Abstract
Cutaneous wound healing is a highly complex process, which includes inflammation, cell proliferation, matrix deposition and remodelling phases. Various growth factors, like epidermal growth factor (EGF), play an important role during wound healing. However, little is known about relationship between EGF and oxidant–antioxidant events in cutaneous wound healing models. Thus we planned to evaluate the connection between EGF therapy and oxidative stress in dermal tissue followed by wounding. Fifty‐four adult male Wistar‐albino rats were randomly divided into three groups: control, untreated and topical EGF administrated group. A linear full‐thickness excision of 40 mm in length on both sides of spinal cord was made on the back of each rat and sutured under anaesthesia and sterile conditions. Excision was closed with 4/0 atraumatic silk suture. EGF solution was freshly prepared at 10 ng/ml dose in thilotears gel under aseptic conditions. Following the surgery, 1 ml of EGF solution was administered to wound strips one time in everyday. The animals were euthanised and wound tissues were collected on days 1, 5, 7 and 14. Thiobarbituric acid reactive substans (TBARS), glutathione (GSH), reactive nitrogen oxide species (NOx), ascorbic acid levels and superoxide dismutase activity were measured spectrophotometrically. TBARS levels decreased and NOx levels increased on day 5 after operation, and GSH levels were increased on day 14 in EGF administered group compared with untreated group. Our data showed that EGF may act like an antioxidant by scavenging toxic oxidation products in wound tissue. In addition, it may contribute healing of the wound tissue in earlier stages and suggest a potential effective role for antioxidant therapies, especially until day 5.
Keywords: Antioxidants, EGF, Skin, Wound healing
INTRODUCTION
Cutaneous wound healing is a highly complex process, which includes inflammation, cell proliferation, matrix deposition and remodelling phases (1). These phases follow each other like a cascade. The wound healing process is affected by any change of this chain of events. Many factors, like growth factors, are active in the wound healing process. Growth factors are structurally proteins or steroid hormones. They are found in small amounts but have a powerful influence on the process of wound repair, growth, differentiation and metabolism of the cells (2, 3, 4, 5, 6, 7). Growth factors such as platelet‐derived growth factor, fibroblast growth factor, transforming growth factor‐β, epidermal growth factor (EGF), insulin‐like growth factor, granulocyte‐macrophage colony‐stimulating factor, interleukin‐1 (IL‐1), IL‐2 and tumour necrosis factor‐α play a vital role during wound healing process (8, 9, 10, 11, 12).
EGF, a crucial growth factor in wound healing, plays an important role in the regulation of cell growth, proliferation and differentiation. EGF is a 6045‐Da protein with 53 amino acid residues and has three intramolecular disulfide bonds (13). EGF, is a low‐molecular weight polypeptide, first purified from the mouse submandibular gland (14) and then found in many human tissues including submandibular and parotid glands. EGF acts by binding to epidermal growth factor receptor (EGFR) with high affinity on the cell surface and stimulating the intrinsic protein‐tyrosine kinase activity of the receptor. EGFR is the cell‐surface receptor for members of EGF family of extracellular protein ligands (15). It is known that many types of cells, including dermal fibroblasts have EGF receptors. With stimulation of these receptors, the growth factors respond by increasing the proliferation of the cells in cell cultures (16). The effects of EGF include: a wide range stimulation of target cells, chemotaxis, regulating mitogenic stimulation of fibroblasts, endothelial cells and keratinocytes (3). EGF has been shown to stimulate wound healing in skin (17), cornea (18), gastric (4) and oral mucosa 19, 20.
During wound healing, various inflammatory cells such as neutrophils, macrophages, endothelial cells and fibroblasts produce reactive oxygen species (ROS) (21). ROS such as superoxide (O2 −), hydrogen peroxide (H2O2) and hydroxyl radical (OH) are natural byproduct of the normal oxygen metabolism (22). However, during environmental stress (e.g. wound, ultraviolet or heat exposure), ROS levels can increase, and ROS which are highly reactive because of the presence of unpaired electrons, may cause significant damage to cell structures. This is a situation known as oxidative stress, and is associated with increased production of oxidising species or a significant decrease in antioxidant defences. Antioxidant defence system arising from both antioxidant enzymes and non enzymatic antioxidant compounds defends the physiological activities of organisms from oxidative stress in all aerobic organisms. During the wound healing, activated neutrophils and macrophages produce a large amount of O2 − and its derivatives via the phagocytic isoform of nicotinamide adenine dinucleotide phosphate (NADPH) of NADPH oxidases. Damaged endothelial cells can also produce high concentrations of O2 −, H2O2 and OH under ischaemic conditions (23). Releasing excessive ROS due to inflammation and ischaemia in wound tissue is a deleterious and destructive phenomenon during the healing process (24) and this activates the antioxidant defence system (25). Antioxidants have been shown to promote wound healing (26, 27, 28, 29, 30). Antioxidant levels protect the wound against ROS, either partially or completely, during impaired wound healing or in normal subjects (31).
Nitric oxide (NO), which is involved in many physiological and pathological processes, is an important signalling molecule in the body of mammals including humans (32). NO is unstable nitrogen radical that is generated in different cell types through the enzyme NO synthase (NOS). There are at least three distinct isoforms of NOS present. Two enzymes, the neuronal NOS (nNOS) and endothelial NOS (eNOS), release NO for a short period in response to physiological stimuli. The inducible NOS (iNOS), induced by endotoxins and cytokines, is continuously active and releases NO for a long period (33). It has both beneficial and detrimental effects in the mammalian systems depending on the NOS isoform (32). There are physiological effects such as neurotransmission and vasodilatation of NO which produced by eNOS and nNOS in nanomolar concentration. However, NO produced by iNOS has a toxic effect and leads to tissue damage when reached mikromolar concentration. It is known that NO is produced by many different cell types in wounded tissues. The cells include platelets (nNOS and iNOS), macrophages (iNOS), fibroblasts (eNOS and iNOS), endothelial cells (eNOS) and keratinocytes (eNOS and iNOS) 34, 35.
Ascorbic acid (AA) is found in the biology of plants, animals and single‐cell organisms (36). Many plants and animals can produce AA from glucose. Humans, some fruit bats, guinea pig and human‐like primates need to get AA from diet. Synthesis and signalling properties are still under investigation (37). AA usually acts as an antioxidant by being available for energetically favourable oxidation. AA can terminate these chained radical reactions by serving as a stable (electron + proton) donor in interactions with free radicals, and converted into the radical ion called ‘semi‐dehydroascorbate’ and then dehydroascorbate. The oxidised forms of AA are relatively unreactive, and do not cause cellular damage. They can be converted back to AA by cellular enzymes. However, excess AA not only promotes, but also initiates free radical reactions in the presence of free metal ions, thus making it a potentially dangerous pro‐oxidative compound in certain metabolic contexts (38). Glutathione (GSH) is a tripeptide that contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of glutamate side chain. It is an antioxidant, preventing damage, which is caused by ROS such as free radicals and peroxides to important cellular components (39). Superoxide dismutases (SODs) are enzymes that catalyse the dismutation of O2 − into oxygen and H2O2. It has been known that treatment with SOD decreases ROS generation and oxidative stress (40).
There is limited research on relationship between growth factors and oxidant–antioxidant events in experimental wound healing in skin models. In light of the above, we planned to investigate that effect of topical EGF application on the lipid peroxidation level, NO production, both enzymatic and non enzymatic antioxidant status on days 1, 5, 7 and 14 after excisional surgery.
MATERIALS AND METHODS
Animals
This study was carried out in accordance with the regulations of Animal Experimentation Ethics Committee at Gazi University. All experiments were performed with 54 adult, male Wistar‐albino rats weighing 200–250 g. They were maintained in a 12‐h light/12‐h dark cycle at room temperature (25 ± 3°C), with standard rat chow and tap water provided ad libitum. Rats were housed in individual cages in Gazi University – Laboratory Animals Breeding and Experimental Research Center.
The animals were randomised into three groups:
-
1
Non wounded animals (n = 6; on day 0)
-
2
Untreated excisional group (n = 6; on day 1)
Untreated excisional group (n = 6; on day 5)
Untreated excisional group (n = 6; on day 7)
Untreated excisional group (n = 6; on day 14)
-
3
EGF therapy group (n = 6; on day 1)
EGF therapy group (n = 6; on day 5)
EGF therapy group (n = 6; on day 7)
EGF therapy group (n = 6; on day 14)
Wound excision
The animals were anaesthetised with xylazine (2–5 mg/kg) and ketamine (40–50 mg/kg) intramuscularly. Their backs were shaved and cleaned with tincture of iodine. A linear full‐thickness excision of 40 mm in length on both sides of spinal cord was made on the back of each rat and sutured under anaesthesia and sterile conditions. Excision was closed with 4/0 atraumatic silk suture.
On days 1, 5, 7 and 14, animals euthanised with the same anaesthetic solution. The wound strips were removed and transported immediately frozen in liquid nitrogen, and then the samples were kept at −30°C until assay. At the same time, skin samples indicating 0 day were also collected from non wounded animals (n = 6, on day 0).
Preparation and administration of EGF
EGF was purchased from Sigma‐Aldrich (St. Louis, MO). EGF solution was freshly prepared at 10 ng/ml dose in thilotears gel under aseptic conditions. Following the surgery, 1 ml of EGF solution was administered to wound strips one time in everyday.
Determination of TBARS levels
Thiobarbituric acid reactive substans (TBARS) level as lipid peroxidation end product was measured in tissues by spectrophotometric method of Casini et al. (41). Briefly, tissue samples were homogenised in ice‐cold 10% trichloroacetic acid (TCA; 1:10, w/v). After centrifugation at 1008 g for 10 minutes, 750 µl of supernatant was added to an equal volume of 0·67% (m/v) TBA and heated at 100°C for 15 minutes. Absorbance of the samples was measured at 535 nm. A 1 mm stock tetraethoxypropane solution was used as standard.
Determination of GSH levels
GSH levels were determined by a modified Ellman method (42). Briefly, tissue samples were homogenised in ice‐cold 10% TCA (1:10, w/v). After centrifugation at 1008 g for 10 minutes, 0·5 ml of supernatant was added to 2 ml of 0·3 M Na2HPO4.2 H2O solutions. A 0·2 ml solution of 0·4 mg/ml 5,5′‐dithiobis‐2‐nitrobenzoic acid (DTNB) was added and absorbance at 412 nm was measured immediately after mixing. GSH levels were calculated using an extinction coefficient of 13 600/mol/cm. Results are expressed as µmol/g tissue.
Determination of NOx levels
Levels of reactive nitrogen oxide species (NOx) stable end products of NO‐in tissue homogenates were determined by Griess reaction (43). Tissue samples were homogenised in phosphate buffer (pH 7·4) and centrifuged at 2000 g for 5 minutes; to supernatants (0·5 ml), 0·25 ml of 0·3 M NaOH was added. After incubation for 5 minutes at room temperature, 0·25 ml of 10% (w/v) ZnSO4 was added for deproteinisation. This mixture was then centrifuged at 14 000 g for 5 minutes and supernatants were used for Griess assay. Nitrate levels in tissue homogenates were determined spectrophotometrically. Nitrate was reduced to nitrite using vanadium trichloride (VCI3) (44). Nitrite levels were measured by the Griess reaction. Sodium nitrite (1, 10, 50 and 100 µM) was used as standard.
Determination of AA levels
AA levels were assayed by a spectrophotometric method 45, 46. Tissues were thawed at 0°C, weighed and homogenised on ice in nine volume of 0·35 M perchloric acid (PCA) with 0·1 mg/ml EDTA. Samples were centrifuged at 15 000 g for 3 minutes at 4°C and supernatants were obtained. A portion (200 µl) of a standard or sample in a centrifugal analyser tube was combined with 50 µl of colour reagent. After incubation for 3 hours at 37°C, samples were brought to 0°C and 300 µl of 65% (v/v) H2SO4 was added. Samples were vortexed and the optical density at 515 nm was measured.
Determination of SOD activity
SOD activity was detected by the method of Sun et al. (47). Exactly tissue samples were homogenised in ice‐cold 0·9% NaCl and centrifuged at 5488 g and stayed +4°C for 30 minutes. A 2·45 ml of assay reagent (containing 3 mm xanthine, 0·6 mm ethylenediaminetetraacetic acid (EDTA), 150 µM NBT, 400 mm Na2CO3 and 1 g/l bovine serum albumin (BSA)) was combined with 500 µl of supernatant sample. Xanthine oxidase (50 µl, 167 U/l) was added to initiate the reaction and the reduction of nitro blue tetrazolium (NBT) by O2 −, which are produced by the xanthine/xanthine oxidase system, was determined by measuring the absorbance at 560 nm. SOD activity was expressed U/l, where 1 U is defined as that amount of enzyme causing half‐maximal inhibition of NBT reduction.
Statistical analysis
The statistical differences between the mean values were evaluated by one‐way analysis of variance. Values of P < 0·05 were considered to be significant. All data were expressed as the mean ± standard deviation.
RESULTS
The results of TBARS, GSH, NOx, AA levels and SOD activity are shown in Table 1 and 1, 2, 3, 4, 5
Table 1.
The effects of EGF therapy on the TBARS, GSH, NOx, AA levels and SOD activity in skin wound tissue after excisional surgery †
| TBARS (nmol/g tissue) | GSH (µmol/g tissue) | NOx (µmol/g tissue) | AA (mg/g tissue) | SOD (U/g tissue) | |
|---|---|---|---|---|---|
| Non wounded group | 256·99 ± 35·19 | 2·448 ± 0·12 | 435·957 ± 35·77 | 0·167 ± 0·01 | 969·15 ± 18·34 |
| Untreated groups | |||||
| Day 1 | 421·50 ± 85·84 * | 2·16 ± 0·39 | 468·25 ± 58·80 | 0·07 ± 0·01 * | 534·80 ± 14·37 * |
| Day 5 | 331·93 ± 59·40 | 2·22 ± 0·35 | 478·74 ± 41·12 | 0·13 ± 0·01 * | 589·12 ± 11·83 * |
| Day 7 | 267·40 ± 32·85 | 2·01 ± 0·22 | 472·79 ± 43·02 | 0·09 ± 0·01 * | 599·16 ± 18·18 * |
| Day 14 | 206·02 ± 20·79 | 2·11 ± 0·17 | 349·41 ± 12·94 | 0·12 ± 0·02 * | 554·39 ± 27·07 * |
| EGF therapy groups | |||||
| Day 1 | 421·13 ± 62·03 * | 2·43 ± 0·10 | 470·34 ± 70·43 | 0·08 ± 0·01 * | 525·94 ± 15·78 * |
| Day 5 | 191·60 ± 31·75 # | 2·33 ± 0·10 | 603·32 ± 92·97 * , # | 0·13 ± 0·02 * | 630·54 ± 15·05 * |
| Day 7 | 462·23 ± 61·27 * , # | 2·14 ± 0·35 | 418·59 ± 44·87 | 0·08 ± 0·01 * | 576·15 ± 23·60 * |
| Day 14 | 259·97 ± 42·94 | 3·49 ± 0·43 * , # | 411·68 ± 51·17 | 0·12 ± 0·02 * | 528·87 ± 13·67 * |
AA, ascorbic acid; EGF, epidermal growth factor; GSH, reduced glutathione; NOx, reactive nitrogen oxide species; SOD, superoxide dismutase; TBARS, thiobarbituric acid reactive substans.
†All data are expressed as mean ± standard deviation.
* P < 0·05 as compared with the non wounded group.
# P < 0·05 as compared with the respective control.
Figure 1.
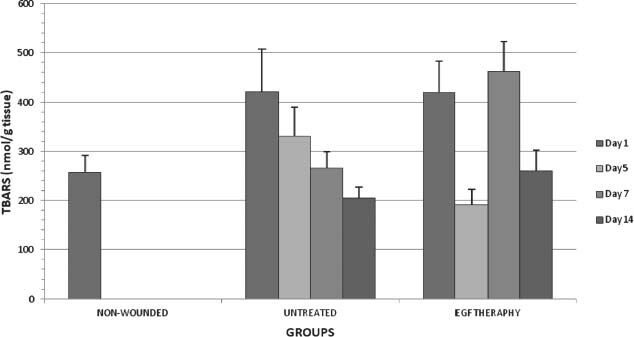
TBARS levels in all groups on days 1,5,7 and 14 after wounding.
Figure 2.
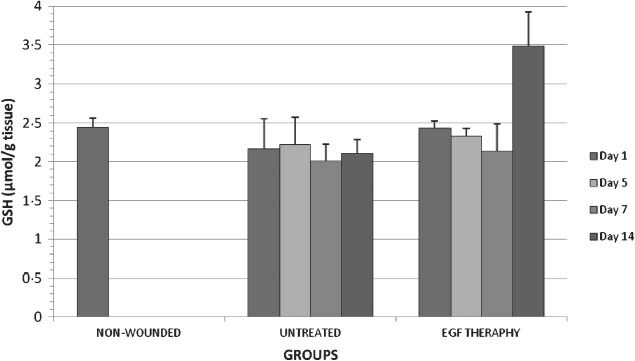
GSH levels in all groups on days 1,5,7 and 14 after wounding.
Figure 3.
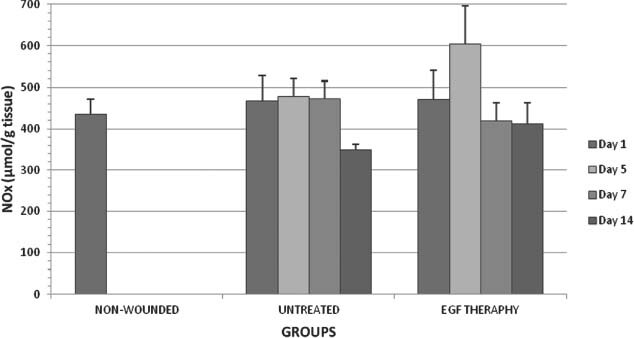
NOx levels in all groups on days 1,5,7 and 14 after wounding.
Figure 4.
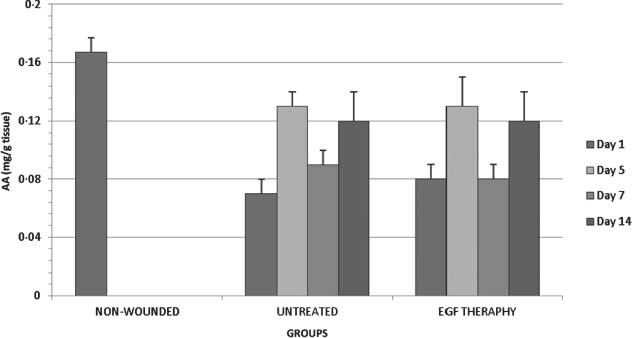
AA levels in all groups on days 1,5,7 and 14 after wounding.
Figure 5.
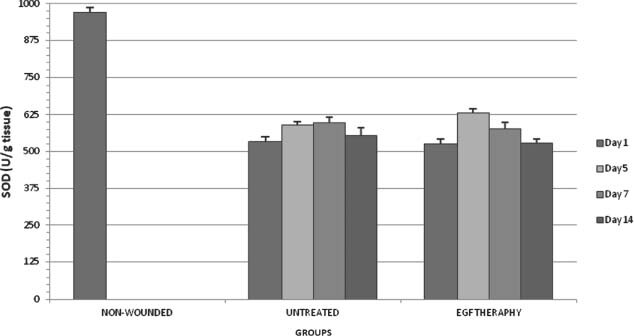
SOD activity in all groups on days 1,5,7 and 14 after wounding.
Thiobarbituric acid reactive substans
As shown in Figure 1, TBARS level was significantly decreased in EGF therapy group when compared with the respective control on day 5 after wounding (P < 0·05). A decline in the lipid peroxidation levels was observed in the EGF therapy group on day 5. EGF may be an antioxidant reducing lipid peroxidation. However, on day 7 after operation TBARS level was statistically increased in the EGF therapy group. There was a significant decrease only on day 14 in the untreated groups when compared with on day 1 (P < 0·05). TBARS levels were statistically decreased on day 5 after operation when compared with on day 1 after operation in the EGF therapy group.
Glutathione
With the application EGF, the wound tissue GSH level was increased statistically (P < 0·05) only on day 14 when compared with the respective control (Figure 2). EGF may be an antioxidant reducing lipid peroxidation. In addition, EGF may promote antioxidant capacity of wound tissue, especially during the delayed wound healing. There was not any significant alteration in all untreated groups. In the EGF therapy groups, GSH level increased on day 14 when compared with the other experimental groups.
Reactive nitrogen oxide species
As shown in Figure 3, NOx level was significantly (P < 0·05) increased on day 5 after wounding in EGF therapy group when compared with the respective control. Excessive NO production appeared wounded tissue on day 5 after surgery caused by proliferation of macrophage which is triggered by EGF. In the untreated groups, there was a significant decrease only on day 14 when compared with the other untreated groups. In the EGF therapy groups, NOx level was significantly increased on day 5 after operation when compared with on day 1. However, NOx levels were significantly decreased both on days 7 and 14 when compared with on day 5 in the EGF therapy groups.
Ascorbic acid
There was no significant (P < 0·05) alteration in the EGF therapy group when compared with the respective control (Figure 4). In the untreated groups, AA levels were statistically increased both on days 5 and 14 after operation when compared with on day 1. In the EGF therapy groups, AA level was significantly increased on day 5 after operation when compared with on day 1. AA level was significantly decreased on day 7 after operation in EGF therapy groups when compared with on day 5.
Superoxide dismutase
As shown in Figure 5, there was no significant (P < 0·05) changes in the EGF therapy group when compared with the respective control. In the untreated groups, SOD levels were statistically increased both on days 5 and 7 after operation when compared with on day 1. SOD level was significantly decreased on day 14 after operation in the untreated group when compared with on day 7. In the EGF therapy groups, SOD level was significantly increased both on days 5 and 7 after operation when compared with on day 1. However, SOD level was significantly decreased on day 7 after operation in EGF therapy group when compared with on day 5. SOD level was significantly decreased on day 14 after operation in EGF therapy group when compared with on days 5 and 7.
DISCUSSION
EGF plays a vital role by stimulating wound healing in skin, cornea, oral and gastric mucosa (2, 3, 4, 5, 6, 7, 18). Many studies have reported the effects of growth factors on the normal healing or impaired healing of skin wounds in animal models (2, 3, 4, 5, 6, 7). But little is known about the relationship between the growth factors and oxidant–antioxidant events in experimental cutaneous wound healing in skin models. Thus we have planned this study to evaluate the relationship between EGF therapy and oxidative stress in the skin. All this values of wound tissue following EGF application were determined after excisional surgery on days 1, 5, 7 and 14.
In this study, TBARS level was significantly decreased in EGF therapy group when compared with the respective control on day 5 after wounding. A decline in lipid peroxidation levels was observed in the EGF therapy group on day 5. For this reason, EGF may be an antioxidant effect reducing lipid peroxidation. Consistent with our study, Akbulut et al. found that EGF reduces lipid peroxidation in rat gastric tissue on stress ulcer healing 4. Similarly, Erkasap et al. reported that EGF administration on ethanol induced gastric ulcer in rats shown to reduce TBARS levels of gastric tissue (48). However, on day 7 after operation TBARS level was statistically increased in the EGF therapy group. EGF administration caused to decrease significantly the tissue TBARS levels depending on the days except on day 7 which is the inflammation phase in the wound healing. On the other hand, EGF may cause unknown negative effects for the organisms after the first 5 days.
In our study, with the application EGF, wound tissue GSH level was increased statistically only on day 14 when compared with the respective control. EGF may promote antioxidant capacity of wound tissue, especially during the delayed wound healing. In addition, the increased levels of GSH may have occurred to eliminate the negative effects of the TBARS levels and EGF therapy in wound tissue. Consistent with our study, Akbulut et al. determined that there were no significant changes in the gastric GSH levels among all the groups with the EGF application 4. Also, Gulec et al. reported that there were no alterations in the GSH levels with EGF therapy on the oral mucosa (49). In addition, Gupta et al. found that GSH levels reduced during wound healing process for 14 days in immunocompromised rats (50).
NO is a critical signal molecule and mediator for wound healing. In addition, excessive NO production in the early wound healing is necessary for the healing process. In our study, NOx level was significantly increased on day 5 after wounding in EGF therapy group when compared with the respective control. Thus excessive NO production appeared wounded tissue on day 5 after surgery may caused by proliferation of macrophage which is triggered by EGF. These findings indicated that EGF administration decreased tissue NOx levels. In addition, there is a relationship with EGF and NO production. In accordance with this finding, Coskun et al. determined that NO production maximised 24 hours after wounding and the EGF administration caused a decrease in NO production (19). Heck et al. pointed that EGF suppresses NO and H2O2 production by keratinocytes. They showed the treatment of both human and mouse keratinocytes with EGF to result in decreased NO production (51). It has been known that the expression of iNOS is localised in macrophages, keratinocytes and fibroblasts in the healing of wounds (52).
In our study, there was no significant alteration in term of AA levels in the EGF therapy group when compared with the respective control. In the EGF therapy groups, AA level was significantly increased on day 5 after operation when compared with on day 1. AA level was significantly decreased on day 7 after operation in EGF therapy groups when compared with on day 5. Cells required AA for collagen production and neutrophil phagocytes during impressive wound healing. These events may cause fluctuations of AA levels in wound healing process. There were no significant alterations in the AA levels of EGF treatment when compared with the respective control. Consistent with our study, Peker et al. reported that there was no change in the AA levels on oral mucosa with EGF treatment (49).
SOD is an inducible enzyme and its activity depends on O2 − concentration (51). Kiyohara et al. pointed that EGF may play an important role in wound healing after burns by increasing SOD activity (53). In addition, Nishiguchi et al. reported that EGF application to cultured fibroblast obtained from rat skin increased SOD activity (54). In this study, there were no significant changes in the EGF therapy group when compared with the respective control. In EGF therapy groups, SOD level was significantly increased both on days 5 and 7 after operation when compared with on day 1. However, SOD level was significantly decreased on day 7 after operation in EGF therapy group when compared with on day 5. SOD level was significantly decreased on day 14 after operation in EGF therapy group when compared with on days 5 and 7. EGF administration may increase SOD level by its antioxidant effect in day 5 of wound healing.
There is strong evidence that EGF may be able to protect tissue from oxidative damage. Our data show that EGF, like other antioxidants, may act by scavenging already formed toxic oxidation products. It may also fail to show the beneficial effects on wounded tissue while also suggesting new and potentially effective causal antioxidant therapies for the first 5 days. Hence, we suggest the use of EGF administration to wound tissue for the first 5 days with regard to inhibit the harmful effects of EGF.
ACKNOWLEDGEMENT
This investigation was supported by Gazi University Research Foundation, Ankara, Turkey (Project Number: 05/2008.35).
REFERENCES
- 1. Clark RAF. Biology of dermal wound repair. Dermatol Clin 1993;11:647–66. [PubMed] [Google Scholar]
- 2. Gonul B, Erdogan D, Ozogul C, Koz M, Celebi C. Effect of EGF dosage forms on alkali burned corneal wound healing of mice. Burns 1995;21:7–10. [DOI] [PubMed] [Google Scholar]
- 3. Fujisawa K, Miyamoto Y, Nagayama M. Basic fibroblast growth factor and epidermal growth factor reverse impaired ulcer healing of the rabbit oral mucosa. J Oral Pathol Med 2003;32: 358–66. [DOI] [PubMed] [Google Scholar]
- 4. Akbulut KG, Gonul B, Turkyilmaz A, Celebi N. The role of EGF formulation on stress ulcer healing of the gastric mucosa. Surg Today 2002;32:880–3. [DOI] [PubMed] [Google Scholar]
- 5. Gonul B, Akbulut KG, Ozer C, Yetkin G, Celebi N. The role of TGF‐α formulation on aspirin induced ulcer healing and oxidant stress in the gastric mucosa. Surg Today 2004;34:1035–40. [DOI] [PubMed] [Google Scholar]
- 6. Yetkin G, Celebi N, Ozer C, Gonul B, Ozogul C. The healing effect of TGF‐alpha on gastric ulcer induced by acetylsalicylic acid in rats. Int J Pharm 2004;277:163–72. [DOI] [PubMed] [Google Scholar]
- 7. Alemdaroglu C, Degim Z, Celebi N, Zor F, Ozturk S, Erdogan D. An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns 2006;32:319–27. [DOI] [PubMed] [Google Scholar]
- 8. Rigler DJ. Inflamation and repair. In: Jones TC, Hunt RD, King NW, editors. Veterinary pathology. Pennsylvania, 1997:150–7. [Google Scholar]
- 9. Pascoe JR. Wound healing. In Gourley IM, Gregory CR, editors. Atlas of small animal surgery. New York, 1991:2–13. [Google Scholar]
- 10. Steed DL. Modifiying the wound healing response with exogenous growth factors. Clin Plast Surg 1998;25:397–405. [PubMed] [Google Scholar]
- 11. Celebi N, Turkyilmaz A, Gonul B, Ozogul C. Effects of EGF microemulsion formulation on the healing of stress‐induced gastric ulcers in rats. J Control Release 2002;83:197–210. [DOI] [PubMed] [Google Scholar]
- 12. Sayan H, Gonul B, Akbulut KG, Turkyilmaz A, Celebi N. Effects of epidermal growth factor formulations on liver malondialdehyde and reduced glutathione levels in stress ulcer model. FABAD J Pharm Sci 2001;26:61–3. [Google Scholar]
- 13. Carpenter G, Cohen S. Epidermal growth factor. J Biol Chem 1990;265:7709–12. [PubMed] [Google Scholar]
- 14. Venturi S, Venturi M. Iodine in evolution of salivary glands and in oral health. Nutr Health 2009;20:119–34. [DOI] [PubMed] [Google Scholar]
- 15. Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys 2004;59:21–6. [DOI] [PubMed] [Google Scholar]
- 16. Ksander GA. Exogeneous growth factors in dermal wound healing. Annu Rep Med Chem 1989;24:223–32. [Google Scholar]
- 17. Pessa ME, Bland KI, Copeland EM. Growth factors and determinants of wound repair. J Surg Res 1987;42:207–17. [DOI] [PubMed] [Google Scholar]
- 18. Gonul B, Kaplan B, Bilgihan K, Budak MT. Effect of EGF in artificial tear on vitamin C levels of corneal wounded eye tissues. Eye 2000;15:213–6. [DOI] [PubMed] [Google Scholar]
- 19. Coskun S, Gulec EG, Balabanli B, Acarturk F. Effects of epidermal growth factor on lipid peroxidation and nitric oxide levels in oral mucosal ulcer healing: a time–course study. Surg Today 2007;37:570–4. [DOI] [PubMed] [Google Scholar]
- 20. Peker EG, Coskun S, Ebegil M, Acarturk F. Effect of exogenous epidermal growth factor (EGF) on nonenzymatic antioxidant capacities and MPO activity of wound tissue. Med Chem Res 2009;19:533–40. [Google Scholar]
- 21. Soneja A, Drews M, Malinski T. Role of nitric oxide, nitroxidative and oxidative stress in wound healing. Pharmacol Rep 2005;57:108–19. [PubMed] [Google Scholar]
- 22. Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress‐induced cancer. Chem Biol Interact 2006;160:1–40. [DOI] [PubMed] [Google Scholar]
- 23. Brovkovych V, Dobrucki LW, Brovkovych S, Dobrucki I, Do Nascimento CA, Burewicz A, Malinski T. Nitric oxide release from normal and dysfunctional endothelium. J Physiol Pharmacol 1999;50:575–86. [PubMed] [Google Scholar]
- 24. Sakallıoglu U, Aliyev E, Eren Z, Aksimsek G, Keskiner I, Yavuz U. Reactive oxygen species scavenging activity during periodontal mucoperiosteal healing: an experimental study in dogs. Arch Oral Biol 2005;50:1040–6. [DOI] [PubMed] [Google Scholar]
- 25. Senel O, Cetinkale O, Ozbay G, Ahcioglu F, Bulan R. Oxygen free radicals impair wound healing in ischemic rat skin. Ann Plast Surg 1997;39:516–23. [DOI] [PubMed] [Google Scholar]
- 26. Martin A. The use of antioxidants in healing. Dermatol Surg 1996;22:156–60. [DOI] [PubMed] [Google Scholar]
- 27. Slater TF. Free radical mechanisms in tissue injury. Biochem J 1984;222:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weinstein GS, Maves MD, Mccormack MI. Deferoxamine decreases necrosis in dorsally based pig skin flaps. Otolaryngol Head Neck Surg 1989;101:559–61. [DOI] [PubMed] [Google Scholar]
- 29. Foschi D, Castoldi L, Radalli E. Hyaluronic acid prevents oxygen free radical damage to granulation tissue: a study in rat. Int J Tissue React 1990;12:333–9. [PubMed] [Google Scholar]
- 30. Silaeva SA, Guliaeva NV, Khatsernova BI, Onufriev MV, Nikolaev A. Effects of 4‐methyluracil and carnosine on healing of skin wounds in rats. Biol-Eksp Biol Med 1990;109:180–2. [PubMed] [Google Scholar]
- 31. Shukla A, Rasik AM, Patnaik GK. Depletion of reduced glutathione, ascorbic acid, vitamin E and antioxidant defence enzymes in a healing cutaneous wounds. Free Rad Res 1997;26:93–101. [DOI] [PubMed] [Google Scholar]
- 32. Hou YC, Janczuk A, Wang PG. Current trends in the development of nitric oxide donors. Curr Pharm Des 1999;5:417–41. [PubMed] [Google Scholar]
- 33. ArunaDevi R, Ramteke VD, Kumar S, Shukla MK, Jaganathan S, Kumar D, Sharma AK, Tandan SK. Neuroprotective effect of s‐methylisothiourea in transient focal cerebral ischemia in rat. Nitric Oxide 2010; 22:1–10. [DOI] [PubMed] [Google Scholar]
- 34. Schwenter A, Vodovotz Y, Weller R, Billiar T. Nitric oxide and wound repair: role of cytokines? Nitric Oxide 2002;7:1–10. [DOI] [PubMed] [Google Scholar]
- 35. Nguyen T, Brunson D, Crespi CL, Penman BW, Wishnok JC, Tannenbaum SR. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci U S A 1992; 89:3030–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stone I. The natural history of ascorbic acid in the evolution of the mammals and primates and its significance for present day man. J Orthomolecular Psychiatry 1972;1:82–9. [Google Scholar]
- 37. Valpuestaa V, Botellaa MA. Biosynthesis of L‐ascorbic acid in plants: new pathways for an old antioxidant. Trends in Plant Science 2004;9:573–7. [DOI] [PubMed] [Google Scholar]
- 38. Young I S, Woodside JV. Antioxidants in health and disease. J Clin Pathol 2001;54:176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol 2003;66:1499–503. [DOI] [PubMed] [Google Scholar]
- 40. Segui J, Gironella M, Sans M, Granell S, Gil F, Gimeno M, Coronel P, Piqué JM, Panés J. Superoxide dismutase ameliorates TNBS‐induced colitis by reducing oxidative stress, adhesion molecule expression, and leukocyte recruitment into the inflamed intestine. J Leukoc Biol 2004;76:537–44. [DOI] [PubMed] [Google Scholar]
- 41. Casini A, Ferrali M, Pompelam A, Maellaro A, Comborti M. Lipid peroxidation and cellular damage in extrahepatic tissues of bromobenzene intoxicated mice. Am J Pathol 1986;123:520–31. [PMC free article] [PubMed] [Google Scholar]
- 42. Aykac G, Uysal M, Yalcin AS, Kocak‐Toker N, Sivas A, Oz H. The effect of chronic ethanol ingestion on hepatic lipid peroxide, glutathione, glutathione peroxidase and glutathione transferase in rats. Toxicology 1985;36:71–6. [DOI] [PubMed] [Google Scholar]
- 43. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analyses of nitrate, nitrite and [15N] nitrate in biological fl uids. Anal Biochem 1982;126:131–8. [DOI] [PubMed] [Google Scholar]
- 44. Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001;5:62–71. [DOI] [PubMed] [Google Scholar]
- 45. Roe J, Kuether C, Zımler R. Distribution of ascorbic acid in blood. Fed Proc 1946;5:151. [PubMed] [Google Scholar]
- 46. Berger J, Shepard D, Morrow F, Taylor A. Relationship between dietary intake and tissue levels of reduced and total vitamin C in the nonscorbutic guinea pig. J Nutr 1989;119:734–40. [DOI] [PubMed] [Google Scholar]
- 47. Sun Y, Oberley LW, Li V. A simple method for clinical assay of superoxide dismutase. Clin Chem 1988;34:497–500. [PubMed] [Google Scholar]
- 48. Erkasap S, Erkasap N, Aral E, Koken T, Kahraman A, Aydın Y, Yılmaz S, Ateş E. Mast cell stabilizator and antioxidant effects of epidermal growth factor (EGF) on gastric mucosal injury by ethanol in rats. Chin J Physiol 2005;48:1–6. [PubMed] [Google Scholar]
- 49. Gulec Peker EG, Coskun S, Ebegil M, Acarturk F. Effect of exogenous epidermal growth factor (EGF) on nonenzymatic antioxidant capacities and MPO activity of wound tissue. Med Chem Res 2010;19:533–40. [Google Scholar]
- 50. Gupta A, Singh RL, Raghubir R. Antioxidant status during cutaneous wound healing in immunocompromised rats. Mol Cell Biochem 2002;241:1–7. [DOI] [PubMed] [Google Scholar]
- 51. Heck DE, Laskin DL, Gardner CR, Laskin JD. Epidermal growth factor suppresses nitric oxide and hydrogen peroxide production by keratinocytes. Potential role for nitric oxide in the regulation of wound healing. J Biol Chem 1992;267:21277–80. [PubMed] [Google Scholar]
- 52. Reichner JS, Meszaros AJ, Louis CA, Henry WL Jr, Mastrofrancesco B, Martin BA, Albina JE. Molecular and metabolic evidence for the restricted expression of inducible nitric oxide synthase in healing wounds. Am J Pathol 1999;154:1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kiyohara Y, Nishiguchi K, Komada F, Iwakawa S, Hirai M, Okumura K. Cytoprotective effects of epidermal growth factor (EGF) ointment containing nafamostat, a protease inhibitor, on tissue damage at burn sites in rats. Biol Pharm Bull 1993;16:1146–9. [DOI] [PubMed] [Google Scholar]
- 54. Nishiguchi K, Kiyohara Y, Komada F, Iwakawa S, Okumura K. Effect of epidermal growth factor on Cu, Zn‐superoxide dismutase expression in cultured fibroblasts from rat skin. Pharm Res 1994;11:1244–9. [DOI] [PubMed] [Google Scholar]


