Abstract
A large number of silver‐based dressings are commonly used in the management of chronic wounds that are at risk of infection, including diabetic foot ulcers. However, there are still controversies regarding the toxicity of silver dressings on wound healing. The purpose of this study was to objectively test the cytotoxicity of silver dressings on human diabetic fibroblasts. Human diabetic fibroblasts were obtained from the foot skin of four diabetic foot ulcer patients and cultured. The effect of five silver‐containing dressing products (Aquacel Ag, Acticoat*Absorbent, Medifoam Ag, Biatain Ag and PolyMem Ag) and their comparable silver‐free dressing products on morphology, proliferation and collagen synthesis of the cultured human diabetic fibroblasts were compared in vitro. In addition, extracts of each dressing were tested in order to examine the effect of other chemical components found in the dressings on cytotoxicity. The diabetic fibroblasts cultured with each silver‐free dressing adopted the typical dendritic and fusiform shape. On the other hand, the diabetic fibroblasts did not adopt this typical morphology when treated with the different silver dressings. All silver dressings tested in the study reduced the viability of the diabetic fibroblasts and collagen synthesis by 54–70 and 48–68%, respectively, when compared to silver‐free dressings. Silver dressings significantly changed the cell morphology and decreased cell proliferation and collagen synthesis of diabetic fibroblasts. Therefore, silver dressings should be used with caution when treating diabetic wounds.
Keywords: Cytotoxicity, Diabetic fibroblasts, Silver dressing
Introduction
Silver has long been considered a powerful antimicrobial agent, dating back to 1000 BC, when the Greeks and Romans used silver as a disinfectant 1, 2. Currently, a large number of silver‐based dressings are available in a variety of complexes, including foams, hydrofibers and hydrocolloids (3), due to the growing threat of antibiotic resistance and heightened concerns regarding the toxicity of topical antiseptics (4). Since silver is regarded as a broad‐spectrum agent and shows strong activity against gram‐negative bacteria, which frequently colonise chronic wounds, it is commonly used to manage chronic wounds that are at risk of infection, including diabetic foot ulcers.
While the primary purpose of topical antimicrobial agents is to reduce infection by providing potent broad‐spectrum antimicrobial activity, ideally, these agents should not compromise the activity of cells that are involved in the wound‐healing process. Issues associated with the toxicity of dressings on host cells are especially important in diabetic ulcers, which commonly show delayed or no healing due to decreased cellular activity for wound healing. Despite the wide spread use of silver, there are still controversies regarding the toxicity of silver dressings for wound healing. Many researchers have reported that silver‐based dressings facilitated the early phase of wound healing 5, 6, 7, 8, 9, promoted reepithelialisation and accelerated the wound‐healing process 10, 11, 12, 13, 14, 15, 16, 17. However, other researchers have found that the silver component in dressing materials was toxic to skin cells 18, 19, 20 and reepithelialisation 18, 21, 22, 23, 24, 25. Moreover, there have been no previous studies that objectively examined and evaluated the toxicity of silver dressings on the healing of diabetic wounds, in which silver dressings are commonly used.
Thus, the purpose of this study was to examine the cytotoxicity of silver dressings on diabetic fibroblasts, which are key cells in the diabetic wound‐healing process.
Materials and methods
Dressings
Five silver‐containing dressing products (Aquacel Ag, Acticoat*Absorbent, Medifoam Ag, Biatain Ag and PolyMem Ag) and their comparable silver‐free dressing products (Aquacel, Algisite*M, Medifoam, Biatain and PolyMem) from the same manufacturers were purchased from company agents. A description of these dressing products is provided in Table 1. All of these dressings were cut into round disks using 6‐mm biopsy punches under aseptic conditions and special care was taken to prevent cross‐contamination.
Table 1.
Dressing information
| Dressing | Manufacturer | Basic dressing composition | Silver modality |
|---|---|---|---|
| Aquacel | ConvaTec (Deeside, UK) | Carboxymethyl cellulose | None |
| Aquacel Ag | ConvaTec | Carboxymethyl cellulose | Ag+ |
| Algisite*M | Smith & Nephew (Hull, UK) | Calcium alginate | None |
| Acticoat*Absorbent | Smith & Nephew | Calcium alginate | Nanocrystalline Ag0 |
| Medifoam | Biopol (Seoul, Korea) | Polyurethane foam | None |
| Medifoam Ag | Biopol | Polyurethane foam | Ag+ |
| Biatain | Coloplast (Humlebaek, Denmark) | Polyurethane foam | None |
| Biatain Ag | Coloplast | Polyurethane foam | Ag+ |
| PolyMem | Ferris Mfg. (Burr Ridge, Chicago, IL) | Polyurethane foam | None |
| PolyMem Ag | Ferris Mfg. | Polyurethane foam | Nanocrystalline Ag0 |
Isolation and culture of diabetic fibroblasts
Diabetic fibroblasts were obtained from the foot skin of four diabetic foot ulcer patients who underwent debridement or toe amputations. Two male patients (60 and 62 years of age) and two female patients (72 and 75 years of age) were included in this study. The duration of diagnosis with diabetes was greater than 10 years in all patients and they provided informed consents for this study. Freshly discarded skin tissue was deepithelialised and minced into 2 × 1 mm2 fragments. These fragments were then incubated in a 0·075% type I collagenase (Sigma, St. Louis, MO) digestive solution at 37°C for 4 hours. Dissociated cells were diluted in Dulbecco's Modified Eagle Medium/Ham's F‐12 (DMEM/F‐12; Gibco, Grand Island, NY), and collected by centrifugation at 450 ×g for 17 minutes. The cells were then washed twice in 40 ml Mg2+‐ and Ca2+‐free Dulbecco's phosphate‐buffered saline (DPBS; Gibco), resuspended in 5ml DPBS and filtered through a 100‐µm nylon mesh filter (Becton Drive, Franklin Lakes, NJ). Cell viability was assessed using the Trypan blue (Gibco) exclusion assay and cell densities were determined by counting in a hemocytometer. After isolation, the cells were cultured in 12 ml DMEM/F‐12 containing 10% fetal bovine serum (FBS; Gibco), 315 mg/dl glucose and 25 g/ml gentamycin on a 100‐mm tissue culture plate at 37°C in a 5% CO2/95% air atmosphere. Upon reaching confluency, the cells were trypsinised for the next passage and the second passage cells were used for the experiments.
Diabetic fibroblast cultures with dressings
The cultured diabetic fibroblasts were seeded onto 24‐well culture plates containing DMEM/ F‐12 supplemented with 10% FBS and 315 mg/dl glucose. Polycarbonate culture plate inserts, which had an 8‐µm pore size (Millipore, Billerica, MA), were placed into the culture well. Punched disks of the 10 types of dressings were placed in the culture plate insert and 1·5 ml of DMEM/F‐12 were added to each well. A well containing the diabetic fibroblasts without a dressing served as the control. The cells were then incubated in a 37°C humidified atmosphere for 3 days. The initial number of diabetic fibroblasts seeded was 3× 104 cells/well.
Cell morphology
The effect of each dressing disk on diabetic fibroblast morphology was examined by inverted photographic microscopy just after removing the inserts and dressing disks.
Cell proliferation
Cell proliferation was determined using a Cell Counting Kit‐8 assay (Dojindo Lab, Kumamoto, Japan). Briefly, the culture medium in each well was replaced with fresh 0·5 ml DMEM/F‐12 and 0·05 ml tetrazolium salt [2‐(2‐methoxy‐4‐nitrophenyl)‐3‐(4‐nitrophenyl)‐5‐ (2,4‐disulfophenyl)‐2H‐tetrazolium, monosod ium salt], which is reduced by dehydrogenases in viable cells to produce a yellow colour product (formazan). The cells were then further incubated for 3 hours at 37°C. The absorbance of the samples was measured at 450 nm and a reference wavelength of 650 nm using an ELISA reader (DOE Joint Genome Institute, Walnut Greek, CA). Each sample was analysed in triplicate and averaged.
Collagen synthesis assay
The supernatant solution of each well was collected. To measure the production of collagen in the supernatant, a collagen type I carboxy‐terminal propeptide (CICP) enzyme immunoassay was performed using a Metra CICP kit (Quidel, San Diego, CA) according to the manufacturer's instruction. Briefly, 100 µl of diluted culture supernatants were added to each well that had been coated with a monoclonal anti‐CICP antibody. The plates were then incubated at room temperature for 2 hours. One hundred microlitres of rabbit anti‐CICP antiserum were added and incubated for 50 minutes. One hundred microlitres of goat anti‐rabbit alkaline phosphatase conjugates were then added and incubated for 50 minutes. The reaction was stopped and the presence of collagen synthesis was detected by measuring the absorbance at 405 nm. Each sample was analysed in triplicate and averaged.
Extraction test of dressings
In order to examine the effects of other chemical components present in the dressings on cytotoxicity, extracts of each dressing were tested in vitro. A polycarbonate culture plate inserts, which had an 8‐µm pore size (Millipore), were placed into the 24‐well culture plate. Punched disks of the 10 types of dressings were placed in the culture plate insert and 1·5 ml DMEM/F‐12 were added into each well. After incubation at 37°C in a 5% CO2/95% air atmosphere for 1 and 3 days, the extracts of each dressing were collected and their chemical components were tested. Two samples were tested for each dressing. Each sample was analysed in triplicate and the average concentrations of the nine different kinds of chemical components, including silver, were measured using the Toshiba TBA 200FR NEO Chemistry Analyzer (Toshiba medical Systems, Tokyo, Japan), AAnalyst 800 spectrometer (PerkinElmer, Waltham, MA) and Electrolyte 16 analyzer (NOVA biomedical, Waltham, MA). A well containing no dressing disk was included as a control.
Statistical analysis
Data were expressed as mean ± standard deviation. Statistical comparisons were performed using the Mann–Whitney U‐test, where P‐values <0·05 were considered statistically significant.
This study was approved by our Institution's Review Board.
Results
Cell morphology
In the silver‐free dressing treated wells and control wells, the diabetic fibroblasts adopted the typical dendritic and fusiform shape. Diffuse cells had extensional cytoplasm with prominent nuclei. A little debris was observed in the background.
In contrast, diabetic fibroblasts cultured in the silver‐containing dressing treated wells did not adopt the typical dendritic or fusiform shape. The cells were diminutively stellate and had round or ovoid cytoplasm with inconspicuous nuclei. The denuded area and particulate cell debris were prominent in the background. However, the cells with PolyMem Ag partially retained the typical fibroblast fusiform apposition with protrusion and vinculum (Figure 1).
Figure 1.
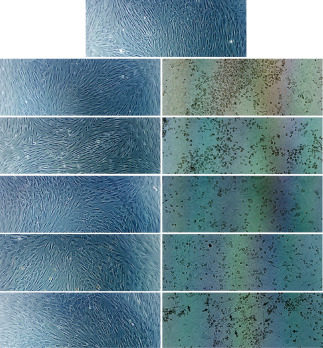
Diabetic fibroblast morphology after 3 days of incubation with the different dressing disks. (Above) Cells without a dressing disk (control). (Lefts, from above to below) Cells treated with Aquacel, Algisite*M, Medifoam, Biatain and PolyMem. (Rights, from above to below) Cells treated with Aquacel Ag, Acticoat*Absorbent, Medifoam Ag, Biatain Ag and PolyMem Ag. The diabetic fibroblasts cultured in wells containing the silver dressings did not adopt the typical dendritic or fusiform apposition morphology. However, the cells treated with PolyMem Ag partially retained the typical fibroblast fusiform apposition morphology (magnification ×200).
Cell proliferation
After 3 days of incubation, the average cell numbers in the wells of Aquacel, Algisite*M, Medifoam, Biatain, PolyMem and control groups were 5·4 ± 0·2 × 104, 6·2 ± 0·3 × 104, 5·2 ± 0·3 × 104, 5·5 ± 0·4 × 104, 5·7 ± 0·2 × 104 and 5·7 ± 0·3 × 104, respectively. However, the cell numbers in the silver‐containing dressing groups were markedly decreased when compared with those of the silver‐free dressing groups. The average cell numbers in the wells of Aquacel Ag, Acticoat*Absorbent, Medifoam Ag, Biatain Ag and PolyMem Ag were 1·8 ± 0·3 × 104, 1·9 ± 0·3 × 104, 1·6 ± 0·3 × 104, 1·7 ± 0·3 × 104 and 2·6 ± 0·2 × 104, respectively.
The difference in cell numbers between the silver‐free and silver‐containing dressing groups indicate that the viability of diabetic fibroblasts after exposure to the silver dressings was reduced when compared to the silver‐free dressing groups. The decrease in cell number was as follows: 67% loss in Aquacel Ag, 69% loss in Acticoat*Absorbent, 70% loss in Medifoam Ag and Biatain Ag and 54% loss in PolyMem Ag (Figure 2).
Figure 2.
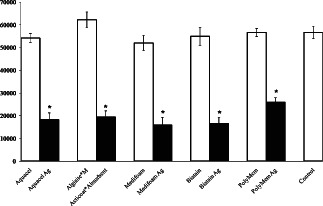
Cell numbers in the control wells and wells containing the dressings. The numbers of silver‐containing dressing treated cells were significantly lower when compared to those of the control and silver‐free dressing group (*P < 0·05).
Collagen synthesis
Collagen levels in the wells of silver‐containing dressing groups were also significantly lower than those of the control and silver‐free dressing groups.
The collagen levels in the Aquacel, Algisite*M, Medifoam, Biatain, PolyMem and control groups were 265 ± 30, 325 ± 23, 260 ± 43, 261 ± 21, 282 ± 44 and 261 ± 22 ng/ml, respectively. The collagen levels of Aquacel Ag, Acticoat*Absorbent, Medifoam Ag, Biatain Ag and PolyMem Ag groups were 92 ± 11, 114 ± 7, 84 ± 8, 98 ± 16 and 146 ± 16 ng/ml, respectively. These results imply that collagen synthesis by the diabetic fibroblasts decreased by the silver dressings when compared with silver‐free dressings. The decrease in collagen synthesis was as follows: 65, 65, 68, 62 and 48% losses in the Aquacel Ag, Acticoat*Absorbent, Medifoam Ag, Biatain Ag and PolyMem Ag, respectively (Figure 3).
Figure 3.
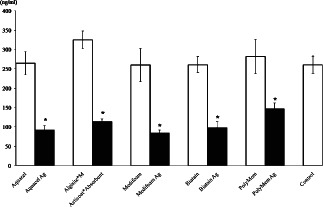
Collagen concentrations in the media of wells containing the dressing and control wells. Collagen synthesis was significantly reduced in the wells containing the silver dressing when compared to the control and silver‐free dressing wells (*P < 0·05).
Extracts of dressings
The extracts of the dressings tested in this study had a similar electrolyte composition to the control extracts, except for the concentration of calcium and silver (Table 2; Figure 4). The calcium concentration was higher in the Algisite*M and Acticoat*Absorbent extracts. The silver content in the extracts of the control and the silver‐free dressings, except for Medifoam, was below the detectable range, whereas the extracts of silver‐free Medifoam contained measurable concentrations of silver. The silver concentrations in the silver‐containing dressing extracts were significantly higher than those in the silver‐free extracts. The highest silver concentration was observed in Acticoat*Absorbent and the lowest was observed in PolyMem Ag. The difference in silver concentration after 1 and 3 days of incubation was not statistically significant in all silver‐contained dressings.
Table 2.
Chemical concentrations in the extracts of the dressing disks
| Sample | Day | T.CO2(mmol/l) | Na (mmol/l) | K (mmol/l) | Cl (mmol/l) | Ca (mmol/l) | Mg (mmol/l) | IP (mmol/l) | pH | Ag (mg/l) |
|---|---|---|---|---|---|---|---|---|---|---|
| Aquacel | 1 | 22·1 | 164·0 | 5·2 | 139·0 | 1·3 | 0·7 | 1·2 | 7·7 | 0·1 |
| 3 | 20·2 | 167·5 | 5·3 | 144·0 | 1·3 | 0·7 | 1·2 | 7·7 | 0·2 | |
| Aquacel Ag | 1 | 21·8 | 166·5 | 5·2 | 141·5 | 1·2 | 0·7 | 1·2 | 7·7 | 11·5 |
| 3 | 20·4 | 169·5 | 5·3 | 143·5 | 1·3 | 0·8 | 1·2 | 7·5 | 9·2 | |
| Algisite*M | 1 | 20·5 | 160·5 | 5·1 | 138·5 | 4·7 | 0·7 | 1·1 | 7·7 | 0·2 |
| 3 | 19·1 | 162·0 | 5·1 | 141·0 | 5·0 | 0·8 | 1·0 | 7·7 | 0·3 | |
| Acticoat*Absorbent | 1 | 21·8 | 161·5 | 5·1 | 138·0 | 3·6 | 0·7 | 1·1 | 7·7 | 12·5 |
| 3 | 19·9 | 162·0 | 5·1 | 140·0 | 3·7 | 0·7 | 0·8 | 7·6 | 18·3 | |
| Medifoam | 1 | 21·7 | 167·0 | 5·2 | 139·5 | 1·3 | 0·8 | 1·2 | 7·8 | 2·8 |
| 3 | 20·0 | 167·0 | 5·2 | 141·0 | 1·3 | 0·8 | 1·2 | 7·7 | 1·7 | |
| Medifoam Ag | 1 | 22·5 | 166·0 | 5·2 | 140·0 | 1·3 | 0·8 | 1·2 | 7·7 | 10·3 |
| 3 | 20·2 | 165·0 | 5·2 | 140·5 | 1·3 | 0·8 | 1·2 | 7·6 | 12·2 | |
| Biatain | 1 | 22·3 | 164·5 | 5·2 | 141·0 | 1·3 | 0·8 | 1·2 | 7·8 | 0·3 |
| 3 | 20·9 | 166·0 | 5·3 | 143·0 | 1·4 | 0·8 | 1·2 | 7·8 | 0·4 | |
| Biatain Ag | 1 | 22·3 | 167·5 | 5·2 | 140·0 | 1·3 | 0·7 | 1·3 | 7·7 | 9·8 |
| 3 | 20·4 | 167·5 | 5·2 | 141·5 | 1·3 | 0·8 | 1·3 | 7·6 | 10·6 | |
| PolyMem | 1 | 21·4 | 166·5 | 5·2 | 141·5 | 1·0 | 0·6 | 1·2 | 7·8 | 0·4 |
| 3 | 19·9 | 168·0 | 5·2 | 143·0 | 1·1 | 0·7 | 1·2 | 7·8 | 0·3 | |
| PolyMem Ag | 1 | 22·3 | 169·5 | 5·3 | 143·5 | 1·0 | 0·6 | 1·3 | 7·7 | 6·2 |
| 3 | 20·8 | 171·5 | 5·3 | 146·5 | 1·0 | 0·6 | 1·3 | 7·7 | 9·0 | |
| Control | 1 | 21·9 | 165·5 | 5·3 | 141·5 | 1·3 | 0·8 | 1·2 | 7·8 | 0·1 |
| 3 | 20·7 | 168·5 | 5·3 | 145·0 | 1·4 | 0·8 | 1·2 | 7·8 | 0·1 |
IP, inorganic phosphate; T.CO2, total carbon dioxide content of a sample.
Figure 4.
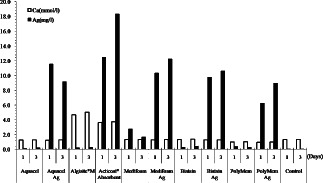
Calcium and silver concentrations in the extracts of the dressings tested in this study.
Discussion
Modern dressing products are designed to be absorbent to keep the wound moist but not macerated, non adherent, prevent secondary injury when the dressings are changed, and allow for long‐term undisturbed wound healing without the need for frequent dressing changes (19). Among the dressings currently used, some contain silver to provide a local environment free of infection.
Due to its broad antimicrobial spectrum, silver is considered an important component of dressings. Silver can exists as a silver atom (Ag0) or in three oxidation states, Ag+, Ag2+ and Ag3+. Of these, only the nanocrystalline silver atom (Ag0) and Ag+ are soluble in water (26) and have been used in large numbers of wound dressings, which originated from Moyer's use of a silver nitrate solution to treat patients with burns (27). Wound care modalities contain silver in the following forms: (a) ionic silver, the active Ag+ form, (b) elemental (metallic) silver (Ag0), in the form of nanocrystalline particles or foil, (c) inorganic compounds or complexes, such as silver nitrate and silver sulfadiazine and (d) organic complexes such as colloidal silver or silver protein complexes (28). In the silver dressings tested in this study, the silver in the Aquacel Ag, Medifoam Ag and Biatain Ag release Ag+. Acticoat*Absorbant and PolyMem Ag release Ag0 in the nanocrystalline form. The functional mechanisms of these silver dressings during the wound‐healing process also varied. In addition, these dressings contained other components aside from silver and the actions promoting wound healing are complicated in topical microenvironments.
Nanocrystalline Ag0 or Ag+ are believed to suppress infection via interference with microbial proliferation by altering DNA and RNA 29, 30, causing fetal structural changes in bacterial cell walls and membranes (31), reducing ATP, increasing production of reactive oxygen species and disrupting the mitochondrial respiratory chain 32, 33, 34, 35. In addition, it was showed that ionic silver competes for cellular entry with essential metals, for example, copper (36), and inhibits the activity of thiol‐group containing enzymes, for example, lactate dehydrogenase (37). In addition, nanocrystalline Ag0 could bind to each other and promote electron transport, resulting in the formation of insoluble complexes in microorganisms 38, 39. Thus, silver is considered a broad‐spectrum bactericide and has a far lower propensity to induce microbial resistance than antibiotics due to its multiple target sites in microorganisms (40).
The results of this study showed that silver in the dressing extraction had significantly toxic effects on morphology, proliferation and collagen synthesis of diabetic fibroblasts regardless of the silver modality. Among the silver‐containing dressings tested in this study, PolyMem Ag was the least toxic to diabetic fibroblasts. The silver concentration in the extract of the PolyMem Ag was the lowest compared to other silver‐containing dressings. This may explain the lower toxicity of the PolyMem Ag.
Interestingly, although Acticoat*Absorbent had a higher silver concentration, it was less toxic than Aquacel Ag, Medifoam Ag and Biatain Ag. The base material of Acticoat*Absorbent is a calcium alginate fabric and high concentrations of calcium were detected in the extraction of Acticoat*Absorbent and Algisite. Extracellular calcium is a potent mediator of the balance between proliferation and differentiation in a number of different cell types (41). Proliferation of human dermal fibroblasts increases in response to elevated extracellular calcium (42). Extracellular calcium may modulate the proliferation of fibroblasts through a proliferative pathway that connects the calcium‐sensing receptor to the activation of c‐Src kinase, tyrosine phosphorylation of known c‐Src kinase substrates, such as focal adhesion kinase, and activation of the extracellular signal‐regulated kinase 1 (41). Doyle et al. showed that exposure to different concentrations of calcium alginate for 1 to 7 days resulted in concentration‐dependent proliferation of human dermal fibroblasts (43). Algisite*M, which is a silver‐free calcium alginate fabric, also induced significantly higher proliferation and collagen synthesis in diabetic fibroblasts than the other silver‐free dressings.
In regards to the lasting effect of antimicrobial function in each silver‐containing dressing, active silver should be released continuously over several days to retain the long‐term effect and avoid frequent dressing changes. However, the difference in the silver concentrations after 1 and 3 days of incubation for each silver‐containing dressing was not significant.
In this study, we used a culture plate insert with an 8‐µm pore size, in which one dressing disk was placed. Cell damage by direct dressing‐to‐cell contacts could be avoided using this system. In addition, this experimental design allowed for the precise measurements of cell proliferation and collage synthesis by preventing the fabric dressings (Aquacel, Aquacel Ag, Algisite*M and Acticoat*Absorbent) from contaminating the samples prior to analysis.
In this study, we examined the cytotoxicity of silver dressings on diabetic fibroblast in vitro. However, we do not suggest that silver should be discarded because of the cytotoxicity showed in this study, since silver is a powerful agent for infection in a diabetic foot ulcer and has not been marketed as a healing agent. Managing infection in a diabetic foot ulcer is important and silver is effective at infection prevention and managing hard‐to‐heal ulcers relating to critical colonisation and local early infection. Although silver dressings may decrease cell proliferation and collagen synthesis in a culture plate, they can result in a 3‐log reduction of infection in the clinic (7). Therefore, the use of silver dressings should be considered from a clinical point of view. In addition, the human body has complex homeostatic mechanisms and silver might have different effects in vivo. Thus, further studies are needed to assess the toxicity of silver dressings on diabetic wound healing in vivo.
Despite some of the limitations of this work, the result of this study may provide helpful information in regards to choosing dressings for the management of diabetic wounds. When treating diabetic wounds with low bacterial bioburden, one should be cautious about applying silver dressings, since their use may also damaging key cells involved in wound healing.
Conclusion
Silver dressings significantly changed the cell morphology and decreased cell proliferation and collagen synthesis of diabetic fibroblasts. Therefore, silver dressings should be used with caution when treating uninfected diabetic wounds.
Acknowledgement
This study was supported by a grant from Korea University.
References
- 1. Russell AD, Hugo WB. Antimicrobial activity and action of silver. Prog Med Chem 1994;31:351–70. [DOI] [PubMed] [Google Scholar]
- 2. Dunn K, Edwards‐Jones V. The role of Acticoat™ with nanocrystalline silver in the management of burns. Burns 2004;30:S1–S9. [DOI] [PubMed] [Google Scholar]
- 3. Tomaselli N. The role of topical silver preparations in wound healing. J Wound Ostomy Continence Nurs 2006;33:367–80. [DOI] [PubMed] [Google Scholar]
- 4. Hollinger MA. Toxicological aspects of topical silver pharmaceuticals. Crit Rev Toxicol 1996;26:225–60. [DOI] [PubMed] [Google Scholar]
- 5. Wright B, Lam K, Buret A, Olson ME, Burrell RE. Early healing events in a porcine model of contaminated wounds: effects of nanocrystalline silver on matrix metalloproteinases, cell apoptosis, and healing. Wound Repair Regen 2002;10:141–51. [DOI] [PubMed] [Google Scholar]
- 6. Fong J, Wood F. Nanocrystalline silver dressings in wound management: a review. Int J Nanomedicine 2006;1:441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mooney EK, Lippitt C. Friedman Jeff. Silver dressings. Plast Reconstr Surg 2006;117:666–9. [DOI] [PubMed] [Google Scholar]
- 8. Lansdown AB, Sampson B, Laupattarakasem P, Vuttivirojana A. A Silver aids healing in the sterile skin wound: experimental studies in the laboratory rat. Br J Dermatol 1997;137:728–35. [PubMed] [Google Scholar]
- 9. Lansdown AB. Metallothioneins: potential therapeutic aids for wound healing in the skin. Wound Repair Regen 2002;10:130–2. [DOI] [PubMed] [Google Scholar]
- 10. Bador K. Organ deposition of silver following silver nitrate therapy for burns. Plast Reconstr Surg 1966;37:550–1. [DOI] [PubMed] [Google Scholar]
- 11. Baños AM, Nogueras Flores I, Palomar Llatas F. Clinical evaluation of a silver dressing in the treatment of infected and colonized ulcers. Rev Enferm 2008;31:42–8. [PubMed] [Google Scholar]
- 12. Tredget E, Shankowsky HA, Groeneveld A, Burrell R. A matched pair randomized study evaluating the efficacy and safety of Acticoat silver coated dressing for the treatment of burn wounds. J Burn Care Rehabil 1998;19:531–7. [DOI] [PubMed] [Google Scholar]
- 13. Coombs CJ, Wan AT, Masterton JP, Conyers RA, Pedersen J, Chia YT. Do burn patients have a silver lining? Burns 1992;18:179–84. [DOI] [PubMed] [Google Scholar]
- 14. Wilkinson LJ, White RJ, Chipman JK. Silver and nanoparticles of silver in wound dressings: a review of efficacy and safety. J Wound Care. 2011;20:543–9. [DOI] [PubMed] [Google Scholar]
- 15. Yin H, Langford R, Burrell R. Comparative evaluation of the antimicrobial activity of Acticoat antimicrobial barrier dressing. J Burn Care Rehabil 1999;20:195–200. [DOI] [PubMed] [Google Scholar]
- 16. Demling RH, DeSanti L. The rate of re‐epithelializa tion across meshed skin grafts is increased with exposure to silver. Burns 2002;28:264–6. [DOI] [PubMed] [Google Scholar]
- 17. Lohsiriwat V, Chuangsuwanich A. Comparison of the ionic silver‐containing Hydrofiber* and paraffin gauze dressing on split‐thickness skin graft donor sites. Ann Plast Surg 2009;62:421–2. [DOI] [PubMed] [Google Scholar]
- 18. Burd A, Kwok CH, Hung SC, Chan HS, Gu H, Lam WK, Huang L. A comparative study of the cytotoxicity of silver‐based dressings in monolayer cells, tissue explant, and animal models. Wound Repair Regen 2007;15:94–104. [DOI] [PubMed] [Google Scholar]
- 19. Paddle‐Ledinek JE, Nasa Z, Cleland HJ. Effect of different wound dressings on cell viability and proliferation. Plast Reconstr Surg 2006;117:110S–8S. [DOI] [PubMed] [Google Scholar]
- 20. Poon VK, Burd A. In vitro cytotoxity of silver: implication for clinical wound care. Burns 2004;30:140–7. [DOI] [PubMed] [Google Scholar]
- 21. Monafo WW, Moyer CA. The treatment of extensive thermal burns with 0.5 percent silver nitrate solution. Ann N Y Acad Sci 1968;150:937–45. [DOI] [PubMed] [Google Scholar]
- 22. Fox CL Jr. Silver sulfadiazine–a new topical therapy for Pseudomonas in burns. Therapy of Pseudomonas infection in burns. Arch Surg 1968;96:184–8. [DOI] [PubMed] [Google Scholar]
- 23. Bellinger CG, Conway N. Effects of silver nitrate and sulfamylon on epithelial regeneration. Plast Reconstr Surg 1970;45:582–5. [DOI] [PubMed] [Google Scholar]
- 24. Scapicchio AP, Constable JD, Opitz B. Comparative effects of silver nitrate and sulfamylon on epidermal regeneration. Plast Reconstr Surg 1968;41:319–22. [DOI] [PubMed] [Google Scholar]
- 25. Niedner R, Schöpf E. Inhibition of wound healing by antiseptics. Br J Dermatol 1986;115:41–4. [DOI] [PubMed] [Google Scholar]
- 26. Lansdown ABG, Williams A. How safe is silver in wound care? J Wound Care 2004;13:131–6. [DOI] [PubMed] [Google Scholar]
- 27. Klasen HJ. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 2001;26:131–8. [DOI] [PubMed] [Google Scholar]
- 28. Bolton L. Are silver products safe and effective for chronic wound management? J Wound Ostomy Continence Nurs 2006;33:469–77. [DOI] [PubMed] [Google Scholar]
- 29. Modak SM, Fox CL Jr. Binding of silver sulfadiazine to the cellular components of Pseudomonas aeruginosa . Biochem Pharmacol 1973;22:2391–404. [DOI] [PubMed] [Google Scholar]
- 30. Rosenkranz HS, Rosenkranz S. Silver sulfadiazine: interaction with isolated deoxyribonucleic acid. Antimicrob Agents Chemother 1972;2:373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hatchett DW, Josowicz M. Composites of intrinsically conducting polymers as sensing nanomaterials. Chem Rev 2008;108:746–9. [DOI] [PubMed] [Google Scholar]
- 32. Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram‐negative bacterium Escherichia coli . Appl Environ Microbiol 2007;73:1712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shrivastava S, Bera T, Singh SK, Singh G, Ramachandrarao P, Dash D. Characterization of antiplatelet properties of silver nanoparticles. ACS Nano 2009;3:1357–64. [DOI] [PubMed] [Google Scholar]
- 34. Sondi I, Salopek‐Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for gram‐negative bacteria. J Colloid Interface Sci 2004;275:177–82. [DOI] [PubMed] [Google Scholar]
- 35. Holt KB, Bard AJ. Interaction of silver(I) ions with the respiratory chain of Escherichia coli: an electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+ . Biochemistry 2005;44:13214–23. [DOI] [PubMed] [Google Scholar]
- 36. Solioz M, Odermatt A. Copper and silver transport by CopB‐ATPase in membrane vesicles of Enterococcus hirae . J Biol Chem 1995;270:9217–21. [DOI] [PubMed] [Google Scholar]
- 37. Rogers KS. Variable sulfhydryl activity toward silver nitrate by reduced glutathione and alcohol, glutamate and lactate dehydrogenases. Biochim Biophys Acta 1972;263:309–14. [DOI] [PubMed] [Google Scholar]
- 38. Dunn K, Edwards‐Jones V. The role of Acticoat with nanocrystalline silver in the management of burns. Burns 2004;30:S1–S9. [DOI] [PubMed] [Google Scholar]
- 39. Lansdown AB. Silver. I: its antibacterial properties and mechanism of action. J Wound Care 2002;11:125–30. [DOI] [PubMed] [Google Scholar]
- 40. Jones SA, Bowler PG, Walker M, Parsons D. Controlling wound bioburden with a novel silver‐containing Hydrofiber dressing. Wound Repair Regen 2004;12:288–94. [DOI] [PubMed] [Google Scholar]
- 41. McNeil SE, Hobson SA, Nipper V, Rodland KD. Functional calcium‐sensing receptors in rat fibroblasts are required for activation of SRC kinase and mitogen‐activated protein kinase in response to extracellular calcium. J Biol Chem 1998;273:1114–20. [DOI] [PubMed] [Google Scholar]
- 42. Huang S, Maher VM, McCormick JJ. Extracellular Ca2+ stimulates the activation of mitogen‐activated protein kinase and cell growth in human fibroblasts. Biochem J 1995;310:881–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Doyle JW, Roth TP, Smith RM, Li YQ, Dunn RM. Effects of calcium alginate on cellular wound healing process modeled in vitro. J Biomed Mater Res 1996;32:561–8. [DOI] [PubMed] [Google Scholar]


