Abstract
To compare the changes in microvascular blood flow in the small intestinal wall, wound contraction and fluid evacuation, using the established V.A.C. abdominal dressing (VAC dressing) and a new abdominal dressing, the ABThera open abdomen negative pressure therapy system (ABThera dressing), in negative pressure wound therapy (NPWT). Midline incisions were made in 12 pigs, which were subjected to treatment with NPWT using the VAC or ABThera dressing. The microvascular blood flow in the intestinal wall, were measured before and after the application at topical negative pressures of −50, −75 and −125 mmHg, using laser Doppler velocimetry. Wound contraction and fluid evacuation were also measured. Baseline blood flow was defined as 100% in all settings. The blood flow was significantly reduced, to 64·6 ± 6·7% (P < 0·05) after the application of −50 mmHg using the VAC dressing, and to 65·3 ± 9·6% (P < 0·05) after the application of −50 mmHg with the ABThera dressing. The blood flow was significantly reduced, to 39·6 ± 6·7% (P < 0·05) after the application of −125 mmHg using VAC, and to 40·5 ± 6·2% (P < 0·05) after the application of −125 mmHg with ABThera. No significant difference in the reduction in blood flow could be observed between the two groups. The ABThera system gave significantly better fluid evacuation from the wound compared to the VAC system. There was no difference between the dressings regarding the reduction in blood flow, but the ABThera dressing afforded better drainage of the abdomen and better wound contraction than the VAC dressing.
Keywords: ABThera, Fluid evacuation, Microvascular blood flow, NPWT, Open abdomen, VAC
Introduction
There is an increasing trend towards managing intra‐abdominal surgical emergencies by leaving the incision open and creating an open abdomen. For patients with abdominal compartment syndrome, wound dehiscence, trauma or intra‐abdominal sepsis, decompression laparotomy may be lifesaving 1, 2, 3.
Negative pressure wound therapy (NPWT), may be an option for patients in which an open abdomen has been created. The technique entails the application of negative pressure to a sealed, airtight wound. The suction force created by the vacuum pump enables the drainage of excess fluid and debris, which leads to the relief of wound oedema and reduction in bacterial count. The technique also allows the abdominal wall to move freely towards the midline without the problem of adhesion between the bowels and the abdominal wall. However, the method has occasionally been associated with an increased risk of the development of intestinal fistulae and enteroatmospheric fistulae 4, 5, 6, 7, 8. The reason for this is unknown. The decrease in intestinal wall blood flow induced by NPWT has been suggested to be a possible cause 9, 10. However, the patient group is often extremely complex, and it is therefore possible that multiple reasons lie behind the development of fistulae.
We have recently shown that the application of NPWT to an open abdomen induces a decrease in microvascular blood flow in the small intestinal wall lying close to the NPWT dressing using the V.A.C. abdominal dressing (VAC dressing) 9. A new abdominal dressing for NPWT has recently been introduced onto the market: the ABThera open abdomen negative pressure therapy system (ABThera dressing). We undertook this study to compare the effects of this new dressing on the microvascular blood flow in the small intestinal wall lying close to the NPWT dressing with the VAC or ABThera dressing. We studied the microvascular blood flow using fibreoptic laser Doppler probes. Changes in blood flow were measured in the small intestinal wall during exposure to pressures of −50, −75 and −125 mmHg. We also investigated the differences in wound contraction and fluid evacuation using the VAC and the ABThera dressings. To our knowledge, no such study has previously been conducted.
Materials and methods
Experimental animals
Twelve domestic pigs of both genders with a median weight of 60 kg were used. The animals were fasted overnight but given free access to water. The investigation complied with the “Guide for the Care and Use of Laboratory Animals” as recommended by the U.S. National Institutes of Health, and published by the National Academies Press (1996).
Anaesthesia
Before commencing surgery, sodium thiopental (5 mg/kg), atropine (0·02 mg/kg) and pancuronium (0·5 mg/kg) were given intravenously. Intubation was performed with a Portex endotracheal tube (7·5 mm internal diameter, Medcompare, South San Francisco, CA). A servo‐ventilator (Siemens Elema 300A, Stockholm, Sweden) was used for mechanical ventilation throughout the experiments. The ventilator settings used were: minute volume = ml/kg, FiO2 = 0·5, breathing frequency = 16 breaths/minute and positive end‐expiratory pressure = 5 cmH2O. Anaesthesia and muscular paralysis were maintained by continuous intravenous infusion of 8–10 mg/kg/hour propofol (Diprivan, AstraZeneca, Sweden), 0·15 mg/kg/hour fentanyl (Leptanal, Lilly, France) and 0·6 mg/kg/hour pancuronium (Pavulon, Organon Teknika, Boxtel, the Netherlands).
Data acquisition
Microvascular blood flow in the small intestinal loops, wound contraction, fluid evacuation, heart frequency and ventilator parameters were recorded throughout the experiments.
Surgical procedure
A 30‐cm long midline incision was performed on each pig. The VAC dressing or the ABThera dressing (both from KCI®, Inc., San Antonio, TX) was used to close the wound. The visceral protective layer was cut to an approximate size of 35 cm wide and 35 cm long, extending into the paracolic gutters on both sides. A layer of polyurethane Granu Foam™ was placed on top of the visceral protective layer between the edges of the wound. The wound was covered with a self‐adhesive polyethylene drape, and a track pad was inserted through the drape (all from KCI, Inc.), and then connected to a continuous vacuum source.
Microvascular blood flow was measured using laser Doppler velocimetry (Peri Flux System 5000, Perimed, Stockholm, Sweden) using a technique that quantifies the sum of the motion of the red blood cells in a specific volume. This method is applied extensively in plastic surgery procedures and employs a fibreoptic probe carrying a beam of light. Light impinging on cells in motion undergoes a change in wavelength (Doppler shift), while light impinging on static objects remains unchanged. The magnitude and frequency distributions of the changes are directly related to the number and velocity of red blood cells. The information is collected by a returning fibre, converted into an electronic signal and analyzed 11.
Laser Doppler probes were inserted into the intestinal wall of the ileac loop, and sutured to the inner surface of the dressing centrally beneath the dressing, and at the anterior abdominal wall. The locations of the probes were confirmed upon completion of the experiments.
Experimental protocol
The microvascular blood flow was measured continuously using the laser Doppler filament probes. Recordings were made before NPWT (baseline = 0 mmHg) and during exposure to NPWT at −50 −75 and −125 mmHg. The pressures were applied in random in order to prevent systematic effects, and baseline was restored between each pressure setting.
A chest tube was inserted through the abdominal wall into the Pouch of Douglas, and 1000 ml albumin solution was infused into the Pouch of Douglas to mimic the fluid in an open abdomen. NPWT was applied at pressures of −50, −75 and −125 mmHg using the VAC dressing or the ABThera dressing. The amount of fluid evacuated into a canister was measured gravimetrically. The abdomen was completely drained between each pressure setting before another 1000 ml albumin solution was infused. The amount of fluid evacuation was recorded after 1, 5 and 10 minutes of active negative pressure.
Wound contraction was determined by measuring the width and length of the wound before and after application of negative pressure, with the VAC dressing or the ABThera dressing.
Calculations and statistics
Laser Doppler velocimetry measurements were performed on 12 pigs. The output was recorded continuously using the Peri Flux System 5000. Microvascular blood flow was expressed in terms of perfusion units (PU). Calculations and statistical analysis were performed using GraphPad 5.0 software (San Diego, CA). The Mann–Whitney test was used to identify differences between in microvascular blood flow of the small intestinal loops. Significance was defined as: P < 0·05 (*), and P > 0·05 (not significant, n.s.). The values are given as the mean of 12 measurements expressed as the percent of the baseline value and the standard error on the mean (SEM).
Results
Microvascular blood flow in the small intestinal loop located at the anterior abdominal wall
The microvascular blood flow in the small intestinal loop sutured to the dressing at the anterior abdominal wall is shown in Figure 1. The results are expressed as the percentage of the baseline blood flow, which was defined as 100%. The blood flow was significantly reduced, to 75·9 ± 5·2% (P < 0·05), after the application of −50 mmHg using the VAC dressing, and to 76·0 ± 5·0% (P < 0·05) after the application of −50 mmHg with the ABThera dressing. The blood flow was significantly reduced, to 56·3 ± 4·2% (P < 0·05), after the application of −75 mmHg using the VAC dressing, and to 53·1 ± 4·8% (P < 0·05) after the application of −75 mmHg with the ABThera dressing. The blood flow was significantly reduced, to 45·7 ± 3·6% (P < 0·05), after the application of −125 mmHg using the VAC dressing, and to 46·6 ± 6·9 % (P < 0·05) after the application of −125 mmHg with the ABThera dressing.
Figure 1.
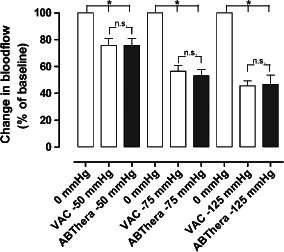
The microvascular blood flow in a small intestinal loop located at the surface of the anterior abdominal wall, when exposed to topical negative pressures of −50, −75 and −125 mmHg. The results shown are the mean values of six measurements ± SEM, expressed as a percentage of the baseline value. Statistical analysis was performed using the Mann–Whitney test. Significance was defined as P < 0·05 (*), and P > 0·05 (not significant, n.s.).
Microvascular blood flow in the small intestinal loop located apically of the abdomen, beneath the dressing
The microvascular blood flow in the small intestinal loop of the abdomen sutured to the dressing, apical of the abdomen, just beneath the dressing is shown in Figure 2. The blood flow was significantly reduced, to 64·6 ± 6·7% (P < 0·05), after the application of −50 mmHg using the VAC dressing, and to 65·3 ± 9·6% (P < 0·05) after the application of −50 mmHg with the ABThera dressing. The blood flow was significantly reduced, to 49·0 ± 4·6% (P < 0·05), after the application of −75 mmHg using the VAC dressing, and to 59·0 ± 5·9% (P < 0·05) after the application of −75 mmHg with the ABThera dressing. The blood flow was significantly reduced, to 39·6 ± 6·7% (P < 0·05), after the application of −125 mmHg using the VAC dressing, and to 40·5 ± 6·2% (P < 0·05) after the application of −125 mmHg with the ABThera dressing.
Figure 2.
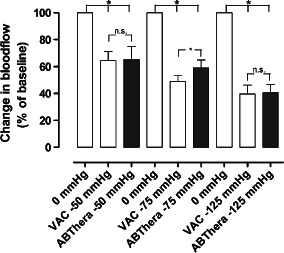
The microvascular blood flow in a small intestinal loop located centrally beneath the abdominal dressing, when exposed to topical negative pressures of −50, −75 and −125 mmHg. The results shown are the mean values of six measurements ± SEM, expressed as a percentage of the baseline value. Statistical analysis was performed using the Mann–Whitney test. Significance was defined as P < 0·05 (*), and P > 0·05 (not significant, n.s.).
Fluid evacuation
The results pertaining to fluid evacuation are shown in Figure 3. At −50 mmHg, the amount of fluid removed from the abdomen using the VAC dressing after 10 minutes was 443 ± 26 ml, compared with 618 ± 18 ml when using the ABThera dressing (P < 0.05) (Figure 3A). At −75 mmHg, the amount of fluid removed from the abdomen using the VAC dressing after 10 minutes was 597 ± 7 ml, compared with 712 ± 6 ml when using the ABThera dressing (P < 0.05) (Figure 3B). During the application of −125 mmHg, the amount of fluid removed from the abdomen using the VAC dressing after 10 minutes was 703 ± 42 ml, compared with 967 ± 16 ml when using the ABThera dressing (P < 0. 05) (Figure 3C).
Figure 3.
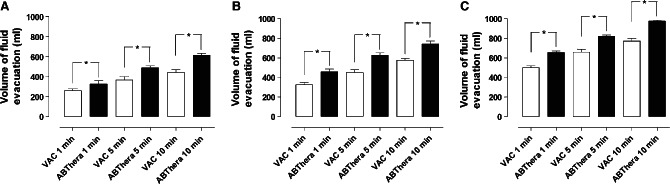
Comparison of fluid removal using the two dressings, measured after 1, 5 and 10 minutes during NPWT at −50 (A), −75 (B) and −125 mmHg (C), using 1000 ml albumin solution. The results are shown as means ± SEM of six experiments. Statistical analysis was performed using the Mann–Whitney test. Significance was defined as P < 0·05 (*) and P > 0·05 (not significant, n.s.).
Wound contraction
The results regarding wound contraction are shown in Figure 4. The ABThera dressing showed a slight but significant reduction in wound width, compared to the VAC dressing.
Figure 4.
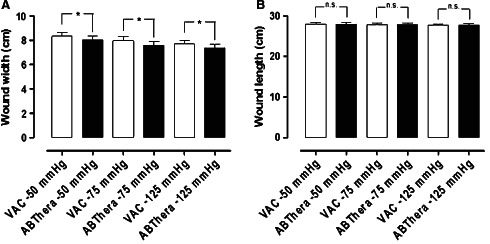
Comparison of wound contraction (wound width and wound length) using the two dressings, resulting from NPWT at −50, −75 and −125 mmHg. The results are shown as means ± SEM of six experiments. Statistical analysis was performed using the Mann–Whitney test. Significance was defined as P < 0·05 (*) and P > 0·05 (not significant, n.s.).
Discussion
We have recently shown that NPWT of the open abdomen induces ischaemia in the small intestinal loop and the omentum lying in close contact with the visceral protective layer, and that the degree of ischaemia seemed to increase with increasing negative pressure 10. This study was carried out using the VAC abdominal dressing. Recently, a new dressing for abdominal NPWT has been released onto the market. In the present study, we compared the VAC abdominal dressing, which has been used by surgeons for several years, with this new abdominal dressing, the ABThera dressing, made by the same manufacturer.
The ABThera dressing has arms of foam covered in a plastic film in the visceral protective layer, which could be expected to lead to better drainage of the abdomen. We indeed found that the ABThera dressing afforded significantly better drainage of the open abdomen than the VAC dressing. Theoretically this would significantly improve the care and recovery of these patients. We also observed that the ABThera dressing afforded significantly better wound contraction than the VAC system. Both these effects could explained by the foam arms, which extend along the anterior abdominal wall and laterally, providing better pressure transduction leading to better drainage and better wound contraction. Good wound contraction will probably create a more stable abdomen, and thus less pain in some patients. It might also counteract lateral facial retraction, improving the situation for the surgeon when the open abdomen is finally closed.
High closure rates of the abdomen have been reported with NPWT compared with other techniques 12, 13, 14, 15, 16. However, the method has occasionally been associated with increased development of intestinal fistulae and enteroatmospheric fistulae 4, 5, 6, 7, 8. NPWT has gained clinical acceptance due to excellent clinical results, but the basic mechanisms by which NPWT affects the open abdomen wound are still unclear. Investigations such as this may provide valuable knowledge allowing the treatment to be improved. We have previously shown that the use of NPWT in the open abdomen induces a decrease in the blood flow in the small intestinal wall lying close to the dressing. This decrease may indicate the relative zone of hypoperfusion found in other NPWT studies 9. We have also shown that the decrease in blood flow becomes greater with increasing negative pressure 9. Interestingly, the blood flow could be restored by inserting a protective thin plastic disc over the intestines 9, but could not be prevented by inserting four layers of paraffin gauze between the visceral protective layer and the intestines 17. Macroscopic changes in the small intestines lying close to the NPWT dressing in laparotomy wounds over 24 and 48 hours were recently studied in 70 kg pigs 18. Half of the animals were treated with a protective thin plastic disc over the intestines, while the other half was treated with conventional NPWT for open abdomen. Slight petechial bleeding was seen in the small intestinal loops lying close to the dressing in both groups 18. The area of petechial bleeding was significantly larger after 24 hours, but especially after 48 hours, in the conventional NPWT group. In contrast, hardly any petechial bleeding was seen in the group treated with a protective disc over the intestines 18. The area of petechial bleeding may indicate signs of ischemia.
Whether the application of NPWT to the open abdomen promotes the development of fistulae or not has been hotly debated. Our pre‐clinical studies indicate that ischaemia is induced in the intestinal loops lying close to the visceral protective layer when negative pressure is applied 10, 17. The effect of these findings in the clinical situation is still not known. In the present study we show that the application of a negative pressure to the open abdomen using the VAC dressing induces ischaemia or a decrease in microvascular blood flow in the small intestinal wall lying close to the visceral protective layer, not only just beneath the vacuum source but also at the anterior abdominal wall. These findings agree with prior findings 10, 17.
In this study, we compared the VAC abdominal dressing, with a new abdominal dressing called the ABThera abdominal dressing system. Prior to the investigation we hypothesised that the ABThera dressing would probably induce less ischaemia due to better pressure transduction, due to the foam arms extending laterally into the abdominal cavity. Unfortunately, we found that both the VAC and the ABThera dressing induced a decrease in microvascular blood flow in the intestinal wall lying close to the visceral protective layer. In the majority of the experiments no difference could be observed, in the decreased microvascular blood flow in the small intestinal wall, between the two different systems. Comparing the two locations investigated, both systems induced a significant ischaemia in the intestinal loops at the anterior abdominal wall and in the intestinal loops lying beneath the dressing. When using the ABThera dressing the measurements at the anterior abdominal wall were made in the intestinal loop lying close to the visceral protective layer, but not in contact with the foam arms. Ischemia may have been even more pronounced in the tissue in contact with the foam arms since these conduct the negative pressure efficiently.
Conclusions
The ABThera dressing afforded better drainage of the open abdomen, and better wound contraction than the VAC dressing. The application of the VAC and ABThera dressings to the open abdomen both resulted in a decrease in the microvascular blood flow in the small intestinal wall lying close to the NPWT dressing. The decrease in blood flow was related to the amount of negative pressure applied, and not the kind of dressing. No difference was found between the two different systems. We speculate that a longstanding decrease in blood flow in the intestinal wall may induce ischemia and secondary necrosis in the intestinal wall, which could promote the development of intestinal and enteroatmospheric fistulae.
References
- 1. Schecter WP, Ivatury RR, Rotondo MF, Hirshberg A. Open abdomen after trauma and abdominal sepsis: a strategy for management. J Am Coll Surg 2006;203:390–6. [DOI] [PubMed] [Google Scholar]
- 2. Swan MC, Banwell PE. The open abdomen: aetiology, classification and current management strategies. J Wound Care 2005;14:7–11. [DOI] [PubMed] [Google Scholar]
- 3. Deenichin GP. Abdominal compartment syndrome. Surg Today 2008;38:5–19. [DOI] [PubMed] [Google Scholar]
- 4. Bee TK, Croce MA, Magnotti LJ, Zarzaur BL, Maish GO 3rd, Minard G, Schroeppel TJ, Fabian TC. Temporary abdominal closure techniques: a prospective randomized trial comparing polyglactin 910 mesh and vacuum‐assisted closure. J Trauma 2008;65:337–42 discussion 342–34. [DOI] [PubMed] [Google Scholar]
- 5. Becker HP, Willms A, Schwab R. Small bowel fistulas and the open abdomen. Scand J Surg 2007;96:263–71. [DOI] [PubMed] [Google Scholar]
- 6. Fischer JE. A cautionary note: the use of vacuum‐assisted closure systems in the treatment of gastrointestinal cutaneous fistula may be associated with higher mortality from subsequent fistula development. Am J Surg 2008;196:1–2. [DOI] [PubMed] [Google Scholar]
- 7. Trevelyan SL, Carlson GL. Is TNP in the open abdomen safe and effective? J Wound Care 2009;18:24–5. [DOI] [PubMed] [Google Scholar]
- 8. Rao M, Burke D, Finan PJ, Sagar PM. The use of vacuum‐assisted closure of abdominal wounds: a word of caution. Colorectal Dis 2007;9:266–8. [DOI] [PubMed] [Google Scholar]
- 9. Lindstedt S, Malmsjo M, Hansson J, Hlebowicz J, Ingemansson R. Microvascular blood flow changes in the small intestinal wall during conventional negative pressure wound therapy and negative pressure wound therapy using a protective disc over the intestines in laparostomy. Ann Surg 2012;255:171–5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10. Hlebowicz J, Hansson J, Lindstedt S. Microvascular blood flow response in the intestinal wall and the omentum during negative wound pressure therapy of the open abdomen. Int J Colorectal Dis 2012;27:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zografos GC, Martis K, Morris DL. Laser Doppler flowmetry in evaluation of cutaneous wound blood flow using various suturing techniques. Ann Surg 1992;215:266–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheatham ML, Malbrain ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppaniemi A, Olvera C, Ivatury R, D'Amours S, Wendon J, Hillman K, Wilmer A. Results from the International Conference of Experts on Intra‐abdominal Hypertension and Abdominal Compartment Syndrome. II. Recommendations. Intensive Care Med 2007;33:951–62. [DOI] [PubMed] [Google Scholar]
- 13. Malbrain ML, De laet I, Cheatham M. Consensus conference definitions and recommendations on intra‐abdominal hypertension (IAH) and the abdominal compartment syndrome (ACS)‐‐the long road to the final publications, how did we get there? Acta Clin Belg Suppl 2007:44–59. [DOI] [PubMed] [Google Scholar]
- 14. Perez D, Wildi S, Demartines N, Bramkamp M, Koehler C, Clavien PA. Prospective evaluation of vacuum‐assisted closure in abdominal compartment syndrome and severe abdominal sepsis. J Am Coll Surg 2007;205:586–92. [DOI] [PubMed] [Google Scholar]
- 15. Stevens P. Vacuum‐assisted closure of laparostomy wounds: a critical review of the literature. Int Wound J 2009;6:259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Svensson S, Monsen C, Kolbel T, Acosta S. Predictors for outcome after vacuum assisted closure therapy of peri‐vascular surgical site infections in the groin. Eur J Vasc Endovasc Surg 2008;36:84–9. [DOI] [PubMed] [Google Scholar]
- 17. Lindstedt S, Hansson J, Hlebowicz J. Comparative study of the microvascular blood flow in the intestinal wall during conventional negative pressure wound therapy and negative pressure wound therapy using paraffin gauze over the intestines in laparostomy. Int Wound J 2012;9:150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lindstedt S, Malmsjo M, Hansson J, Hlebowicz J, Ingemansson R. Macroscopic changes during negative pressure wound therapy of the open abdomen using conventional negative pressure wound therapy and NPWT with a protective disc over the intestines. BMC Surg 2012;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]


