Abstract
Transmetatarsal amputation (TMA) represents an effective surgical procedure used to treat several clinical conditions such as forefoot infection, gangrene and chronic ulceration in diabetic patients. TMA permits walking without the need for prosthesis, but nevertheless is burdened with a high complications rate. The aim of this study was to evaluate the possibility to use platelet gel (PG) as an adjuvant therapy when performing TMA procedure in diabetic patients. In a 6‐year period, 26 diabetic patients had undergone TMA procedure followed by autologous PG applications (group A) and 32 patients had undergone TMA as sole procedure (group B). After TMA procedure, the treatment is based on outpatient management and consists of a weekly platelet‐rich plasma gel application on the surgical wound for 1 month in group A and on clinical evaluation only for group B. For group A, healing rate was of 96·15% and one patient (3·84%) presented wound dehiscence, and no postoperative wound infections occurred. For group B, healing rate was of 59·37%; severe infection of the stump prompted to the proximal amputations in 40·62% of patients during the follow‐up period. PG application may be an effective adjuvant treatment to improve wound healing in diabetic dysvascular patients.
Keywords: Diabetic foot, Diabetic patients, Platelet gel, Transmetatarsal amputation, Wound healing
Introduction
Transmetatarsal amputation (TMA) represents an effective surgical approach used to treat several clinical conditions such as forefoot infection, gangrene and chronic ulceration in diabetic dysvascular patients 1. The primary goal of a TMA is to remove the non viable tissue and preserve the limb function. The main advantages of TMA are represented by the possibility to walking without prosthesis and using low energy.
The main disadvantage of this amputation is the high complication rates with the development of infection and/or ischaemia, which raise the doubt whether it is worthwhile performing TMA in patients with a diabetic foot 2, 3. In fact, wound healing rates from multiple series range from approximately 40% to 70% 4.
Studies on the restoration of tissue integrity have shown the involvement of platelets in the wound‐healing process. Platelet activation can modulate wound healing by interacting with molecular signals, primarily, cytokines and growth factors (GFs). Platelet gel (PG) allows an exogenous in situ addition of GF with homeostasis restoration and tissue reparation and regeneration 5. In this light, the purpose of this study was to evaluate the success rate of TMA in patients with diabetic foot treated with platelet‐rich gel.
Patients and methods
In a 6‐year period (1 January 2005 to 31 December 2010), 58 diabetic patients had undergone TMA procedure. The demographics are shown in Table 1. Indications for TMA were chronic forefoot ulceration, forefoot infection, forefoot gangrene or a combination of these. Twenty‐six TMAs (group A) were followed by autologous PG applications as described below in the text. Thirty‐two TMAs (group B) were conducted as sole procedure without application of PG.
Table 1.
Characteristics of patients
| Characteristics | Total | Group A | Group B |
|---|---|---|---|
| Number of patients | 58 | 26 | 32 |
| Gender | |||
| Male | 46 (79·31%) | 19 (73·07%) | 27 (84·37%) |
| Female | 12 (20·68%) | 7 (26·92%) | 5 (15·62%) |
| Age (years) | |||
| Range | 41–85 | 43–85 | 41–79 |
| Mean | 65·5 | 67·5 | 63·5 |
| Measurable ABI | 39 (67·24%) | 17 (65·38%) | 22 (68·75%) |
| Non compressible ABI | 19 (32·75%) | 9 (34·61%) | 10 (31·25%) |
| Diabetes mellitus type II | 58 (100%) | 26 (100%) | 32 (100%) |
| Coronary artery disease | 33 (56·89%) | 18 (69·23%) | 15 (46·87%) |
| Hypertension | 39 (67·24%) | 17 (65·38%) | 22 (68·75%) |
| Cerebral vascular accident | 9 (15·51%) | 5 (19·23%) | 4 (12·05%) |
| End‐stage renal disease | 8 (13·79%) | 5 (19·23%) | 3 (9·37%) |
| Palpable pedal pulses | 18 (31·03%) | 8 (30·76%) | 10 (31·25%) |
| Previous toe amputation | 16 (27·58%) | 6 (23·07%) | 10 (31·25%) |
| Pre‐TMA revascularisation | 43 (74·13%) | 19 (73·07%) | 24 (75·00%) |
ABI, ankle brachial index; TMA, transmetatarsal amputation.
All patients were treated after collecting appropriate information on the type of treatment and obtaining informed consent. Institutional Review Board approval was obtained. In 43 TMAs (74·13%), the patients underwent either surgical or endovascular revascularisation beforehand, whereas in 15 TMAs (25·86%) the patients were either believed to be adequately perfused or did not have revascularisation options. Thirty‐nine patients (67·24%) had a measurable ankle brachial indices, whereas 19 (32·75%) had non compressible ankle brachial indices.
TMA was performed as follows for both groups of patients: the viable soft tissue envelope determined the incisional approach (Figures 1 and 2). The incision was begun on the dorsal surface of the foot directly down to the level of the bone without undermining the flaps. As the medial and lateral extents of the dorsal flap were reached, the plantar incision was begun, leaving an acute angulation to avoid dog ears with closure. Soft tissue coverage of the metatarsals was provided by the long plantar flap.
Figure 1.
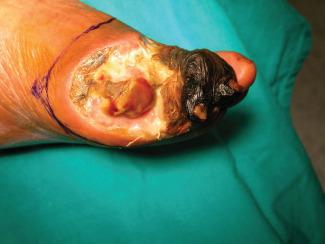
Image showing the incisional approach.
Figure 2.
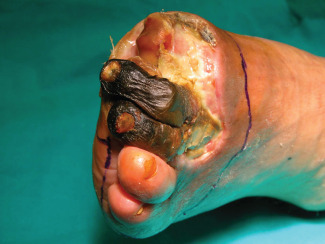
Imaging showing the incisional approach around the forefoot.
Once haemostasis has been achieved, the small‐bladed oscillating saw was used to divide the metatarsal bones approximately 1 cm proximal to the dorsal skin flap. The cut edges were smoothed with a rasp. The plantar soft tissues were divided to release the amputated segment. The long plantar flap was covered with PG before suturing it on the dorsal flap (Figure 3). The skin was then closed using non absorbable suture and the surgical wound was also covered with PG.
Figure 3.
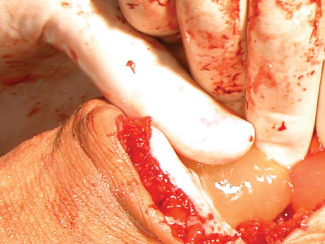
Application of platelet gel on plantar flap.
The autologous platelet‐rich plasma was prepared as follows: the whole blood (15 ml) was centrifuged at 399 g for 11 minutes at 22°C to separate red blood cells and platelet‐rich plasma (PRP). PRP was centrifuged at 959 g for 11 minutes at 22°C to separate platelet concentrate (PC) and platelet‐poor plasma (PPP). PC was then placed in a mixer, whereas PPP was immediately frozen at −80°C and then stored in a blood bank refrigerator at 4°C overnight so that it can slowly thaw and produce cryoprecipitate. The next morning the bag containing thawed PPP was centrifuged (704 g for 11 minutes); PPP and cryoprecipitate were separated, the latter being sterilely transferred into the bag containing PC. The bag containing cryoprecipitate and PC was sterilely connected to four 150‐ml transfer bags. The remaining 5% of the product in the connecting tube was tested for bacteria, and the platelet count was carried out in all samples: in the main bag the platelet concentration was usually about 2 000 000/ml. Aliquots were weighted, numbered, recorded and stored at a temperature ranging from −30°C to −80°C, on an average at −40°C. During the processing phases, several quality controls were carried out: the platelets found in the final mix must be >1–1·5 million/µl; average fibrinogen concentration (mg/U) in the cryoprecipitate must be equal to (target > 140): 642·7 – factor VIII (>70 IU/l). Asepsis of stored products is verified as well as the GF ratios: PDGF‐TGFβ‐IGF in the aliquots of frozen‐activated gel.
After TMA procedure, the treatment is based on outpatient management and consists of a weekly PRP gel application on the surgical wound for a month. The response to topical haemotherapy with PRP gel was evaluated following stump healing. The Student's t‐test was used to evaluate the differences between each group, and P values <0·05 were considered statistically significant.
Results
A healed stump was achieved in 25 (96·15%) of the 26 amputations (in Figure 4, an example of early result after 1 week is shown) of group A. Only one patient of this group had wound dehiscence, which occurred 38 days after surgery. In this case, the stump was surgically revised and underwent skin grafting procedure and a subsequent healed stump was achieved. No postoperative wound infections occurred.
Figure 4.
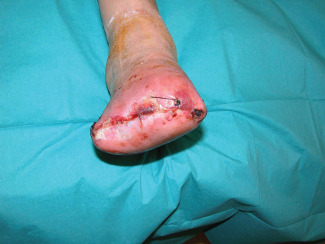
Example of final result after 1 week from procedure.
A healed stump was achieved in 19 (59·37%) of the 32 amputations of group B. Severe infection of the stump has determined the need for more proximal amputations in the remaining 13 patients (40·62%) of this group in the follow‐up period. No calcaneus or equinus gait had developed in any patient of both groups. Median follow‐up was 36 months for both groups. The effects of demographic factors on TMA healing for both groups are listed in Table 2.
Table 2.
Demographics data associated with healed and non healed TMAs for all cohort of patients (group A + group B)
| Characteristics | Healed | Non healed | P value |
|---|---|---|---|
| Number of patients | 44 | 14 | |
| Gender | |||
| Male | 35 (79·54%) | 11 (78·57) | 0·75 |
| Female | 9 (20·45%) | 3 (21·42%) | 0·93 |
| Measurable ABI | 30 (68·18%) | 9 (64·28%) | 0·78 |
| Non compressible ABI | 13 (29·54%) | 6 (42·85%) | 0·45 |
| Coronary artery disease | 24 (54·54%) | 9 (64·28%) | 0·52 |
| Hypertension | 30 (68·18%) | 9 (64·28) | 0·78 |
| Cerebral vascular accident | 7 (15·90%) | 2 (14·28%) | 0·88 |
| End‐stage renal disease | 5 (11·36%) | 3 (21·42%) | 0·34 |
| Palpable pedal pulses | 13 (29·54%) | 5 (35·71%) | 0·66 |
| Previous toe amputation | 11 (25·00%) | 5 (35·71%) | 0·43 |
| Pre‐TMA revascularisation | 36 (81·81%) | 11 (78·57%) | 0·78 |
ABI, ankle brachial index; TMA, transmetatarsal amputation.
Discussion
The goal of TMA is twofold: to control forefoot infection or ischaemia by removing all necrotic, ischaemic or infected tissues to an extent that allows healing; to maximise limb function by salvaging the midfoot and rearfoot, thus leaving a plantigrade platform on which the patient can adequately bear weight and walk. However, the main disadvantage of this amputation is the low healing rate, especially in diabetic dysvascular patients, which raise the doubt whether it is worthwhile performing TMA in these kinds of patients 2, 3.
McKittrick et al. reported a healing rate of 72% in a series of 215 TMA procedures to treat infection or gangrene in patients with diabetes mellitus 6; Thomas et al. reported a 46% healing rate after TMA procedures 7 and Pollard et al. reported a healed stump in 57·4 % of cases 8. Studies on the restoration of tissue integrity have shown the role of the platelets in the wound‐healing process.
The mechanisms through which PG delivers its beneficial effects are still indistinct. But it is quite evident that this is due to the GFs with abundant platelet alpha‐granules that are slowly but steadily released through the gel; however, the cytokines and other chemical mediators also play a major role. These intercellular mediators are usually found in the body; under these particular circumstances and in huge concentration, they release their regenerative potential and trigger a chain reaction giving rise to and magnifying a virtuous circle whose ultimate result is the healing of the lesion 9, 10, 11.
There are several GFs for this study we purposefully mention:
Platelet‐derived GF (PDGF): it plays a significant role in angiogenesis and mitosis, and also regulates other GFs.
Transforming GF‐beta (TGFβ): it plays a role in chemotaxis and activates fibroblasts and osteoblasts, whereas inhibits osteoclasts.
Insulin‐like GF 1 and 2 (IGF‐1 and IGF‐2): it acts on osteoblasts.
Epidermal GF (EGF): it stimulates epithelial and mesenchymal cells.
Vascular endothelial GF (VEGF) and fibroblast GF (FGF): they are involved in angiogenesis and stimulate endothelial cells.
PG is a haemocomponent obtained associating activated hyperconcentrated platelets and cryoprecipitate; it allows an exogenous in situ addition of GF improving tissue reparation and regeneration 5.
Previously, experimental and clinical studies documented that several drugs, such as glucocorticoid, and statins are able to modulate the cytokine and interleukin secretion inducing a modulation in cell proliferation or activation 12, 13, 14. Moreover, other studies revealed that drug–drug interactions are able to induce several clinical manifestations such as blood dysfunction 15. Therefore, these drugs may modulate the healing in our patients; however, as we are not able to document the role of each drug in the clinical response to PG, it is important to remark that all patients enrolled in our study presented several clinical disease and were treated with several drugs, without differences between the groups. In addition, in our study, there were no significant demographic differences between patients with healing wounds and those with non healing wounds, as shown in Table 2.
In our study, we documented that in 96·15% of patients, PG promoted the functional recovery of physiological tissue reparation after a TMA procedure. Therefore, we can assume that PG application may be an effective adjuvant treatment to improve wound healing in diabetic dysvascular patients.
Serra R, Buffone G, Dominijanni A, Molinari V, Montemurro R, de Franciscis S. Application of platelet‐rich gel to enhance healing of transmetatarsal amputations in diabetic dysvascular patients.
References
- 1. Pollard J, Hamilton GA, Rush SH, Ford LA. Mortality and morbidity after transmetatarsal amputation: retrospective review of 101 cases. J Foot Ankle Surg 2006;45:91–7. [DOI] [PubMed] [Google Scholar]
- 2. Anthony T, Roberts J, Modrall JG, Huerta S, Asolati M, Neufeld J, Parker B, Yang W, Sarosi G. Transmetatarsal amputation: assessment of current selection criteria. Am J Surg 2006;192:e8–11. [DOI] [PubMed] [Google Scholar]
- 3. Dudkiewicz I, Schwarz O, Heim M, Herman A, Siev-Ner I,. Trans‐metatarsal amputation in patients with a diabetic foot: reviewing 10 years experience. Foot 2009;19:201–4. [DOI] [PubMed] [Google Scholar]
- 4. Landry GJ, Silverman BS, Liem TK, Mitchell EL, Moneta GL. Predictors of healing and functional outcome following transmetatarsal amputations. Arch Surg 2011;146:1005–9. [DOI] [PubMed] [Google Scholar]
- 5. Crovetti G, Martinelli G, Issi M, Barone M, Guizzardi M, Campanati B, Moroni M, Carabelli A. Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci 2004;30:145–51. [DOI] [PubMed] [Google Scholar]
- 6. McKittrick LS, McKittrick JB, Risley TS. Transmetatarsal amputation for infection or gangrene in patients with diabetes mellitus. Ann Surg 1949;130:826–42. [PubMed] [Google Scholar]
- 7. Thomas SR, Perkins JM, Magee TR, Galland RB. Transmetatarsal amputation: an 8‐year experience. Ann R Coll Surg Engl 2001;83:164–6. [PMC free article] [PubMed] [Google Scholar]
- 8. Pollard J, Hamilton GA, Rush SM, Ford LA. Mortality and morbidity after transmetatarsal amputation: retrospective review of 101 cases. Journal of Foot and Ankle Surgery. 2006;45:91–7. [DOI] [PubMed] [Google Scholar]
- 9. D'Agostino E, Fratellanza G, Caloprisco G, Formisano P, Giacco F, Cavalcanti M, Perna E, Nigro M, Formisano S. Effetto del gel piastrinico sulla proliferazione cellulare in vitro. La Trasf del Sangue 2002;47:429–33. [Google Scholar]
- 10. Weiser L, Bhargava M, Attia E, Torzilli PA. Effect of serum and platelet‐derived growth factor on chondrocytes grown in collagen gels. Tissue Eng 1999;5:533–44. [DOI] [PubMed] [Google Scholar]
- 11. Lucarelli E, Beccheroni A, Donati D, Sangiorgi L, Cenacchi A, Del Vento AM, Meotti C, Bertoja AZ, Giardino R, Fornasari PM, Mercuri M, Picci P. Platelet‐derived growth factors enhance proliferation of human stromal stem cells. Biomaterials 2003;24:3095–100. [DOI] [PubMed] [Google Scholar]
- 12. Falone D, Gallelli L, Di Virgilio A, Tucci L, Scaramuzzino M, Terracciano R, Pelaia G, Savino R. Effects of simvastatin and rosuvastatin on matrix metalloproteinase 9 and NF‐kB in lung cancer and in normal pulmonary tissues. Cell Prolif 2012;48:212–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pelaia G, Gallelli L, Renda T, Fratto D, Falcone D, Caraglia M, Busceti MT, Terracciano R, Vatrella A, Maselli R, Savino R. Effects of statins and farnesyl transferase inhibitors on ERK phosphorylation, apoptosis and cell viability in non‐small lung cancer cells. Cell Prolif 2012;45:557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gallelli L, Pelaia G, Fratto D, Muto V, Falcone D, Vatrella A, Curto LS, Renda T, Busceti MT, Liberto MC, Savino R, Cazzola M, Marsico SA, Maselli R. Effects of budesonide on P38 MAPK activation, apoptosis and IL‐8 secretion, induced by TNF‐alpha and Haemophilus influenzae in human bronchial epithelial cells. Int J Immunopathol Pharmacol 2010;23:471–9. [DOI] [PubMed] [Google Scholar]
- 15. Siniscalchi A, Gallelli L, Calabrò G, Tolotta GA, De Sarro G. Phenobarbital/Lamotrigine coadministration‐induced blood dyscrasia in a patient with epilepsy. Ann Pharmacother 2010;44:2031–4. [DOI] [PubMed] [Google Scholar]


