Abstract
Annona squamosa L. (Annonaceae), commonly known as custard apple, mainly used for its edible fruit, is also recognised with numerous medicinal properties. As there is no report on the efficacy of this plant for wound healing, we examined the efficacy of ethanolic extract of A. squamosa leaves on wound repair in streptozotocin–nicotinamide‐induced diabetic rats. Open excision wounds were made on the back of rats. The drug at a dosage of 100 mg/kg body wt was reconstituted in 200 µl of phosphate buffered saline and applied topically once daily for the treated wounds. The control wounds were left untreated. Wound tissues formed on days 4, 8, 12 and 16 (post‐wound) were used to estimate DNA, total protein, total collagen, hexosamine and uronic acid. Levels of lipid peroxides were also evaluated along with tensile strength and period of epithelialisation. A. squamosa L. increased cellular proliferation and collagen synthesis at the wound site as evidenced by increase in DNA, protein and total collagen. The treated wounds were observed to heal much faster as proved by enhanced rates of epithelialisation and wound contraction, which was also confirmed by histopathological examinations. The results strongly substantiate the beneficial effects of the topical application of A. squamosa L. in the acceleration of normal and diabetic wound healing.
Keywords: Annona squamosa, Collagen, Streptozotocin, Tensile strength, Wound healing
INTRODUCTION
Wound healing is a process of well‐recognised orchestrated and predictable events, in which there are four distinct inter‐related phases: haemostasis, inflammation, proliferation and remodelling (1). Interplay between blood cells, endothelial cells, fibroblasts, keratinocytes and the local release of growth factors and cytokines influence the rate of wound repair. Any disruption in this interplay delays this process (2).
Diabetes is responsible for delayed or impaired wound healing, leading in many instances, to chronic ulcer formation. Peripheral neuropathy and peripheral vascular diseases are thought to be the underlying factors for the delayed wound healing in diabetic condition (3).
Although the mechanisms involved in delayed wound repair in diabetic patients are not completely understood, it is evident that all phases of the healing process are disrupted. In fact, delay in collagen synthesis, impairment in epithelialisation and reduced angiogenesis have been described during the proliferative phase of the healing process (4). Earlier reports suggested that many factors like decreased production of growth factors such as transforming growth factor‐β, insulin‐like growth factor‐1 and vascular endothelial growth factor, reduced collagen deposition, excessive protease activity, delayed inflammatory response and impaired nitric oxide synthesis contribute to the impaired wound healing observed in diabetes mellitus 5, 6. In addition, fibroblasts do not produce adequate amounts of extracellular matrix (ECM) and the keratinocytes do not reepithelialise the wound (7). As a result, the skin microvasculature becomes damaged, tissue ischaemia ensures and chronic diabetic wounds develop (8).
The process of wound healing is promoted by several natural products (9), plant products which are composed of active principles such as triterpenes, alkaloids, flavanoids (10) and biomolecules (11). These agents usually influence one or more phases of the healing process. We have already shown in our earlier reports the wound healing potential of some medicinal plants 12, 13, 14.
Annona squamosa L. (Annonaceae), commonly known as custard apple, is cultivated throughout India, mainly for its edible fruit. Traditionally, in India, the tribes and villagers of the Aligarh district and Chotanagpur regions broadly use the young leaves of A. squamosa along with the seeds of Piper nigrum for the treatment of diabetes (15). A. squamosa has also been traditionally used for the management of several diseases such as epilepsy, dysentery, cardiac problems, worm infestation, constipation, haemorrhage, antibacterial infection, dysuria, fever and ulcer (16). It also possesses antifertility, antitumour and abortifacient properties 17, 18.
From the leaves of A. squamosa, several flavonoids and a tetrahydroisoquinoline alkaloid with cardiotonic activity (19) have been isolated. Partially purified flavonoids of aqueous A. squamosa leaf extract possess antimicrobial and insecticidal activity (20) and antioxidant activity (21). Antidiabetic activity of cold aqueous extract in streptozotocin (STZ)–nicotinamide type 2 diabetic rats has already been reported 22, 23. The twigs of A. squamosa possess antiulcer activity (24).
However, the medicinal values of this plant pertaining to wound healing have not yet been reported. Hence, in this study, we examined the efficacy of topical application of the ethanolic extract of A. squamosa leaves by macroscopical, physical, biochemical and histological methods in the process of wound repair in diabetic rats.
MATERIALS AND METHODS
Plant collection and extraction
Young leaves of A. squamosa were shade dried and crushed to make fine powder. A 100 g of this powder was macerated with 70% ethanol in dark and filtered to harvest a viscous supernatant. The supernatant was then dried under vacuum below 40°C. The viscous residue was collected, weighed and kept at 4°C until use (yield 22·08 g).
Chemicals
l‐Hydroxyproline, d‐glucuronic acid, chloramine‐T, glucosamine, gallic acid, calf thymus DNA, 1,1,3,3‐tetraethoxypropane and bovine serum albumin were purchased from Sigma Chemical Company (St Louis, MO). P‐dimethyl aminobenzaldehyde, diphenylamine and Folin's Phenol reagent were from Loba Chemie (Mumbai, India). Methyl cellosolve was obtained from Merck (Darmstadt, Germany). All other reagents were of high analytical grade.
Determination of total phenolic content
The total phenolic content of the crude extract was estimated using Folin–ciocalteu reagent (FCR) based assay (25). To 200 µl of the aliquot taken from a stock solution (1 mg/ml) of the extract, 2·45 ml of water and 150 µl of FCR were added. The mixture was kept at room temperature for 5 minutes and then 300 µl of 1 N sodium carbonate solution was added. The mixture was kept at room temperature for 30 minutes and the colour developed was recorded at 765 nm. Total phenols (mg/g) in the crude extract were expressed as gallic acid equivalent (GAE), using a standard curve prepared from gallic acid (0·1 mg/ml) solution.
Experimental design and diabetes induction
Healthy male Wistar rats, weighing between 150 and 200 g were used for this study. The rats were housed in wire topped cages with sterilised rice husk bedding under controlled conditions of light/dark cycle (12:12 hours), temperature at 29–31°C and rats were fed with commercial rat feed and water ad libitum. All procedures were carried out according to the stipulations of the Institutional Animal Care and Use Committee. A formal approval from the Institutional Animal Ethical Committee has also been obtained.
Diabetes was induced by a single intraperitoneal injection of STZ (50 mg/kg body wt) dissolved in 0·1 M of cold citrate buffer (pH 4·5) 15 minutes after the intraperitoneal administration of nicotinamide (110 mg/kg body wt) in overnight fasted rats (26). Induction of diabetes was confirmed by tail vein blood glucose estimation using glucometer (One Touch Horizon, Johnson & Johnson, Mumbai, India) after 72 hours. After 2 weeks, rats with blood glucose level >250 mg/dl were deemed as diabetic and used for the experiment.
The rats were divided into four groups, each group containing six rats:
-
1
group I: control rats, left untreated;
-
2
group II: rats treated with A. squamosa (200 µl at a concentration of 100 mg/kg body wt);
-
3
group III: diabetic control rats also left untreated; and
-
4
group IV: diabetic rats treated with A. squamosa (200 µl at a concentration of 100 mg/kg body wt).
Wound creation and drug administration
After shaving the back of the rats, a 2 cm2 full thickness open excision wound was made by removing a patch of skin under light ether anaesthesia as reported in our earlier studies (12). The control rats were left untreated and the treated rats received 200 µl (100 mg/kg body wt) of the extract applied topically once daily, until the wounds healed completely. Wound tissues formed were removed on days 4, 8, 12 and 16 post‐wounding and used for different biochemical analyses. The same dosage was followed for the diabetic treated rats also.
Biochemical analyses
Nucleic acids were first extracted by the method of Porat et al. (27). A 100 mg of the wound tissue was homogenised in 5 ml of ice‐cold distilled water. 5 ml of 10% trichloroacetic acid (TCA) was added and the samples were kept in an ice bath for 30 minutes to precipitate the proteins and nucleic acids. The contents were centrifuged and the pellets were first washed with 1 ml of 10% of TCA and then with 3 ml of absolute alcohol. The lipid free sediment was resuspended in 5 ml of 5% TCA and kept at 90°C for 15 minutes to separate the nucleic acids. An aliquot from this was taken and used to estimate DNA (28), total protein (29). The total collagen content in granulation tissues was estimated based on the hydroxy proline index (30). Hexosamine was estimated by the method of Elson and Morgan (31). Schiller method was used to estimate uronic acid (32). Lipid peroxides level in wound tissues was also measured (33).
Biophysical analyses
Reduction in wound size was calculated as a percentage of the original size which was determined by tracing the wound area on to a transparent graph sheet and measuring the surface area planimetrically.
A reduction in epithelialisation period is a good sign as it denotes the number of days taken for complete closure of the wounds.
To measure the tensile strength, a 6 cm linear incision was made on the back of the rats. Intermittent sutures were placed 1 cm apart with black cotton thread. Wounds were treated as described above. The sutures were removed on day 7 and the tensile strength of the wound was determined on day 10.
From each wounded skin, 6–8 dumb bell‐shaped specimens of 4 mm width and 12 mm length were punched out parallel to the back bone. The thickness of the skin samples was measured using screw gauge. The broadened ends were gripped by the jaws of the Instron Tensile Tester (Model 4301, Instron Corporation, Canton, MA) and the stress– strain curves were recorded at an extension rate of 0·5 cm/minutes. The specimens were kept cold by spraying ice‐cold physiological saline during the experiment. The skins were strained to rupture and the ultimate load (kg) and the ultimate strain (percent extension) were calculated. Tensile strength (kg/cm2) was obtained by dividing the ultimate load (kg) by the area of original cross section (thickness × width). The average of 4–6 readings per rat (each group containing 6 rats) were pooled and mean and standard deviation (SD) were calculated (34).
Histopathology
The rats were sacrificed and the wound tissues were removed. These samples were then separately fixed in 10% formalin–saline, dehydrated through graded alcohol series, cleared in xylene and embedded in paraffin wax (melting point 56°C). Serial sections of 5 µm were cut and stained with haematoxylin and eosin and Van Gieson's. The sections were examined under light microscope and photomicrographs were taken.
Statistical analysis
Data were expressed as mean ± SD of six animals in each group and the results were statistically evaluated using Student's paired and one‐way analysis of variance. All statistical analyses were performed using graph pad prism version 5·0. Values corresponding to P < 0·05 were considered as significant.
RESULTS
Total polyphenolic content
Total polyphenolic content in the crude extract was found to be 264 mg/g in terms of GAE.
Effect of A. squamosa on biochemical analyses
Table 1 depicts the DNA, total protein and collagen content of control and treated wounds. In group II, A. squamosa treatment significantly increased the DNA content from day 4 (91%) to day 12 (41%) when compared with group I. A similar trend in the group IV treated (diabetic) was observed, with a significant (P < 0·05) increase on days 4, 8 and 12.
Table 1.
Effect of Annona squamosa on DNA, protein and total collagen content of wound tissues †
| Day 4 | Day 8 | Day 12 | Day 16 | |
|---|---|---|---|---|
| DNA (mg/100 mg wet weight) | ||||
| Control | 1·36 ± 0·13 | 5·39 ± 0·31 | 3·90 ± 0·25 | 1·48 ± 0·31 |
| Treated | 2·60 ± 0·28 * | 7·23 ± 0·53 * | 5·50 ± 0·28 * | 1·61 ± 0·25 |
| Diabetic control | 1·56 ± 0·27 | 2·47 ± 0·28 | 1·38 ± 0·28 | 0·84 ± 0·05 |
| Diabetic treated | 2·14 ± 0·30 * | 4·48 ± 0·26 * | 1·87 ± 0·21 * | 0·8 ± 0·08 |
| Protein (mg/100 mg wet weight) | ||||
| Control | 3·57 ± 0·19 | 5·15 ± 0·65 | 4·79 ± 0·77 | 2·28 ± 0·44 |
| Treated | 5·66 ± 0·17 * | 9·11 ± 0·53 * | 5·38 ± 0·32 | 2·20 ± 0·66 |
| Diabetic control | 2·83 ± 0·21 | 4·62 ± 0·44 | 2·10 ± 0·40 | 1·46 ± 0·24 |
| Diabetic treated | 4·81 ± 0·42 * | 6·45 ± 0·34 * | 2·29 ± 0·28 | 1·32 ± 0·25 |
| Collagen (mg/100 mg dry wt) | ||||
| Control | 1·54 ± 0·26 | 3·59 ± 0·41 | 2·34 ± 0·39 | 1·39 ± 0·32 |
| Treated | 2·59 ± 0·26 * | 5·55 ± 0·39 * | 4·35 ± 0·30 * | 2·11 ± 0·20 * |
| Diabetic control | 1·09 ± 0·23 | 1·78 ± 0·30 | 1·01 ± 0·29 | 0·91 ± 0·30 |
| Diabetic treated | 1·50 ± 0·18 * | 2·82 ± 0·44 * | 1·29 ± 0·31 | 1·36 ± 0·38 * |
†Values (as mg/100 mg) are expressed as mean ± standard deviation (n = 6 animals).
* P < 0·05 (significant, when compared with corresponding control).
In treated groups, a marked increase in the collagen content (68–85%) in group II animals and 27–45% in group IV animals was observed, when compared with their respective controls. A concomitant increase in protein content was also observed in treated groups (P < 0·05).
Hexosamine and uronic acid, the ground substrate on which collagen is laid down, gradually increased till day 8 in both the treatment group, when compared with their respective controls (Table 2).
Table 2.
Effect of Annona squamosa on hexosamine and uronic acid content of wound tissues †
| Day 4 | Day 8 | Day 12 | Day 16 | |
|---|---|---|---|---|
| Hexosamine (µg/100 mg dry wt) | ||||
| Control | 453·5 ± 82·0 | 731·5 ± 41·8 | 374·3 ± 51·6 | 105·9 ± 22·2 |
| Treated | 907·2 ± 30·1 * | 1223·0 ± 168·7 * | 548·9 ± 40·8 * | 129·7 ± 26·5 * |
| Diabetic control | 271·0 ± 45·7 | 576·8 ± 70·9 | 148·2 ± 23·9 | 26·4 ± 5·4 |
| Diabetic treated | 457·4 ± 43·9 * | 666·6 ± 56·6 * | 159·0 ± 27·1 | 28·1 ± 5·0 |
| Uronic acid (µg/100 mg dry wt) | ||||
| Control | 77·3 ± 15·0 | 113·2 ± 24·2 | 71·2 ± 12·6 | 54·0 ± 9·0 |
| Treated | 132·1 ± 22·5 * | 171·5 ± 18·3 * | 92·6 ± 9·1 * | 61·3 ± 6·6 |
| Diabetic control | 59·5 ± 11·8 | 104·5 ± 13·5 | 37·7 ± 9·9 | 22·6 ± 3·3 |
| Diabetic treated | 78·7 ± 11·3 * | 133·9 ± 20·0 * | 38·7 ± 7·3 | 17·7 ± 2·7 |
†Values (as mg/100 mg) are expressed as mean ± standard deviation (n = 6 animals).
* P < 0·05 (significant differences from corresponding treated and untreated groups).
Rate of wound contraction
Figure 1A shows the photographs of the periodical observation of the wounds on different days. Wounds which received A. squamosa contracted completely on day 17 in group II rats as against the control wounds which showed only 85% of contraction (Figure 1B). Similar results obtained in group III and IV animals also (Figure 2A and B). Significant reduction in wound size on all the days in the treatment groups substantiates the efficacy of A. squamosa on wound healing.
Figure 1.
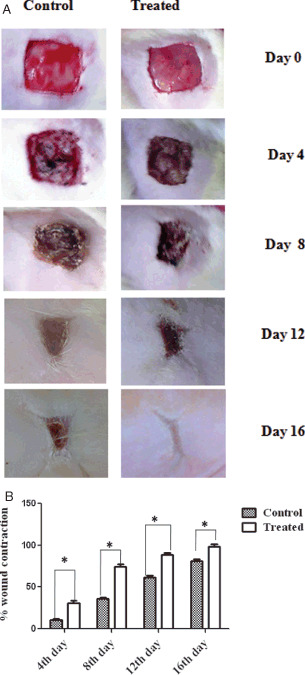
(A) Photographical representation of contraction rate on different days of control and treated animals. (B) Graphical representation of percentage wound contraction on various days of control and treated animals (groups I and II). Values are expressed as mean ± standard deviation for six animals * P < 0·05, as significant when compared with the control. Scale bar 1 cm.
Figure 2.
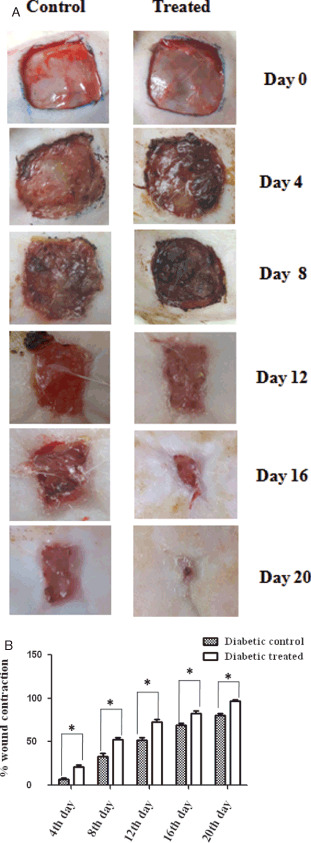
(A) Photographical images of contraction rate on different days of control and treated wounds of diabetic animals. (B) Graphical representation of percentage wound contraction on various days of both diabetic control and diabetic treated wounds. Values are expressed as mean ± standard deviation for six animals * P < 0·05, as significant compared with the control. Scale bar 1 cm.
As shown in Figure 3A, level of lipid peroxidation was significantly decreased by 70% in animals of groups II and IV when compared with the control groups.
Figure 3.
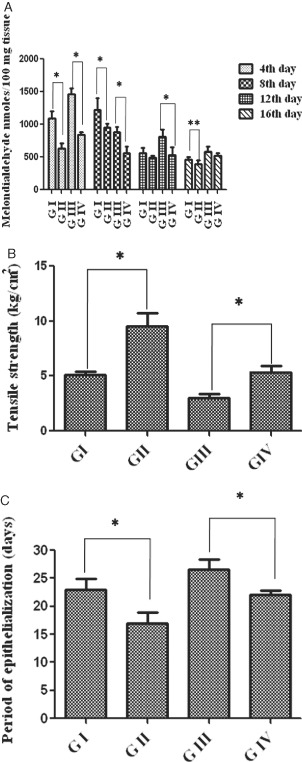
(A) Lipid peroxides of wound tissues on various days. Values are expressed as mean ± standard deviation (SD) for six animals and level of significance is expressed as * P < 0·05, respectively, compared with the corresponding control. (B) Tensile strength of incision wounds from control and treated rats. Values are expressed as mean ± SD for six animals, * P < 0·01. (C) Period of epithelialisation (measured as the number of days required for complete healing) in control and treated rats. Values are expressed as mean ± SD for six animals, * P < 0·01.
Effect of A. squamosa on biophysical analyses
The biophysical changes studied are (1) reduction in wound size, (2) tensile strength and (3) epithelialisation days of the wounds for complete healing. Figure 3B compares the tensile strength of control and treated incision wounds (day 8). A significant increase (89%) in tensile strength was observed in group II and 77% in group IV rats when compared with the corresponding control groups.
The macroscopic analysis of the wounds showed that the A. squamosa treated groups required a total period of 17 (non diabetic) and 22 days (diabetic), respectively, for complete healing, whereas the control groups took 23 (non diabetic) and 28 days (diabetic) (Figure 3C), respectively.
Microscopic analyses of wound tissues
Figure 4 shows the histological specimen of the weeks 1 and 2 of groups I and II. Untreated wound shows less number of cells and collagen deposition with minimal cellular infiltration (Figure 4A), whereas in treated wounds, fibroblasts and macrophages with moderate collagen deposition was seen. Hyalinished collagen, stroma and proliferating capillaries were also found in week 1 of group II animals (Figure 4B).
Figure 4.
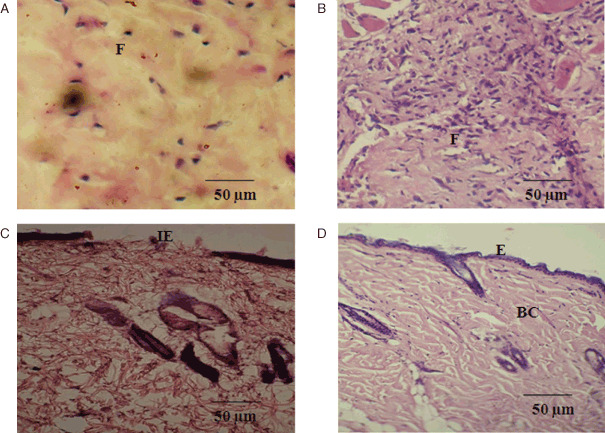
Photomicrographs of histological study of control and Annona squamosa treated wound tissues on weeks 1 and 2 of post‐wounding, respectively (stained with haematoxylin–eosin and Van Gieson's; magnification 20×). During week 1, control (A) showing loosely packed collagen with irregular epithelialisation and less fibroblasts treated tissue (B) showing new blood vessel formation and high fibroblasts with dense collagen deposition. On week 2, control (C) shows thin epithelial layer with less collagen and treated (D) shows complete epithelialisation with regularly arranged dense collagen. Scale bar 50 µm. BC, blood capillaries; E, epithelialisation; F, fibroblast; IE, incomplete epithelialisation.
In the week 2, loosely packed collagen fibres with irregular pattern and undifferentiated keratinocytes under the basal layer of lamina were observed. Incomplete epithelialisation with lesser fibrous tissue at the wound site was also noted in untreated tissues (Figure 4C). Histology of treated wound for week 2 showed keratinocytes, which were clearly differentiated from epidermal layer and accumulated in the basal lamina of epidermis. Followed by this, collagen fibres were densely packed and parallelly arranged. More accumulation of collagen fibres in the ECM region had also been noticed with prominent thick bundles of collagen fibres embedded with proliferating fibroblasts (Figure 4D).
Histological evaluation was also carried out for weeks 1–3 of wound tissues for group III and IV animals. Figure 5A shows lesser number of fibroblasts and blood vessels than the treated one in week 1 (Figure 5B). In the week 2, very few proliferating capillaries macrophages were observed with mild collagen formation in untreated wound (Figure 5C). However, large number of proliferating capillaries, complete fibrous tissues and increased macrophages were located with dense collagen in treated tissues (Figure 5D). Incomplete epithelialisation with less collagen fibres were observed at the wound site in untreated group in week 3 (Figure 5E). However, complete epithelialisation, increased and thick collagen bundle deposition were observed in the treated group (Figure 5F).
Figure 5.
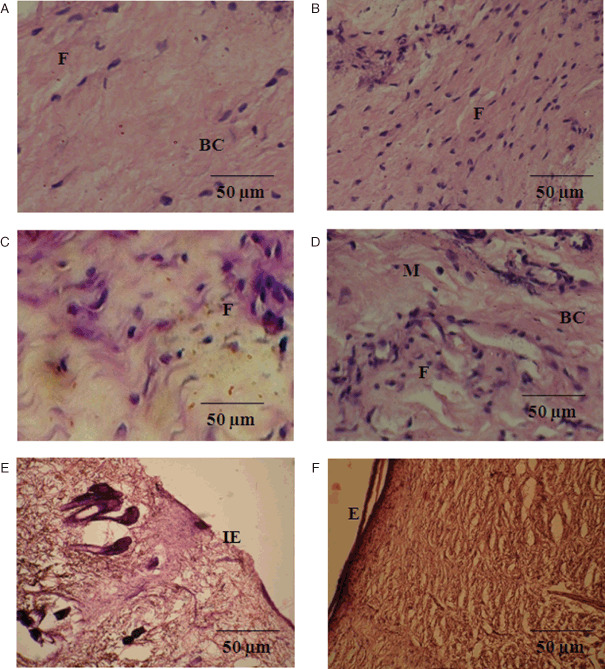
Photomicrographs of histological study of diabetic control and Annona squamosa treated wound tissues on weeks 1–3 of post‐wounding, respectively (stained with haematoxylin–eosin and Van Gieson's; magnification 20×). First week control (A) shows less fibroblasts and blood capillaries at wound site, whereas treated tissue (B) showing angiogenesis and fibroblasts. Second week control (C) shows minimal cellular infiltration and less macrophages but treated (D) shows slightly higher capillaries, collagen fibre with macrophages. Third week control (E) explores incomplete epithelialisation with thin collagen fibre at the wound site. Treated tissue (F) shows complete epithelialisation, high fibrous tissues with uniform collagen deposition at the wound site. Scale bar 50 µm. BC, blood capillaries; E, epithelialisation; F, fibroblast; IE, incomplete epithelialisation; M, monocytes.
DISCUSSION
Diabetes mellitus is known to be associated with a variety of alterations in connective tissue metabolism, as a result of which diabetic patients face a huge problem of poor and delayed wound healing. Loss of collagen determined in diabetes may be due to decreased levels of synthesis and enhanced catabolism of newly synthesised collagen (35).
Diabetes mellitus induces poor wound healing of cutaneous lesions by a mechanism which is still unclear. Therefore, diabetic animal models have been used for the better understanding of the healing process of these lesions. In acute wound healing, inflammatory response should occur rapidly to permit the development of subsequent phases of wound healing. Reepithelialisation is a process of restoring the epidermis and involves proliferation and migration of keratinocytes. Cell proliferation is an essential event during reepithelialisation, so proliferating keratinocytes ensure an adequate supply of cells to migrate and cover the wound. Synthesis of ECM is also a key feature of wound healing. Dermal reconstruction is characterised by the formation of granulation tissue, which includes cell proliferation, ECM deposition, wound contraction and angiogenesis (36).
Earlier reports promised that plant products are potential agents for wound healing and largely preferred because of their widespread availability, non toxicity, effectiveness and absence of unwanted side effects as crude preparations (37).
Treatment of wounds with A. squamosa was found to produce a marked increase in DNA content of the wound tissues. The increase in DNA represents hyperplasia of the cells. This growth was accompanied by a concomitant increase in hexosamine, uronic acid and collagen indicating active synthesis and deposition of matrix proteins. Collagen forms an important component of the ECM and the healing process depends, to a great extent, on the regulated biosynthesis and deposition of new collagen and their subsequent maturation (38). Assessment of collagen content in control and extract treated wound tissues clearly suggests that A. squamosa enhanced the production of new collagen.
Hexosamine and uronic acid are matrix molecules which act as ground substratum on which collagen is laid down. It is reported that there is an increase in the levels of these compounds during the early stage of wound healing following which normal levels are restored (39). Similar trends were observed in A. squamosa treated wounds. Increased DNA and protein content of granulation tissues indicate the level of cellular proliferation and protein synthesis. Increased cellular proliferation may be due to the mitogenic activity of the plant extract, which might have significantly contributed to healing process. Early dermal and epidermal regeneration in treated rats also confirmed that the extract had a positive effect towards cellular proliferation, granular tissue formation and epithelialisation. In addition, it has been reported that the definite increase in collagen and hexosamine showed a positive correlation with that of DNA in cultures of swine aortic media (40). Higher protein and collagen contents of treated wounds suggest that A. squamosa stimulates the proliferation of cells which actively synthesise the ECM.
The collagen synthesised is laid down at the wound site and become cross linked to form fibres. Wound strength is acquired from both remodelling of collagen and the formation of stable intra and intermolecular cross links (41).
Furthermore, A. squamosa administration significantly enhanced the healing of both excision and incision wounds. Faster wound contraction in excised wounds and greater tensile strength in incised wounds could infer that A. squamosa not only increases collagen content but also aids in cross linking of this protein. Hydroxyproline, the main constituent of collagen serves as a marker of collagen biosynthesis at the wound site. Collagen not only deliberates strength and integrity to the tissue matrix but also plays a vital role in homeostasis and in epithelialisation at the later phase of healing. Improved ground substratum contents such as hexosamine and uronic acid reproduces the stabilisation of collagen molecules by accelerating electrostatic and ionic interactions (42).
The free radicals and oxidative reaction products produce tissue damage and play a major role in the aggravation of tissue damage during wound healing (43). Decreased levels of lipid peroxides suggest that A. squamosa possesses significant antioxidant activity, which facilitated the inhibition of oxidative damage and enhance the healing process. Presence of large amount of bioactive principles such as alkaloids, flavonoids in A. squamosa and its well‐known antioxidant property decreased the period of epithelialisation and helped the wounds to heal significantly faster (44). Earlier reports showed that the wound healing activity of Clitoria ternatea could be attributed to the presence of flavonoids and phenolic compounds (45).
The high amount of total phenolic content of A. squamosa could also be a reason for increased wound healing activity because they play an important role in wound healing because of their free radical scavenging activity (46).
Histological evaluations further support these results. A greater degree of epithelialisation and fibroblastic deposition observed in A. squamosa treated wounds implies prohealing efficacy of the plant extract. Capillary vessels were distributed among most of the granulation tissue, but increased at the centre of the wound, especially during week 2. At the week 3, the percentage of collagen increased gradually. These changes indicate a shift in wound healing from the proliferative phase to the maturation phase. In an excision wound, all three phases coexist together. A. squamosa also accelerates scar formation.
Topically administered drugs are effective in faster wound contraction because of the larger availability at the wound site (47). From this study, we confirm that the rate of wound contraction in treated rats was significantly higher and period of epithelialisation was shorter in both normal and diabetic treated rats. These results further support the efficacy of A. squamosa on wound repair.
In conclusion, topical administration of ethanolic extract of A. squamosa promotes various stages of wound healing such as fibroplasia, collagen synthesis, wound contraction and epithelialisation.
ACKNOWLEDGEMENTS
T. Ponrasu acknowledges Council of Scientific and Industrial Research (CSIR), New Delhi, for the award of Senior Research Fellowship. We wish to thank Dr. T. Narasimhaswamy, CLRI, for his help in microscopical analysis.
REFERENCES
- 1. Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Sur 1998;176:26S–38S. [DOI] [PubMed] [Google Scholar]
- 2. Shukla A, Dubey MP, Srivastava R, Srivastava BS. Differential expression of proteins during healing of cutaneous wounds in experimental normal and chronic models. Biochem Biophys Res Commun 1998;244:434–9. [DOI] [PubMed] [Google Scholar]
- 3. Mustoe T. Understanding chronic wounds: a unifying hypothesis on their pathogenesis and implications for therapy. Am J Surg 2004;187:65S–70S. [DOI] [PubMed] [Google Scholar]
- 4. Snyder RJ. Treatment of nonhealing ulcers with allografts. Clin Dermatol 2005;23:388–95. [DOI] [PubMed] [Google Scholar]
- 5. Perez Gutierrez RM, Vargas SR. Evaluation of the wound healing properties of Acalypha langiana in diabetic rats. Fitoterapia 2006;77:286–9. [DOI] [PubMed] [Google Scholar]
- 6. Teoh SL, Latiff AA, Das S. The effect of topical extract of Momordica charantia (bitter gourd) on wound healing in nondiabetic rats and in rats with diabetes induced by streptozotocin. Clin Exp Dermatol 2009;34:815–22. [DOI] [PubMed] [Google Scholar]
- 7. Mansbridge JN, Liu K, Pinney RE, Patch R, Ratcliffe A, Naughton GK. Growth factors secreted by fibroblasts: role in healing diabetic foot ulcers. Diabetes, Obes Metabol 1999;1:265–79. [DOI] [PubMed] [Google Scholar]
- 8. Sheetz MJ, King GL. Molecular understanding of hyperglycemia's adverse effects for diabetic complications. JAMA 2002;288:2579–88. [DOI] [PubMed] [Google Scholar]
- 9. Song JJ, Salcido R. Use of honey in wound care: an update. Adv Skin Wound Care 2011;24:40–44. [DOI] [PubMed] [Google Scholar]
- 10. Sharma SP, Aithal KS, Srinivasan KK, Udupa A, Kumar V, Kulkarni DR. Antiinflammatory and wound healing activities of the crude alcoholic extracts and flavanoids of vitex leucoxylon. Fitoterapia 1990;61:263–5. [Google Scholar]
- 11. Manikandan P, Sumitra M, Gayathri VS, Suguna L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem 2006;290:87–96. [DOI] [PubMed] [Google Scholar]
- 12. Suguna L, Singh S, Sivakumar P, Sampath P, Chandrasekaran G. Influence of Terminalia chebula on dermal wound healing in rats. Phytother Res 2002;16:227–31. [DOI] [PubMed] [Google Scholar]
- 13. Sumitra M, Manikandan P, Suguna L. Efficacy of Butea monosperma on dermal wound healing in rats. Int J Biochem Cell Biol 2005;37:566–73. [DOI] [PubMed] [Google Scholar]
- 14. Sumitra M, Manikandan P, Gayathri VS, Mahendran P, Suguna L. Emblica officinalis exerts wound healing action through up‐regulation of collagen and extracellular signal‐regulated kinases (ERK1/2). Wound Repair Regen 2009;17:99–7. [DOI] [PubMed] [Google Scholar]
- 15. Atique A, Iqbal M, Ghouse AKM. Use of Annona squamosa and Piper nigrum against diabetes. Fitoterapia 1985;56:190–2. [Google Scholar]
- 16. Topno KK. Plants used by tribals of Chotanagpur against diabetes. Botanica 1997;47:99–1. [Google Scholar]
- 17. Asolkar LV, Kakkar KK, Chakre OJ. Glossary of Indian medicinal plants with active principles. New Delhi: Publication and Information Directorate, 1992:72–3. [Google Scholar]
- 18. Yoganarasimhan SN. Medical plants of India–Tamil Nadu (vol. II). Bangalore: International Book Publisher, Print Cyper Media, 2000:48–62. [Google Scholar]
- 19. Wagner H, Reiter M, Ferstl W. New drugs with cardiotonic activity I. Chemistry and pharmacology of the cardiotonic active principle of Annona squasmosa. Planta Medica 1980;40:77–85. [DOI] [PubMed] [Google Scholar]
- 20. Kotkar HM, Mendki PS, Sadan SV, Jha SR, Upasani SM, Maheswari VL. Antimicrobial and pesticidal activity of partially purifed flavonoids of Annona squamosa. Pest Manag Sci 2002;58:33–7. [DOI] [PubMed] [Google Scholar]
- 21. Kaleem M, Asif M, Ahmed QU, Bano B. Antidiabetic and antioxidant activity of Annona squamosa extract in streptozotocin‐induced diabetic rats. Singapore Med J 2006;47:670–5. [PubMed] [Google Scholar]
- 22. Shirwaikar A, Rajendran K, Kumar CD, Bodla R. Antidiabetic activity of aqueous leaf extract of Annona squamosa in streptozotocin nicotinamide type 2 diabetic rats. J Ethnopharmacol 2004;91:171–5. [DOI] [PubMed] [Google Scholar]
- 23. Gupta RK, Kesari AN, Murthy PS, Chandra R, Tandon V, Watel G. Hypoglycemic and antidiabetic effect of ethanolic extract of leaves of Annona squamosa L. in experimental animals. J Ethnopharmacol 2005;99:75–81. [DOI] [PubMed] [Google Scholar]
- 24. Yadav DK, Singh N, Dev K, Sharma R, Sahai M, Patil G, Maurya R. Anti‐ulcer constituents of Annona squamosa twigs. Fitoterapia 2011;82:666–75. [DOI] [PubMed] [Google Scholar]
- 25. Swain T, Hills WE. Phenolic constituents of pronus domestica. Quantitative analysis of Phenolic constituents. J Sci Food Agric 1959;10:63–8. [Google Scholar]
- 26. Masiello P, Broca C, Gross R, Roye M, Manteghetti M, Hillaire‐Buys D, Novelli M, Ribes G. Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes 1998;47:224–9. [DOI] [PubMed] [Google Scholar]
- 27. Porat S, Rousso M, Shosan S. Improvement of the gliding function of flexor tendons by topically applied enriched collagen solution. J Bone Joint Surg Br 1980;62B:208–13. [DOI] [PubMed] [Google Scholar]
- 28. Burton K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J 1956;62:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenaol reagent. J Biol Chem 1951;193:265–75. [PubMed] [Google Scholar]
- 30. Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 1961;93:440–7. [DOI] [PubMed] [Google Scholar]
- 31. Elson LA, Morgan WTJ. A colorimetric method for the determination of glucosamine and chondrosamine. Biochem J 1933;27:1824–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schiller S, Slover GA, Dorfman A. A method for the separation of acid mucopolysaacharides: its application to the isolation of heparin from the skin of rats. J Biol Chem 1961;236:983–7. [PubMed] [Google Scholar]
- 33. Santos MT, Valles J, Aznar J, Vilches J. Determination of plasma malondialdehyde‐like material and its clinical application in stroke patients. J Clin Pathol 1980;33:973–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vogel HG. Studies antagonistic effect of aminoacetonitrile and prednisolone on mechanical properties of rat skin. Biochim Biophys Acta 1971;252:580–5. [DOI] [PubMed] [Google Scholar]
- 35. Lien YH, Tseng MM, Stern R. Glucose and glucose analogs modulate collagen metabolism. Exp Mol Pathol 1992;57:215–21. [DOI] [PubMed] [Google Scholar]
- 36. Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol 2007;25:9–18. [DOI] [PubMed] [Google Scholar]
- 37. Sandhya S, Sai Kumar P, Vinod KR, Banji D, Kumar K. Plants as potent anti diabetic and wound healing agents – a review. Hygeia JD Med 2011;3:11–9. [Google Scholar]
- 38. Dunphy JE, Udupa KN, Edwards LC. Wound healing: a new perspective with particular reference to ascorbic acid deficiency. Ann Surg 1956;144:304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dunphy JE, Udupa KN. Chemical and histochemical sequences in the normal healing of wounds. New Eng J Med 1955;253:847–51. [DOI] [PubMed] [Google Scholar]
- 40. Daoud AS, Fritz KE, Jarmolych J, Augustyn J, Nawhinney TP. Production of glycosaminoglycans, collagen, and elastic tissue by aortic medial plants. Adv Exp Med Biol 1977;82:928–33. [DOI] [PubMed] [Google Scholar]
- 41. Chithra P, Sajithlal GB, Chandrakasan G. Influence of Aloe vera on the glycosaminoglycans in the matrix of healing dermal wounds in rats. J Ethnopharmacol 1998;59:179–86. [DOI] [PubMed] [Google Scholar]
- 42. Siegel RC. Collagen cross‐linking. Synthesis of collagen cross‐links in vitro with highly purified lysyl oxidase. J Biol Chem 1976;251:5786–92. [PubMed] [Google Scholar]
- 43. Sonel O, Cetinkale O, Ozbay G, Ahcloglu F, Bulan R. Oxygen free radicals impair wound healing in ischemic rat skin. Ann Plast Surg 1997;39:516–23. [DOI] [PubMed] [Google Scholar]
- 44. Solanki YB, Jain SM. Wound healing activity of Clitoria ternatea L. in experimental animal models. Pharmacologia 2012;3:160–8. [Google Scholar]
- 45. Baskar R, Rajeswari V, Kumar TS. In vitro antioxidant studies in leaves of Annona species. Ind J Exp Biol 2007;45:480–5. [PubMed] [Google Scholar]
- 46. Muthusamy SK, Kirubanandan S, Sripriya R, Sehgal PK. Triphala promotes healing of infected full‐thickness dermal wound. J Surg Res 2008;144:94–101. [DOI] [PubMed] [Google Scholar]
- 47. Akkol EK, Acıkara OB, Suntar I, Citoglu GS, Keles H, Ergene B. Enhancement of wound healing by topical application of Scorzonera species: determination of the constituents by HPLC with new validated reverse phase method. J Ethnopharmacol 2011;137:1018–27. [DOI] [PubMed] [Google Scholar]


