Abstract
This case study illustrates the option of treating poorly healing diabetic wounds with shock waves. A case study was performed with a 75‐year‐old male patient with diabetic gangrene of both feet facing the prospect of imminent amputation. On a visual analogue pain scale (0–10), the patient reported a pain score of between 7 and 9. In the past, focused shock waves have been used to successfully treat poorly healing wounds and in this case are adopted for the treatment of severe peripheral arterial occlusive disease. Over a time interval of nearly a year, 11 treatments were delivered. At the end of the treatment the necrotic areas vanished. By then the pain score decreased to 2 and no further pain medication was needed.
Keywords: Diabetic wound, Gangrene, Shock wave, Wound healing
INTRODUCTION
Focused shock waves have been used to treat common orthopaedic indications (1) such as tendinosis calcarea, humeral, radial and ulnar epicondylitis, heel spurs, pseudoarthrosis (2) and trigger points (3) with great success for around 15 years. Extracorporeal shock wave therapy (ESWT) was originally developed for use in urology, where it has been used for the non invasive fragmentation of kidney stones for around 30 years (extracorporealshock wave lithotripsy). These techniques found wider application with the introduction of radial pressure waves approximately 10 years ago which achieved comparably positive results in many indications.
Recently, extracorporeal (usually planar) shock waves have also been started to be used in dermatology. Poorly healing wounds, such as leg ulcers, burns or diabetic leg ulcers, are today being treated with remarkable success 4, 5.
A fundamental distinction is made between focused shock waves and radial pressure waves (6). Shock waves are characterised by a very high pressure amplitude (up to 1000 bar/100 MPa), very short pulse length in the range of 300 ns and extremely short pulse rise time of around 10 ns.
Treatment using focused shock waves is known as ESWT. Radial pressure waves are significantly slower (by a factor of 1000) and pulse amplitude is also usually no more than 10–100 bar (1–10 MPa). Despite this – probably as a result of the characteristic pulsatile, asymmetrical pressure profile – radial pressure waves exert a physiological effect similar to that of shock waves with many indications. Although they are not shock waves, this treatment is also known as radial shock wave therapy (RSWT). The term ‘extracorporeal pulse activation therapy’ appears to represent a more accurate and general description of this method and is now being used more and more frequently.
Although the biological mechanism of action is not yet fully understood, shock wave therapy is being used successfully to improve blood supply and metabolic processes. This ultimately stimulates biological regeneration processes which lead to long‐term healing.
The mechanisms of action which are known with certainty to give rise to the observed positive results before and during wound healing include the following effects:
-
•
Immediate increases in blood flow – particularly in the case of focused shock waves – which are caused not by a pulsed massage effect arising from vibration during pulse application, but through a release of nitrogen monoxide [endothelial nitric oxide synthesis (ENOS)] (7). ENOS stimulates a biochemical vasodilatation and is involved in producing other tissue factors through its role as a multifunctional messenger molecule.
-
•
An increase in cell wall permeability (8).
-
•
A resultant general increase in metabolism.
-
•
The release of additional tissue factors, of which the most significant, with regard to wound healing, is vessel endothelial growth factor, responsible for neovascularisation (9).
-
•
A further key effect is the proliferation and differentiation of stem cells which leads to the formation of new, healthy tissue with almost no scarring (10).
MATERIALS AND METHODS
A 75‐year‐old male patient with diabetic gangrene of both feet faced in April 2007 the prospect of imminent amputation. In addition to peripheral arterial occlusive disease (PAOD) and type II diabetes mellitus, the patient also suffered from arrhythmias, cardiac insufficiency as a result of coronary heart disease, hyperuricaemia, benign prostate hypertrophy, diabetic polyneuropathy and arterial hypertension. Previous long‐term medication history involves insulin (diabetes mellitus), oral anticoagulation (cardiac arrhythmia), cardiac insufficiency medication, antihypertensive medication, prostate hypertrophy medication and pain medication.
Before, a painful inflammatory process with pus formation appeared underneath the nail of the great right toe (June/July 2006). Personal physician recommended curd soap baths, the antiseptic betaisodona (salve) and finally the antibiotic clindamycin. As the condition of the great toe did not improve as desired, the personal physician advised treatment in the hospital. There, in addition to intensive wound treatment from 28 September 2006 to 6 October 2006, a prostavasin therapy was also given (total of 16 infusions), leading to measurable improvement of peripheral perfusion.
On 20 October 2006, a treatment by vascular surgeon was performed – careful removal of the nail of the right great toe; antibiotic intensive cephalexin was prescribed. Following this an Intensive wound treatment through regular visits to doctor's surgery, approximately once per week, was given. Around the end of 2006, an infection also developed in the left great toe which was, however, not as severe as on the right and which also later healed more quickly.
At the recommendation of the surgeon, additional measures for the improvement of peripheral perfusion were taken (November/ December 2006 percutaneous transluminal angioplasty (PTA) of the right and then of the left Arteria tibialis posterior). No visible improvement resulted until the beginning of treatment with shock waves.
The wound treatment performed daily at home are the following: spraying of the feet with Octenisept wound disinfectant, patting dry with sterile gauze compresses, application of lipid cream 10% urea, covering of wounds with Atrauman Ag (salve compress containing silver), covered with sterile compresses for cushioning purposes, securing of these layers with sterile gauze bandages and encased in a tube bandage.
The results of the patient's pretherapy vascular studies showed that colour‐coded duplex sonography shows a relatively well‐perfused Arteria femoralis superficialis in the initial part and an Arteria profunda femoris on both sides. The Arteria poplitea is then, however, detectable on both sides with only a weak monophasic flow signal. Some vascular interventions were performed in 2006 without success like prostavasin therapy and PTA of the right Arteria tibialis.
Arterial blockage‐pressure measurement of the arteries to the foot is as follows:
-
1
Arteria dorsalis pedis right 110 mmHg
-
2
Arteria dorsalis pedis left 80 mmHg
-
3
Arteria tibialis posterior right 95 mmHg
-
4
Arteria tibialis posterior left 105 mmHg
-
5
Systemic pressure 160 mmHg
Measurable PAOD, more advanced on the left than on the right side, and significant necrosis of the great toe were observed (on 20 April 2007). Using a visual analogue pain scale (0–10), the patient reported a pain score of between 7 and 9. Medication taken for pain consisted of Pregabalin 75 mg two to three times in combination with Tramal® long 150 mg, Tramal solution and Novaminsulfon solution.
Treatment with shock waves commenced on 20 April 2007 (Figure 1). A combination approach with focused shock waves and radial pressure waves was used. Treatment was provided using the DUOLITH® SD1, a combination shock and pressure wave device (Storz Medical AG, Tägerwilen, Switzerland). Treatment was carried out through the balls of the feet, without direct contact with the necrotic tissue, initially using a combination of radial pressure waves (RSWT) and focused shock waves (ESWT).
Figure 1.
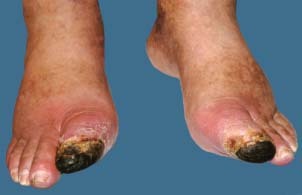
Photograph from 20 April 2007 – start of therapy.
The second treatment was administered 4 days later, on 24 April 2007. Treatment parameters are as follows: focused shock waves (ESWT) 1000 pulses, 0·07 mJ/mm2; radial pressure waves (RSWT) 1000 pulses, 2·6 bar.
One week later, the patient reported an inflammatory reaction above the necrotic areas. A prophylactic course of antibiotics was given and the treatment suspended by mutual consent. More pain medication was administered. One month later the patient reported that his pain had improved and that the necrotic areas of the feet had begun to heal.
By mutual consent, treatment was recommenced on 28 August. On this occasion, however, only focused shock waves were applied (0·03–0·10 mJ/mm2 with a frequency of 4 Hz and 1000–1500 impulses per foot). Treatments were carried out at intervals of 2–4 weeks or longer. A total of 11 treatments were delivered in the period up to 11 March 2008. The patient experienced – for the first time in many years – a pleasant sensation of warmth in the balls of his feet during shock wave application (Figure 2). He also reported a significant reduction in pain.
Figure 2.
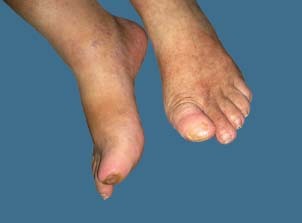
Photograph from 11 March 2008 – after 11 treatments.
No direct surgery was performed during the therapy period except light wound debridement and the excellent wound dressing at home.
RESULTS
The gangrene resolved spontaneously in conjunction with shock wave treatment. Wound recovery was achieved through autolysis of the necrosis during shock wave treatment. In accordance with the healing process with shock wave therapy, starting from the middle of 2008, rebandaging can be reduced to three to two times per week. Atrauman can be dispensed and Mepilex (silicone‐coated foam bandage) is used instead. Only light, conventional hygiene since the end of 2009 was necessary. The patient's pain medication was gradually reduced and then completely discontinued on 26 June 2008. On the visual analogue pain scale, the patient now reported a pain score of just 2. The shock wave treatment was then terminated. A follow‐up was carried out on 18 November 2009 (Figure 3) and a final follow‐up was carried out on 23 March 2010 (Figure 4). The patient required no pain medication in the intervening period. No post‐therapy vascular studies have been performed and the patient is doing very well as of today. Necroses have been completely resolved.
Figure 3.
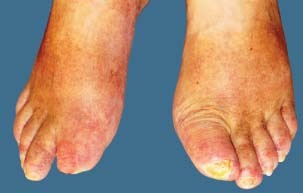
Photograph from 18 November 2009 – follow‐up visit.
Figure 4.
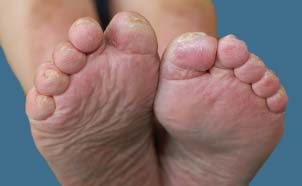
Photograph from 25 March 2010 – follow‐up visit.
DISCUSSION
This case study of a patient with diabetic foot gangrene illustrates the option of treating poorly healing wounds with shock waves. The next step – amputation – was planned and the shock wave treatment was initiated successfully as a final attempt to avoid this. Two to four weeks appear to represent the ideal treatment interval. In the authors' opinion, the reason for the overreaction following the second treatment was the short interval between treatments of just 4 days. In this case, an interval of 2–4 weeks between treatments proved to be optimal.
REFERENCES
- 1. Dreisilker U, Wess O, Novak P. Extrakorporeal erzeugte Stoss‐ und Druckwellen – eine wirksame Therapieform für die Geweberegeneration. Orthopädie‐Report Spezial 2007; 147–51.
- 2. Valchanou VD, Michailov P. High energy shock waves in the treatment of delayed and nonunion of fractures. Int Orthop 1991;15:181–4. [DOI] [PubMed] [Google Scholar]
- 3. Piontkowski U, Sommer S. Combined EPAT/focussed shock wave therapy and trigger points in sports medicine. ISMST Congress, Juan les Pins, 2008.
- 4. Schaden W, Thiele R, Kölpl C, Pusch M, Nissan A, Attinger CE, Maniscalco‐Theberge ME, Peoples GE, Elster EA, Stojadinovic A. Shock wave therapy for acute and chronic soft tissue wounds: a feasibility study. J Surg Res 2007;143:1–12. [DOI] [PubMed] [Google Scholar]
- 5. Moretti B, Notarnicola A, Maggio G, Patella S, Foscarini P, Patella V. ESWT‐induced healing of diabetic foot ulcers. ISMST Congress, Juan les Pins, 2008.
- 6. Wess O. Physik und Technik der Stoss‐ und Druckwellentherapie. Medizinisch-Orthopädische Technik 2005;5:7–32. [Google Scholar]
- 7. Mariotto SW, Cavalieri E, Amelio E, Ciampa AR, Carcereri de Prati A, Marlinghaus E, Russo S, Suzuki H. Extracorporeal shock waves: from lithotripsy to anti‐inflammatory action by NO production. Nitric Oxide 2005;12:89–96. [DOI] [PubMed] [Google Scholar]
- 8. Delius M, Ueberle F, Guo L. Anwendung von Stosswellen für den Transfer von Molekühlen in Zellen. Biomedizinische Technik 2002;47:382. [DOI] [PubMed] [Google Scholar]
- 9. Gutersohn A, Caspari G, Erbel R. Autoangiogenesis induced by cardiac shock wave therapy (CSWT) increases perfusion and exercise tolerance in endstage CAD patients with refractory angina. Circ J 2005;69 (Suppl I):379. [Google Scholar]
- 10. Delhasse Y, Neuland H, Steingen C, Schmidt A, Bloch W. Comparative study between the effects and mode of application if focused and radial shock wave treatment on the behaviour of human mesenchymal stem cells (MSC). ISMST Congress, Juan les Pins, 2008.


