Abstract
The polysaccharide hyaluronan (HA) (synonyms – hyaluronic acid, hyaluronate) is a versatile, polymorphic, glycosoaminoglycan with vast biological functions. HA is found throughout the body, primarily residing in skin, thus playing an important role in wound healing. Research regarding HA's function has changed over the years, primarily focussing on a particular aspect or function. The contribution of HA in each stage of normal wound healing as well as its clinical wound dressing applications will be examined.
Keywords: Hyaluronan; Clinical application; Dermatology; Wound healing; Wound dressings
Introduction
The biology of wound healing is an innate science. Broadly speaking, normal wound healing involves three key phases: inflammatory, proliferative and remodelling (1), with each phase exhibiting overlapping processes of coordinated cellular activities (2). Cytokines mediate these cellular processes, enabling wound healing cells to produce the necessary structural proteins and polymers required for wound healing (3).
One extracellular polysaccharide involved in wound healing is the glycosoaminoglycan (GAG) hyaluronan (HA). HA is found in every human tissue and body fluid (4), displays unique physiochemical and biological properties (5) and its function can change depending on its size (6). HA is involved in each phase of wound healing and has been studied extensively 6, 7, 8, 9 with a few of these studies focussing on the role of HA in one component of wound healing, that is, Borgnoni et al. (1996) (8) examined the role in angiogenesis, whereas David‐Raoudi et al. (2008) (6) studied fibroblasts and HA. The key aspects of HA's role in wound healing as well as its influence on clinical practice and its use in wound dressings will be reviewed.
HA – structure, properties and physiology
In order to properly appreciate the function of HA in wound healing, an overview of HA's basic structure and physiology is required. HA exists in vivo as a negatively charged, disaccharide polymer of repeating d‐glucuronic acid and n‐acetylglucosamine (10) (Figure 1). HA polymers exist in varying lengths, with each molecular size uniquely functioning on each wound healing phase (Table 1). In short, large HA molecules are space‐filling molecules with regulatory structural functions, whereas small HA fragments are involved in angiogeneis, inflammation and immunostimulation (11).
Figure 1.
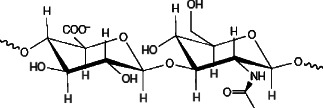
HA molecule (12).
Table 1.
Summary of hyaluronan function during wound healing
| Inflammatory phase: |
| • Binds to fibrinogen to commence clotting pathway. |
| • Allows inflammatory cell migration. |
| • Creates oedema to allow cell infiltration. |
| • Inhibits neutrophil migration to dampen inflammatory response. |
| Proliferative phase: |
| • Draws fibroblasts to wound site. |
| •‘Fills in gaps' of newly formed ECM, creating cushioning and structural organisation. |
| • Stimulates MMPs for angiogenesis. |
| • Promotes keratinocyte migration and proliferation. |
| Remodelling phase: |
| • Contributes to normal and pathological scarring. |
The long, heavy HA polymers (4000–2000 kDa, 1000–5000 saccharides) seen in wound healing create long, twisting, hydrophilic chains (11). These water‐attracting properties give HA its characteristic elastic and cushioning properties (10) illustrated by its concentration in skin and cartilage (4) (Figure 2). When HA exists in high concentrations in this form, it creates a porous scaffolding network, allowing for select diffusion of cells and proteins, creating pathways for cell migration (12). This is a key feature of HA, as cell migration and proliferation are essential to successful wound healing (5). Smaller molecular size HA fragments also encourage cell migration, with very short, low‐weight (four saccharides) HA fragments inducing chemotaxis and medium‐sized HA chains (1000–1250 saccharides) stimulating inflammatory cytokines expression (11).
Figure 2.

Hyaluronan hydrophilic properties.
As HA is required in various shapes and weights for each wound healing phase, its catabolism and synthesis are vital for normal wound healing. HA is manufactured on the cell surface by three enzymes that are embedded within the cell surface, called HA synthases (HAS1, HAS2 and HAS3) (12). This differentiates HA from other GAGs as it does not bind to a core protein (13). So too, while other GAGs, such as heparan sulphate, are manufactured within the cell and then released via exocytosis (13), HAS manufacture HA on the outside of the cell wall, enabling it to interact readily with other cells and HA‐binding proteins, hyaladherins (12). HA catabolism primarily occurs in two main ways, through six hyalurinodases (HYALs) and reactive oxygen species (ROS). HYALs are responsible for altering the various HA sizes needed during each wound healing phase (11). HYAL‐induced breakdown is quite systematic in contrast to the ROS catabolism, which is often induced extrinsically by UV (14) or intrinsically by neutrophils (15). However, it is unknown whether the results of ROS‐induced HA catabolism create functional HA fragments (16). HA's antioxidant and free‐radical scavenging properties have been used in various HA‐based wound dressings.
In addition to HA's unique structural properties, these cell surface interactions are crucial to understand HA's role in wound healing, as HA's size and shape dictates its differing roles and interactions at each wound stage.
HA and the inflammatory phase
During the inflammatory phase of wound healing, HA synthesis increases rapidly (7). Upon breach of the skin, large, heavy molecular size HA fragments are synthesised from platelets and from available HA in the blood stream (11). These HA fragments are able to bind to fibrinogen to commence the extrinsic clotting pathway (11). Because of the large amounts of HA released at wounding, the wound site is saturated with fluid leading to oedema (11). HA's hydrophilic properties cause swelling of the tissue surrounding the wound (17), creating a porous framework for cells to migrate to the injury site (12).
Oedema can be observed macroscopically in this initial stage of wound healing, allowing for microscopic chemotaxis of inflammatory cells (18). This inflammatory process is driven by the primary cytokines tumour necrosis factor‐alpha (TNF‐α), interleukin‐1 beta (IL‐1β) and IL‐8 and is stimulated when concentrations of HA are high (5). These inflammatory cytokines increase blood vessel dilation, allowing increased cellular recruitment to the wound (18). This vasodilation presents as heat and redness, serving as a clinical indicator of wound inflammation and progression (19). Inflammation and its associated symptoms are essential for successful wound repair (11).
Conversely, HA also has a role in reducing and moderating the inflammatory response, through its interaction with the hyaladherin TNF‐stimulated gene‐6 (TSG‐6) (20). TSG‐6 is stimulated by inflammatory cytokines IL‐1 and TNF‐α, causing fibroblasts and other inflammatory cells to express the TSG‐6 protein (21). Once expressed by cells, TSG‐6 proteins are retained in HA‐rich environments, binding to high‐molecular‐weight HA polymers that form heavy chains (22). These heavy HA chains prevent inflammation by inhibiting neutrophil migration (23) and inhibition of plasmin through negative feedback loops (21). This mechanism was tested in a murine pouch study by Wisniewski et al. (1996) (24) where inflammation was induced by IL‐1 or carrageenan. Following administration of TSG‐6, reduction of inflammation was achieved, comparable with the corticosteroid dexamethasone. Although this study shows TSG‐6s anti‐inflammatory properties, the laboratory conditions in the study are not typical for normal human wound healing. The short timeframe used to induce inflammation (4 hours), the use of corticosteroids and the narrow spectrum of wound proteins examined may not reflect clinical conditions.
As the wound progresses out of the inflammatory phase, the inflammatory signal reduces. A delay or lengthening in this phase can render an acute wound chronic, halting its progress to healing (25). The cellular changes associated with reduction of the inflammatory phase are visible, as the classic inflammatory signs recede and granulation tissue begins to form in preparation for the proliferative phase (18). This phase is too heavily influenced by HA's multifaceted properties.
HA and the proliferative phase
Following on from the late inflammatory phase, the proliferative phase of wound healing marks the arrival of fibroblast migration (19). These dermal cells are drawn to wound tissue by small HA fragments (6–20 saccharides) (11) and growth factors. Fibroblasts create collagen and GAGS (including HA), constructing and anchoring the newly formed extracellular matrix (ECM) (2).
In their study, David‐Raoudi et al. (2008) (6) tested the differential effects of HA fragments of varying size (small, medium and large) on human dermal fibroblasts (HDF). The researchers found that multiwell plates coated with different lengths of HA produced greater HDF adhesion than the control group. Additionally, David‐Raoudi et al. also found in an assay to examine proliferation that varying lengths of HA and in particular native HA promoted HDF proliferation at statistically significant levels compared with the control group (P < 0·05). These results reflect physiological conditions with altering HA lengths at each wound healing stage (11).
In contrast, Ferguson et al. (2011) (26) found that differing HA fragments did not significantly influence fibroblast migration and its subsequent collagen production. Additionally, Ferguson et al. found that the molar concentrations of HA influenced fibroblast proliferation more so than molecular size, with low molar concentrations of HA, inhibiting fibroblast migration. This may explain the discrepancies between Ferguson et al. and David‐Raoudi et al. and suggests potential pharmacological wound healing applications, with lower HA concentrations acting as a delivery system for wound therapies.
Another finding of David‐Raoudi et al. was that differing HA lengths stimulate the production of specific collagen types, which can promote or inhibit scar formation. One important function that fibroblasts exert on the developing ECM is collagen synthesis (27). Collagen, which is the body's main connective protein, is crucial to the developing ECM, as it provides tensile strength to the healing wound (13). Various collagen types are produced by fibroblasts, each with its own structure and function (2).
The developing ECM is visibly identifiable as granulation tissue, displaying the structural properties of HA. With collagen and elastin providing the fibrous scaffolding, HA ‘fills in the gaps' to form a cushioning gel (5). As shown during the inflammatory phase of wound healing, long, heavy HA chains form this gel because of HA's hydrophilic nature. Upon saturation, HA displays the elastic recoil properties similar to cartilage (12). This malleability is an important clinical feature of granulation tissue, as wound healing often occurs in areas of high movement or pressure that is joints, plantar surface of feet. Without HA's microscopic, hydrophilic and porous networking abilities, the macroscopic granulation tissue would not able to hold its shape to allow for normal wound healing.
Although granulation tissue is elastic, sharp trauma causes bleeding (2). This hallmark feature of granulation tissue is primarily because of angiogenesis (27). New blood capillaries visibly form within granulation tissue, because of increased metabolic demands from the cells present in the wound. HA contributes to this process by its short‐chain lengths called oligomers, which are as small as 6–20 molecules in length (11). These oligomers bind to the hyaladherin CD44 and act as stimulating fragments for matrix metalloproteinases (MMPs) (23). The MMPs are essential for new capillary sprouting by breaking down the basement membrane of the wound. This allows for new capillary buds to sprout from existing ones (28). Borgognoni et al. (1996) (8) studied low‐weight (short chain) HA and its effects on angiogenesis. In their study, primary (sutured) and secondary (open) wounds were created in rats. Both groups were treated with HA gel. The results showed an increased microvasculature compared with control groups, but only wounds healing by secondary intention demonstrated accelerated healing.
Although this study helps to elucidate the contribution of HA to angiogenesis, its clinical influence is unclear. Of significance, the group with primary wounds and HA treatment showed delayed healing, suggesting negative wound healing outcomes when topical HA is used on primary wounds. Conversely, the group with secondary wounds and HA treatment showed accelerated healing overall. This hastening of healing may positively influence clinical practice, as accelerated healing has potential time and money reductions to both patient and practitioner, thereby promoting the use of HA‐impregnated dressings.
However, HA's direct role in accelerated secondary wound healing in the study by Borgognoni et al. (1996) is questionable for a number of reasons. The authors used a low concentration (0·2%) of HA, in a sodium alginate dressing. This raises two main problems; first, at such a low concentration, HA might not be therapeutic and more importantly, sodium alginate is considered a wound dressing and resembles HA structurally and physically (29). This casts doubt over which component may have stimulated angiogenesis. Thus, it is difficult to clearly determine the effect of sodium alginate alone on angiogenesis, as the results were not analysed separately.
The final component of the proliferative phase of wound healing is epithelialisation, which begins very early after wounding (27). The skin contains most of the body's HA, which is concentrated in the deeper, intercellular layers (stratum basale) of the epidermis as well as in the dermis (30). The main cell type in this basal layer is the keratinocyte (31), which expresses hyaladherin CD44 in large amounts (23). In the skin, HA functions to hydrate the stratum basale, creating the aforementioned porous structures for nutrient channelling (5).
Upon wounding, keratinocytes and their HA structures are torn apart, commencing the inflammatory phase of wound healing (11). Through CD44 interactions, keratinocytes migrate to the wound site, collecting at wound edges (5). Keratinocytes then form a delicate cover over the new wound from the wound borders. These cells then ‘leapfrog’ over each other to form a cover of epithelial cells over the new wound (2). These new cells then differentiate to create the various epidermal skin layers, providing a protective barrier against infection and fluid loss (1).
Successful epithelialisation is not only merely a protective function but also morphological. Kaya et al. (1997) (9) produced transgenic mice whose cells expressed an antisense CD44. This genetic change impaired keratinocyte migration, producing gross morphological changes such as reduced skin elasticity and wound healing delay. This early study highlighted the requirement of the presence of HAs for effective epithelialisation, for without its signalling and physiochemical properties, excessive scarring and delayed wound healing may occur.
HA and the remodelling phase
Although pathological scar formation can be a reality of wound care, normal wound healing does result in scar formation (25). As tissue continues to repair itself, wound edges contract from fibroblast and ECM interactions and collagen is continuously synthesised and degraded, equilibrating at 3 weeks (2). Wound strength increases progressively over time, albeit still less than the original unwounded tissue (2). Gradually, the wound‐specific cells, structures and HA are degraded and replaced by an avascular, collagenous scar (2). The collagen produced in wound scarring never reaches the same strength as injured skin due to its disorganised structure (1).
The associated physiological changes of remodelling are also seen within the wound as its appearance gradually normalises. The previously raw, granulated wound is now a healing scar, functioning concurrently with surrounding healthy skin, steadily and subtlety improving over time.
Clinically, minimal scar tissue also occurs with advancing age because of HA being present primarily in the upper dermis, as is also seen in foetal subjects (30). This might lead to the assumption that HA skin concentrations change at various ages. However, Meyer and Stern (1994) (32) found that the distribution and size of HA remain consistent throughout a life time. This consistency was seen in tissue samples of foetal, middle‐aged and elderly skin. Meyer and Stern (1994) suggest that although the amount of HA remains the same, the amount of extractable HA changes with age because of hyaladherin binding. This shows that while HA is present in human skin of all ages, its location and the extent of hyaladherin binding influences HA's contribution to scarring (30).
The research by Meyer and Stern (1994) in addition to other work in scarless 6, 33 and foetal wound healing (34) has shown promising real‐life applications.
HA in wound dressings
Aside from its role in biology of wound healing, HA's properties have recently been successfully used in a number of wound dressings. HYALOFILL (Anika), HYALOMATRIX (Anika), IALUSET (Laboratoires Genévrier) and HYIODINE (Contipro) are current examples featuring HA, which have been studied and have been shown to be clinically effective in a variety of wounds (35).
Dereure et al. (2012) (36) found that Ialuset resulted in faster wound healing and reduced pain in venous leg ulcers compared with control in a double‐blinded, randomised control trial (RCT). However, the rate of complete healing was found to be similar in both groups at the secondary end point of 60 days. Moseley et al. (2003) (15) compared the antioxidant properties of HA‐based dressings (HA benzyl ester), low‐ and high‐molecular weight HA and AQUACEL (Convatec) in vitro. The results showed that HA benzyl ester, Aqaucel and high‐molecular‐weight HA exhibit free‐radical scavenging properties, with HA benzyl ester showing the highest antioxidant properties. Although consistent with HA's known antioxidant properties, Moseley et al. research may not have yielded the same results in vivo. Other studies have shown success with the use of hyiadine 37, 38, 39, yet none of these have been RCTs. Therefore, future research comparing to use a variety of HA‐based dressings in RCTs may be of further clinical benefit.
Conclusion
Research into HA's role in wound healing is an evolving science. Much of the early research reviewed appeared to focus on HA's role in scar formation and its presence in skin. As scientific research of each phase and process of wound healing has progressed, understanding of HA's roles has deepened, implicating it in every major wound healing event.
HA's functions during wound healing change with its size. Large, heavy and long HA chains appear to have structural functions such as porous networks during inflammation and as a space filler in granulation tissue. This contrasts with the small, light and short HA fragments that have stimulatory and attracting properties such a fibroblast migration and collagen production (40).
The influence of HA on clinical practice can be viewed in differing ways. From a purely educational perspective, HA gives the clinician insight into the physiological depth of wound healing because of HA presence in many wound healing processes. When this cellular understanding is applied to HA research, the clinical context of wound treatment is promoted from a series of biological events to a real‐time science.
Care must be taken when applying the reviewed HA research clinically, as some of the research reviewed does not replicate clinical conditions. Wound research, however, is challenging to perform in real‐life conditions. Apart from the ethical and financial considerations, due to the complex nature of wound healing, consistency in human wound healing research can be difficult to attain (41). These limitations aside, many clinical applications such a scarless healing and HA‐based dressing can be drawn from the reviewed research, with promising future developments.
Acknowledgements
The authors thank Samantha Holloway, Professional Tudor, Wound Healing Research Unit, for her many constructive and helpful suggestions. They also thank Lisa Frenkel for her encouragement, editorial skills and personal support.
References
- 1. Broughton G , Janis J , Attinger C. Wound healing: an overview. Plast Reconstr Surg 2006. ; 117 : 1e – s. [DOI] [PubMed] [Google Scholar]
- 2. Enoch S , Leaper D. Basic science of wound healing. Surgery 2005. ; 23 : 37 – 42. [Google Scholar]
- 3. Slavin J. The role of cytokines in wound healing. J Pathol 1996. ; 178 : 5 – 10. [DOI] [PubMed] [Google Scholar]
- 4. Fraser JRE , Laurent TC , Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med 1997. ; 242 : 27 – 33. [DOI] [PubMed] [Google Scholar]
- 5. Chen J , Abetangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen 1999. ; 7 : 79 – 89. [DOI] [PubMed] [Google Scholar]
- 6. David‐Raoudi M , Tranchepain F , Deschrevel B , Vincent JC , Bogdanowicz P , Boumedene K , Pujol JP. Differential effects of hyaluronan and its fragments on fibroblasts: relation to wound healing. Wound Repair Regen 2008. ; 16 : 274 – 87. [DOI] [PubMed] [Google Scholar]
- 7. Oksala O , Salo T , Tammi R , Kakkien L , Jalkanen M , Inki P , Larjava H. Expression of proteoglycans and hyaluronan during wound healing. J Histochem Cytochem 1995. ; 43 : 125 – 35. [DOI] [PubMed] [Google Scholar]
- 8. Borgognoni L , Reali UM , Santucci M. Low‐molecular‐weight hyaluronic‐acid induces angiogenesis and modulation of the cellular infiltrate in primary and secondary healing wounds. Eur J Dermatol 1996. ; 6 : 127 – 31. [Google Scholar]
- 9. Kaya G , Rodriguez I , Jorcano JL , Vassalli P , Stamenkovic I. Selective suppression of CD44 in keratinocytes of mice bearing an antisense CD44 transgene driven by a tissue‐specific promoter disrupts hyaluronate metabolism in the skin and impairs keratinocyte proliferation. Genes Dev 1997. ; 15 : 996 – 1007. [DOI] [PubMed] [Google Scholar]
- 10. Tammi MI , Day AJ , Turley EA. Hyaluronan and homeostasis: a balancing act. J Biol Chem 2001. ; 277 : 4581 – 4. [DOI] [PubMed] [Google Scholar]
- 11. Stern R , Asari AA , Sugahara KN. Hyaluronan fragments: an information‐rich system. Eur J Cell Biol 2006. ; 85 : 699 – 715. [DOI] [PubMed] [Google Scholar]
- 12. Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 2004. ; 4 : 528 – 39. [DOI] [PubMed] [Google Scholar]
- 13. Alberts B , Johnson A , Lewis J , Raff M , Roberts K , Walter P. Molecular Biology Of The Cell. Part V: Cells in their social context: Cell junctions, Cell Adhesion and the Extracellular Matrix 5 ed., 2008. ; pp. 1131 – 1204.
- 14. Stern R , Maibach HI. Hyaluronan in skin: aspects of aging and its pharmacologic modulation. Clin Dermatol 2008. ; 26 : 106 – 122. [DOI] [PubMed] [Google Scholar]
- 15. Moseley R , Walker M , Waddington RJ , Chen WYJ. Comparison of the antioxidant properties of wound dressing materials ‐ carboxymethylcellulose, hyaluronan benzyl ester and hyaluronan, towards polymorphonuclear leukocyte‐derived reactive oxygen species. Biomaterials 2003. ; 24 : 1549 – 57. [DOI] [PubMed] [Google Scholar]
- 16. Jiang D , Liang J , Nobel PW. Hyaluronan in tissue injury and repair. Ann Rev Cell Dev Biol 2007. ; 23 : 435 – 61. [DOI] [PubMed] [Google Scholar]
- 17. Gerdin B , Hällgren R. Dynamic role of hyaluronan in connective tissue activation and inflammation. J Intern Med 1997. ; 242 : 49 – 55. [DOI] [PubMed] [Google Scholar]
- 18. Hart J. Inflammation 1: its role in the healing of acute wounds. J Wound Care 2002. 11 : 205 – 9. [DOI] [PubMed] [Google Scholar]
- 19. Halloran CM , Slavin J. Pathophysiology of wound healing. Surgery 2002. ; 20 : i – v. [Google Scholar]
- 20. Milner CM , Day AJ. TSG‐6: a multifunctional protein associated with inflammation. J Cell Sci 2003. ; 116 : 1863 – 73. [DOI] [PubMed] [Google Scholar]
- 21. Wisniewski HG , Vilček J. TSG‐6: an IL‐1/TNF‐inducible protein with anti‐inflammatory activity. Cytokine Growth Factor Rev 1997. ; 8 : 143 – 156. [DOI] [PubMed] [Google Scholar]
- 22. Fries E , Kaczmarczyk A. Inter‐α‐inhibitor, hyaluronan and inflammation. Acta Biochim Pol 2003. ; 50 : 735 – 42. [PubMed] [Google Scholar]
- 23. Noble PN , Jiang D , Liang J. Hyaluronan as an immune regulator in human diseases. Physiol Rev 2011. ; 91 : 221 – 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wisniewski HG , Hua JC , Poppers DM , Naime D , Vilcek J , Cronstein BN. TNF/IL‐1 ‐inducible protein TSG‐6 potentiates plasmin inhibition by inter‐α‐inhibitor and exerts a strong anti‐inflammatory effect in vivo. J Immunol 1996. ; 156 : 1609 – 15. [PubMed] [Google Scholar]
- 25. Moore K. Cell biology of chronic wounds: the role of inflammation. J Wound Care 1999. ; 8 : 345 – 48. [PubMed] [Google Scholar]
- 26. Ferguson EL , Roberts JL , Moseley R , Griffiths PC , Thomas DW. Evaluation of the physical and biological properties of hyaluronan and hyaluronan fragments. Int J Pharm 2011. ; 420 : 84 – 92. [DOI] [PubMed] [Google Scholar]
- 27. Singer AJ , Clark RAF. Cutaneous wound healing. New Engl J Med 1999. ; 341 : 738 – 46. [DOI] [PubMed] [Google Scholar]
- 28. Pardue EL. Role of hyaluronan in angiogenesis and its utility to angiogenic tissue engineering. Organogenesis 2008. ; 4 : 203 – 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qin Y. The characterisation of alginate wound dressings with different fibre and textile structures. J Appl Polym Sci 2006. ; 100 : 2516 – 20. [Google Scholar]
- 30. Juhlin L. Hyaluronan in skin. J Intern Med 1997. ; 242 : 61 – 66. [DOI] [PubMed] [Google Scholar]
- 31. Venus M , Waterman J , McNab I. Basic physiology of the skin. Surgery 2010. ; 28 : 469 – 72. [Google Scholar]
- 32. Meyer LJM , Stern R. Age‐dependant changes of hyaluronan in human skin. J Invest Dermatol 1994. ; 102 : 385 – 9. [DOI] [PubMed] [Google Scholar]
- 33. Iocono JA , Ehrlich HP , Keefer KA , Krummel TM. Hyaluronan induces scarless repair in mouse limb organ culture. J Paediatr Surg 1998. ; 33 : 564 – 7. [DOI] [PubMed] [Google Scholar]
- 34. Longaker MT , Chiu ES , Adzick NS , Stern M , Harrision MR , Stern R. Studies of foetal wound healing. Ann Surg 1990. ; 213 : 292 – 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kutting KF. Wound healing through synergy of hyaluronan and iodine complex. J Wound Care 2011. ; 20 : 424 – 30. [DOI] [PubMed] [Google Scholar]
- 36. Dereure O , Czubek M , Combemale P. Efficacy and safety of hyaluronic acid in treatment of leg ulcers: a double‐blind RCT. J Wound Care 2012. ; 21 : 131 – 9. [DOI] [PubMed] [Google Scholar]
- 37. Brenes R , Ajemian MS , Macaron SH , Panait L , Dudrick SJ. Initial experience using hyaluronate‐iodine complex for wound healing. Am Surg 2011. ; 77 : s355 – 9. [PubMed] [Google Scholar]
- 38. Sobotka L , Smahelova A , Pastarova J , Kusalova M. A case report of the treatment of diabetic foot ulcers using a sodium hyaluronate and iodine complex. Int J Low Extrem Wounds 2007. ; 6 : 143 – 7. [DOI] [PubMed] [Google Scholar]
- 39. Wild T , Ponweise D , Rahbarina A , Bruckner M , Czala K , Eberlein T. New galenic antiseptic substance containing iodine (KI3 complex) and hyaluronic acid for treatment of chronic, hardly healing wounds. J Wound Technol 2010. ; 7 : 63 – 65. [Google Scholar]
- 40. Moseley R , Waddington RJ , Embery G. Hyaluronan and its potential role in periodontal healing. Dent Update 2002. ; 29 : 144 – 8. [DOI] [PubMed] [Google Scholar]
- 41. Dorsett‐Martin W. Rat models of skin wound healing: a review. Wound Repair Regen 2004. ; 12 : 591 – 9. [DOI] [PubMed] [Google Scholar]


