, not applicable n = 3; data shown as means ± SEM.
Discussion
There is abundant literature about the cleansing effectiveness of lavage. The question that many of the authors of these reports pose, though, is ‘does the cleansing capacity of lavage outweigh the damaging effects to the soft and bone tissue caused by this treatment?’ In short, is the trauma caused to the healthy tissues a clinically relevant concern? Many researchers have found the answer to be ‘yes’. Several studies have shown that although there is better cleansing with higher lavage pressures, the consequences include further dissemination of cleansing fluid into the interstices of the soft tissue, causing considerable gross and microscopic disruption that may lead to more cell death, higher infection rates and greater bacterial retention 8, 9, 10. In contrast, others have found that not only is a higher pressure lavage more detrimental to bone structure and to healing but it is also no more efficacious in removing foreign material than a low‐pressure lavage cleansing regimen 6, 7.
While animal models are routinely used in wound studies, an ex vivo wound model provides a cost‐effective, readily available venue for performing experiments pertaining to wound healing aspects, such as cleansing, tissue trauma and bacterial penetration in soft tissue 8, 10. Raw, porcine meat has also been used successfully in creating an in vitro wound model for showing the bacterial growth kinetics in a contaminated wound (22). Our study also incorporated the use of an ex vivo bench top wound model for preliminary assessment and screening of high‐pressure lavage, low‐pressure lavage and NPWTi to qualitatively assess tissue disruption after cleansing treatments.
The digital and SEM wound images of the ex vivo wound model successfully illustrate the differences in traumatic effects of wound cleansing; there was visually less tissue disruption in NPWTi‐treated soft tissue compared with lavage‐treated tissue. The degree of blanching also increased in proportion to the pressure of lavage treatment. In addition, the tissue trauma observed in the SEM images was in the form of ruptures and fissures in the tissue, and the intensity of the trauma could be visually assessed to be relative to the pressure of the irrigant used to cleanse the wound. Within a field, the number of cavities and/or grooves formed on the wound bed because of the cleansing treatments was clearly visible and damage to the tissues even more apparent with high‐pressure lavage treatment. High‐pressure lavage appeared to cause the most tissue trauma, whereas NPWTi appeared to cause the least damage out of all the cleansing regimens.
In order for a wound to properly heal, the wound bed must be free of excessive exudate, eschar and foreign matter, which are mitigating factors that impair wound healing (1). Therefore, wound cleansing is an integral part of good wound bed preparation. While lavage has been the conventional method for wound cleansing, there have been more than 30 NPWTi reports published since the first clinical application of instillation therapy with NPWT described by Fleischmann et al. in 1998 (23). Many of these publications report clinical benefits of instillation therapy (24), including NPWTi's wound cleansing attributes. For instance, in 2008, Gabriel et al. stated that, in their experience, NPWTi actively removes exudate and microscopic debris ‘to an extent that we have not experienced with other wound‐care modalities (20)’. Furthermore, in an agar model study, Rycerz et al. demonstrated that, compared with continuous irrigation, NPWTi provided significantly better wound instillant distribution and coverage of not only the simulated wound bed but also tunnels and areas with undermining, thereby facilitating a more thorough cleansing (25). As noted by Wolvos, NPWTi also helped to change the viscosity of wound exudate, thereby enabling easier removal of drainage (18).
Because the ex vivo model demonstrated that high‐pressure lavage caused substantial soft tissue trauma, a full‐thickness acute wound healing model in normal healthy swine was used to assess the effectiveness of debris and contaminant removal by NPWTi in comparison to that of low‐pressure lavage. A thin layer of granulation tissue was first established in the created wounds before the application of simulated debris. The molecular weight of the dextran used to simulate particulate debris was selected to imitate proteinaceous materials found in wound exudate. Wound cleansing via normal saline solution was used in both cleansing treatments, because saline minimised the risk of damaging the newly formed granulation tissue. The results of our study support the previous findings that NPWTi was as effective at cleansing as low‐pressure lavage; both regimens cleaned >90% of the simulated debris.
As with many of the reported findings and our own ex vivo results, the in vivo study also resulted in considerable tissue trauma to the wound bed after lavage cleansing. There was an immediate increase in blanching and swelling (as measured by wound volume and depth) associated with low‐pressure lavage. Upon gross examination of the wounds after cleansing treatments, it was noted that 83% of the wounds treated with low‐pressure lavage exhibited blanching. There was no blanching or swelling detected in association with NPWTi.
Histological analyses showed no significant difference in tissue disruption, oedema and haemorrhage between NPWTi and low‐pressure lavage. This is not entirely unexpected, because the process of fixation and further tissue processing per se can introduce dehydration artifacts in the samples.
In addition to the abundant literature about lavage's cleansing capacity and tissue disruption propensities as a possible ‘side effect', there are also numerous reports of infection control issues regarding lavage that contributed to HAIs 15, 26. HAIs are a foremost concern in many health care settings, not only because of the increased morbidity and mortality associated with them but also because of the economic implications. The Centers for Disease Control and Prevention (CDC) estimates about 100 000 deaths from the 1.7 million HAIs per year in the USA alone, resulting in approximately $4.5 billion in additional health care costs annually 27, 28. One of the ways the US government has responded in an attempt to curb the health care spending was in 2008, when Centers for Medicare and Medicaid Services (CMS) no longer reimbursed hospitals for conditions that were not present on admission; in short, hospitals had to pay for HAIs. Reed and Kemmerly stress in their report about HAIs that ‘these infections, hospitalisations, intangibles, such as grief and anxiety, and dollars spent are all preventable (27)’.
Reports of environmental contamination of A. baumannii associated with low‐pressure lavage have been published within the past decade emphasising significant morbidity and expense 15, 26. In contrast to these previously published results, Ho et al. concluded from their study that low‐pressure pulsed lavage was not associated with an increased rate of cross‐contamination of A. baumannii (29). The authors did state, though, that their findings may have underestimated the true potential for contamination. They also mentioned that, although many health care facilities have instituted infection control measures that are adequate in limiting the spread of cross‐contamination, less stringent infection control measures may be followed during standard wound care that could contribute to environmental cross‐contamination.
The results of our study in comparing the susceptibility of bacterial cross‐contamination between NPWTi and several low‐pressure lavage cleansing regimens indicate that the lavage treatments using the cleansers caused significant cross‐contamination, whereas NPWTi caused none. Approximately half of the bacterial particles inoculated after use of the cleansers were detected at the distances measured, and it may be inferred that the other half were spread even further than the distances at which the collection plates were placed. Cleansing with the low‐pressure pulsed lavage device spread bacterial particles at even further distances than the other lavage treatments. This was of some surprise because the device included an attached splash shield and, most especially, because the device also had conjoined suction capability. These aerosolised particles can hypothetically contribute to an increased risk of HAIs by cross‐contamination of different wounds on the patient and various surfaces of the health care setting. This is a concern especially in the case of immunocompromised patients, particularly if skin microlesions exist. This study also showed that nearly all of the inoculated particles were recovered in the canisters, confirming that cross‐contamination is decreased with the use of NPWTi with all of the foams tested, while also demonstrating that bacteria were actively removed from the wound bed during the instillation process. The sealed environment virtually eliminated chances of bacterial aerosolisation while the therapy was applied.
There are some assumptions and limitations to this study. The assumptions are that the dextran would behave with the same migration characteristics as particulate debris and that the bacterial particles would aerosolise similarly to live bacteria when treated with the cleansing regimens. Some may view the use of the stage IV sacral pressure wound model for the bacterial cross‐contamination portion of the study as the ‘worst‐case’ scenario and may consider this as a limitation. This was intended so that a thorough cleansing of the tunnelling and undermining present on the model was supposed to have been challenging, at the very least. Despite a relatively small sample size, significant differences were apparent between the low‐pressure lavage and NPWTi groups in all areas assessed regarding this area of investigation.
A future direction regarding tissue disruption resulting from this study is the assessment of bacterial penetration after repeated lavage cleansing in comparison to NPWTi. Considering that traditional gauze dressings can be changed up to several times a day and lavage cleansing performed alongside the dressing changes, it would be of great clinical interest to determine if bacteria penetrate into tissue that is repeatedly challenged with trauma and disruption. Also, the coauthors of this study underestimated the degree of environmental cross‐contamination the low‐pressure pulsed lavage cleansing can cause and splatter collection plates should have been placed at further distances. Measurement of the distance of aerosolisation and splatter is a definite area of interest and is vital in clinical relevancy.
On the basis of the data obtained from these studies, it can be concluded that NPWTi has cleansing capacity comparable to low‐pressure lavage but without causing the tissue trauma and disruption associated with the latter treatment. In addition, wound cleansing via NPWTi proved to be a very effective way to decrease bacterial aerosolisation and, thus, potentially reduce the risk of environmental cross‐contamination that could lead to HAIs.
Acknowledgements
The authors would like to thank the staff at Surpass preclinical facility for their assistance with wound creation and cleansing therapies; A. Jimenez of Vel‐Lab Research for histology processing; M. Manwaring of KCI for his help with the wound template for the ex vivo wound model portion of the study and Z. Liu of KCI for statistical analyses. This study was supported by KCI. Many of the authors are employees of KCI. All authors report no other conflicts of interest relevant to this study.
References
- 1. Barr JE. Principles of wound cleansing. Ostomy Wound Manage 1995. ; 41 (7A Suppl.) : 15S – 21S ; discussion 22S. [PubMed] [Google Scholar]
- 2. Wysocki AB. Evaluating and managing open skin wounds: colonization versus infection. AACN Clin Issues 2002. ; 13 : 382 – 97. [DOI] [PubMed] [Google Scholar]
- 3. Edlich RF , Rodeheaver GT , Thacker JG , Lin KY , Drake DB , Mason SS , Wack CA , Chase ME , Tribble C , Long WB 3rd , Vissers RJ. Revolutionary advances in the management of traumatic wounds in the emergency department during the last 40 years: part I. J Emerg Med 2010. ; 38 : 40 – 50. [DOI] [PubMed] [Google Scholar]
- 4. Fernandez R , Griffiths R. Water for wound cleansing. Cochrane Database Syst Rev 2008. ; 23 : CD003861. [DOI] [PubMed] [Google Scholar]
- 5. Bergstrom N , Bennett MA , Carlson CE. Treatment of pressure ulcers: clinical practice guidelines no. 15. Rockville : US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; , 1994. : 6 – 7 , 47 – 53. AHCPR Publication No. 95‐0652. [Google Scholar]
- 6. Draeger RW , Dirschl DR , Dahners LE. Debridement of cancellous bone: a comparison of irrigation methods. J Orthop Trauma 2006. ; 20 : 692 – 8. [DOI] [PubMed] [Google Scholar]
- 7. Adili A , Bhandari M , Schemitsch EH. The biomechanical effect of high‐pressure irrigation on diaphyseal fracture healing in vivo. J Orthop Trauma 2002. ; 16 : 413 – 7. [DOI] [PubMed] [Google Scholar]
- 8. Boyd JI 3rd , Wongworawat MD. High‐pressure pulsatile lavage causes soft tissue damage. Clin Orthop Relat Res 2004. ; 427 : 13 – 7. [DOI] [PubMed] [Google Scholar]
- 9. Wheeler CB , Rodeheaver GT , Thacker JG , Edgerton MT , Edilich RF. Side‐effects of high pressure irrigation. Surg Gynecol Obstet 1976. ; 143 : 775 – 8. [PubMed] [Google Scholar]
- 10. Hassinger SM , Harding G , Wongworawat MD. High‐pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop Relat Res 2005. ; 439 : 27 – 31. [DOI] [PubMed] [Google Scholar]
- 11. Snyder GM , Thom KA , Furuno JP , Perencevich EN , Roghmann MC , Strauss SM , Netzer G , Harris AD. Detection of methicillin‐resistant Staphylococcus aureus and vancomycin‐resistant enterococci on the gowns and gloves of healthcare workers. Infect Control Hosp Epidemiol 2008. ; 29 : 583 – 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zachary KC , Bayne PS , Morrison VJ , Ford DS , Silver LC , Hooper DC. Contamination of gowns, gloves, and stethoscopes with vancomycin‐resistant enterococci. Infect Control Hosp Epidemiol 2001. ; 22 : 560 – 4. [DOI] [PubMed] [Google Scholar]
- 13. Boyce JM , Potter‐Bynoe G , Chenevert C , King T. Environmental contamination due to methicillin‐resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol 1997. ; 18 : 622 – 7. [PubMed] [Google Scholar]
- 14. Weber DJ , Rutala WA , Miller MB , Huslage K , Sickbert‐Bennett E. Role of hospital surfaces in the transmission of emerging health care‐associated pathogens: Norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control 2010. ; 38 ( 5 Suppl. 1 ): S25 – S33. [DOI] [PubMed] [Google Scholar]
- 15. Maragakis LL , Cosgrove SE , Song X , Kim D , Rosenbaum P , Ciesla N , Srinivasan A , Ross T , Carroll K , Perl TM. An outbreak of multidrug‐resistant Acinetobacter baumannii associated with pulsatile lavage wound treatment. JAMA 2004. ; 292 : 3006 – 11. [DOI] [PubMed] [Google Scholar]
- 16. Fuller J. Cover up and clean up to prevent deadly infections. Originally published 2005. . Medical Devices Alerts and Notices. U.S. FDA. URL http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/TipsandArticlesonDeviceSafety/ucm230232.htm [accessed on 13 February 2012].
- 17. Bernstein BH , Tam H. Combination of subatmospheric pressure dressing and gravity feed antibiotic instillation in the treatment of post‐surgical diabetic foot wounds: a case series. Wounds 2005. ; 17 : 37 – 48. [Google Scholar]
- 18. Wolvos T. Wound instillation – the next step in negative pressure wound therapy. Lessons learned from initial experiences. Ostomy Wound Manage 2004. ; 50 : 56 – 66. [PubMed] [Google Scholar]
- 19. Jerome D. Advances in negative pressure wound therapy: the VAC instill. J Wound Ostomy Continence Nurs 2007. ; 34 : 191 – 4. [DOI] [PubMed] [Google Scholar]
- 20. Gabriel A , Shores J , Heinrich C , Baqai W , Kalina S , Sogioka N , Gupta S. Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J 2008. ; 5 : 399 – 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leung BK , LaBarbera LA , Carroll CA , Allen D , McNulty AK. The effects of normal saline instillation in conjunction with negative pressure wound therapy (NPWT) on wound healing in a porcine model. Wounds 2010. ; 22 : 179 – 87. [PubMed] [Google Scholar]
- 22. Assadian O , Assadian A , Stadler M , Diab‐Elschahawi M , Kramer A. Bacterial growth kinetic without the influence of the immune system using vacuum‐assisted closure dressing with and without negative pressure in an in vitro wound model. Int Wound J 2010. ; 7 : 283 – 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fleischmann W , Russ M , Westhauser A , Stampehl M. Vacuum sealing as a carrier system for controlled local drug administration in wound infection [in German]. Unfallchirurg 1998. ; 101 : 649 – 54. [DOI] [PubMed] [Google Scholar]
- 24. McNulty AK , Nguyen K. What is the benefit of instillation therapy? Int J Low Extrem Wounds 2010. ; 9 : 68 – 9. [DOI] [PubMed] [Google Scholar]
- 25. Rycerz AM Jr , Slack P , McNulty AK. Distribution assessment comparing continuous and periodic wound instillation in conjunction with negative pressure wound therapy using an agar-based model . Int Wound J 2012. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Young LS , Sabel AL , Price CS. Epidemiologic, clinical, and economic evaluation of an outbreak of clonal multidrug‐resistant Acinetobacter baumannii infection in a surgical intensive care unit. Infect Control Hosp Epidemiol 2007. ; 28 : 1247 – 54. [DOI] [PubMed] [Google Scholar]
- 27. Reed D , Kemmerly SA. Infection control and prevention: a review of hospital‐acquired infections and the economic implications. Ochsner J 2009. ; 9 : 27 – 31. [PMC free article] [PubMed] [Google Scholar]
- 28. Curtis LT. Prevention of hospital‐acquired infections: review of non‐pharmacological interventions. J Hosp Infect 2008. ; 69 : 204 – 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ho CH , Johnson T , Miklacic J , Donskey CJ. Is the use of low‐pressure pulsatile lavage for pressure ulcer management associated with environmental contamination with Acinetobacter baumannii? Arch Phys Med Rehabil 2009. ; 90 : 1723 – 6. [DOI] [PubMed] [Google Scholar]


