Abstract
Advanced multimodal therapies are being used with increasing frequency in the management of difficult or complex wounds. Although the primary goal remains to expedite complete healing, secondary goals include avoidance of superimposed infection, repeated hospitalisations and subsequent amputations. We describe a case involving a limb‐ and life‐threatening necrotising infection in a diabetic patient in which we successfully applied negative pressure wound therapy, dermal replacement therapy and pulsed radio frequency energy to achieve definitive healing. Further study is warranted to elucidate the most effective combinations of such therapies to promote healing of similarly complex wounds.
Keywords: Complex wound, Diabetic foot, Multimodal therapies
INTRODUCTION
Necrotising fasciitis (NF), occasionally complicating chronic diabetic foot ulcers, is a severe and increasingly common condition that frequently requires amputation 1, 2, 3. NF is a potentially life‐threatening infection often caused by group A Streptococcus. These organisms aggressively invade superficial soft tissues (skin and subcutaneous fat) and extend to the deep fascia. NF can occur in any extremity following ostensibly minor trauma causing a breach in the integument. Very minor superficial epidermal breaches can act as a portal of entry for this severe infection (4). It is also important to realise that in its early stage NF can often be mistaken for simple cellulitis. A high index of suspicion is therefore required to diagnose this important clinical entity. Tenderness, erythema and raised local temperature are the common signs in the early stage (5). As the infection progresses, skin changes such as bullae become evident as well as signs of soft tissue gas on plain radiographs. In the diabetic patient, such infections can also complicate long standing neuropathic or neuroischaemic foot ulcerations. As limb‐ and life‐threatening hand and foot infections in diabetic patients account for a large proportion of amputations and a substantial number of deaths, NF infections must be recognised at their initial presentation and aggressively treated (6). These infections require emergent surgical care in order to control the ascending infection. Delay in the diagnosis and consequently delayed operative debridement has been shown in multiple studies to increase mortality in these patients 7, 8. Antimicrobial therapy, while necessary, is actually adjunctive to surgical treatment. Although wide incision and drainage is mandatory to appropriately manage the acute infection, partial foot or even major limb amputations are sometimes required. Accordingly, large soft tissue wounds remain after the infection has been controlled. Nonetheless, there is no widely accepted guideline for the treatment of these subsequent soft tissue defects and healing such wounds often presents a challenge to successful limb salvage.
In recent years a number of new or ‘advanced therapies' have become available to clinicians managing chronic or complex postsurgical wounds. Studies have shown that the percentage area reduction (PAR) in foot ulcer area after 4 weeks is a predictor of healing at 12 weeks. Four‐week PAR can therefore serve as a pivotal clinical decision point in the treatment algorithm of diabetic foot ulcers for early identification of patients who may not respond to standard care and may require advanced therapies 9, 10. Thus, it has been recommended that the failure to reduce the size of an ulcer at 4 weeks using standard wound care should prompt consideration of an advanced therapy (10). There have been only several studies on advanced wound care technologies that have proven their value in accelerating diabetic foot ulcer healing in prospective, randomised trials. These products include becaplermin (Regranex®, Systagenix Wound Management, Quincy, MA, USA), a living skin equivalent in the form of a bilayered skin substitute (Apligraf®, Organogenesis, Canton, MA) and a human fibroblast‐derived dermal substitute (Dermagraft®, Advanced BioHealing, La Jolla, CA) (10).
The term ‘multimodal therapies' has been adopted to reflect the common practice of using several such modalities either sequentially or concurrently to bring difficult wounds to final closure. Negative pressure wound therapy (NPWT), skin or dermal replacement therapies (DRT) and pulsed radio frequency energy (PRFE) are several of the advanced therapies commonly used in such situations. We herein describe such a case that required the combined use of multiple advanced therapies in order to salvage the limb of this high‐risk diabetic patient.
PATIENT HISTORY
A 61‐year‐old male veteran presented to our VAMC podiatry clinic with a chronic right plantar ulcer that had increased in size and severity over 5 days. He reported having had fever, nausea, chills and malaise over the preceding several days. His oral temperature on presentation was 100·2°F. The patient had a significant medical history of type 2 diabetes of greater than 10 years duration with documented peripheral neuropathy and loss of protective sensation (LOPS). Other comorbidities included hyperlipidaemia and hypertension. He denied a current or past history of smoking nor was there a prior history of peripheral arterial disease (PAD) or renal dysfunction.
Physical exam of the right foot showed erythema with cellulitis, oedema and a full thickness ulceration plantar to the first metatarsal head with purulence and malodor. Cellulitis and oedema extended proximal to the ankle and a haemorrhagic bulla was noted over the anterior distal leg. The ulcer had direct exposure of bone and tendon at its base with an abundance of necrotic soft tissue on its periphery (Figure 1). It tracked proximally into the first interspace and crepitance was felt on range of motion of the great toe. Pedal pulses were lightly palpable through the oedema and Doppler examination confirmed biphasic arterial signals. Radiographs showed gas within the soft tissues that extended proximally to the anterior and lateral compartments of the leg (Figure 2) as well as erosion and cortical reaction of the first metatarsal and hallux. Upon admission, the WBC count was 36 000/µl and his haemoglobin was 8·8 g/dl. The patient was also in acute renal failure with a BUN of 68 mg/dl, creatinine of 2·9 mg/dl and an estimated glomerular filtration rate (eGFR) of 22 ml/minute. Diabetes was not well controlled as evidenced by a haemoglobin A1c level of 9·5% and a presenting blood glucose of 312 g/dl. A deep tissue culture was taken of the wound and preliminary Gram stain showed gram positive cocci, gram negative cocci, gram negative bacilli and anaerobic gram negative bacilli.
Figure 1.
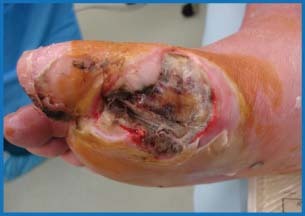
Initial presentation of the wound. Note the exposed tendon and bone with peripheral tissue necrosis.
Figure 2.
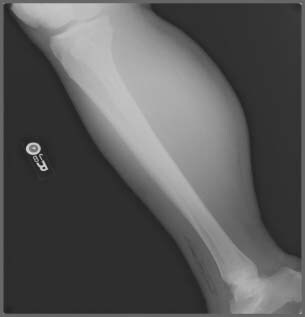
Lateral leg radiograph at initial presentation. Note the air within the soft tissues in the anterior compartment.
Our working diagnosis was an acute necrotising soft tissue infection (non clostridial gas gangrene) with ascending fasciitis of the right foot and leg and accompanying bacteremia/ sepsis. Osteomyelitis of the right hallux and distal first metatarsal was also evident.
TREATMENT AND OUTCOME
The patient was admitted and treated emergently with a partial first ray open amputation and anterior leg fasciotomy for drainage and debridement of necrotic tissue. Intravenous broad spectrum antimicrobial therapy with vancomycin, piperacillin/tazobactam and metronidazole was administered concurrently. After initial aggressive surgical control of the acute infection, the forefoot developed further gangrenous changes because of persistent infection. Additional operative debridement including an open transmetatarsal amputation (TMA) was subsequently performed several days later (Figure 3). Surgical tissue cultures grew group A beta‐haemolytic streptococci, enterococcus and enterobacter species. No anaerobic organisms were recovered. His parenteral antimicrobial therapy was then narrowed to Ertapenem 1 g every 24 hours.
Figure 3.
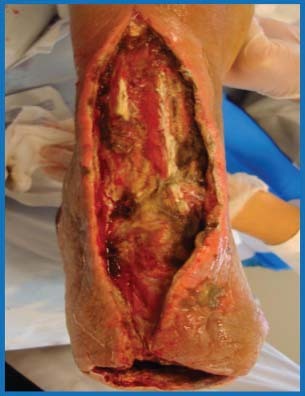
Wounds after an open trans‐metatarsal amputation and anterior compartment incision and drainage.
Although the infection had been adequately controlled, the wounds required periodic operative debridement over the next several weeks. His postsurgical wounds were managed with NPWT (V.A.C®.; KCI, San Antonio, Texas, USA) and changed every 48 hours. Once the patient's acute sepsis and renal dysfunction had been stabilised, he was transferred to a skilled nursing facility and was seen in our clinic weekly. By week 4, the patient was discharged to home where his NPWT dressings continued with the help of home nursing services. Our long‐term plan was to improve the quality of the wounds to the point that split thickness skin grafts might be applied to expedite definitive healing. Concurrently, we expected to revise the open TMA to a more proximal level (Chopart or Syme) because of the soft tissue defects remaining on his distal and plantar foot.
Through several weeks of VAC® therapy, a granular wound base was obtained. At 8 weeks, dermal replacement therapy (DRT) with Dermagraft® (Advanced BioHealing) was initiated (Figure 4). PRFE with twice daily Provant® (Regenesis Biomedical, Inc., Scottsdale, AZ) therapy was initiated concurrently with the DRT tissue substitute. Weekly consecutive Dermagraft® applications were applied to the wounds in the clinic for a total of 14 weeks with continued twice daily use of Provant® by the patient as an outpatient (see Table 1 for therapeutic timelines).
Figure 4.
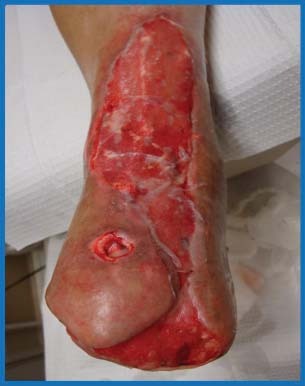
Presentation of wounds after 8 weeks of NPWT therapy. Note the beefy red granular wound base that is now ready for combination therapy.
Table 1.
Timelines

Figure 5 shows the distal TMA wound after 5 weeks of multimodal therapy with Provant®, Dermagraft® and VAC® therapies. Figure 6 shows the anterior wound at 9 weeks (when NPWT was discontinued). Figure 7 shows closure of the distal wound after 14 weeks of combination therapy. Through the use of this multimodal therapy, the anterior ankle wound had been reduced to a size appropriate for a small split thickness skin graft at 24 weeks (Figure 8). Because of the extended period of non weightbearing in a posterior splint and the loss of insertions for his anterior muscle group, the patient developed a fixed equinus deformity. Therefore, a Tendo‐Achilles Lengthening (TAL) was also performed to take the foot out of fixed equinus position. Figure 9 shows complete closure of all wounds by week 39. After initially transitioning to a fixed ankle removable walking brace, the patient currently independently ambulates in therapeutic footwear with custom multidensity insoles.
Figure 5.
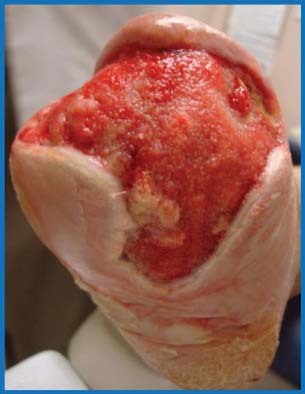
Distal transmetatarsal amputation wound after 5 weeks of combination therapy.
Figure 6.
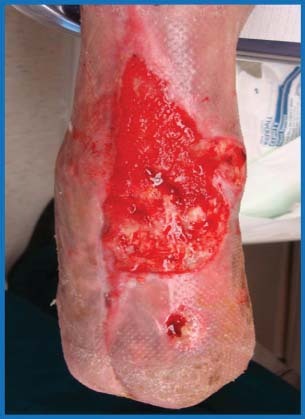
Anterior leg wound after 9 weeks of combination therapy.
Figure 7.
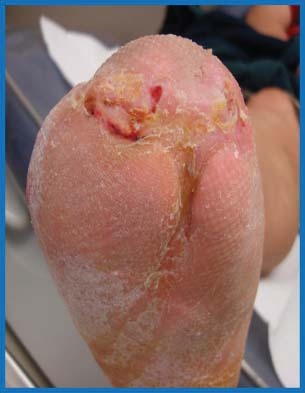
Closure of distal transmetatarsal amputation wound after 14 weeks of combination therapy.
Figure 8.
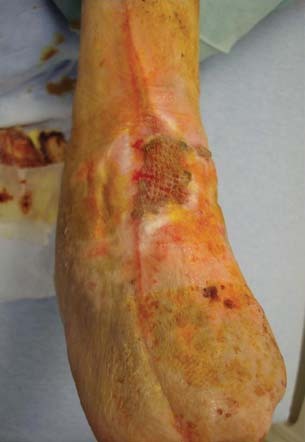
Anterior leg wound after 24 weeks of combination therapy. The wound was now at appropriate size for a split thickness skin graft to be applied.
Figure 9.
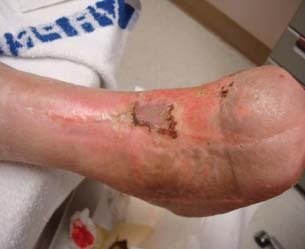
Week 39: complete closure of all wounds with the use of combination therapy.
DISCUSSION
NF is a relatively rare, rapidly progressive life‐threatening infectious process. The most common comorbidity associated with this condition is now diabetes mellitus (1). Its prognosis depends upon rapid diagnosis and immediate surgical treatment (1). The aggressive surgical methods used to treat NF leave wounds that are particularly difficult to manage when they occur in the extremities of the diabetic patient. Patients with this presentation often require extended hospitalisation with revision of emergent open amputations as well as extensive skin grafting while still being at risk for losing the involved limb. In order to optimise limb salvage and minimise the need for further hospitalisation and surgery, we showed multi‐modality wound therapy to effect rapid healing. NPWT has long been a mainstay for our management of chronic wounds and, especially, for large postsurgical defects. On the basis of its known positive impact on dermal fibroblasts, we were also particularly interested in the possibility that PRFE would stimulate both endogenous wound healing and the activity of the exogenous fibroblasts in the dermal tissue substitute 5, 11.
As summarised in several guidelines, chronic non healing diabetic lower extremity wounds, such as diabetic foot ulcers, are a common medical problem that may precede severe complications such as infection, limb loss and death 10, 12, 13, 14, 15, 16. Current standard methods of treatment are aimed at removing necrotic debris, controlling infection, revascularisation and relieving chronic pressure on the wound (10). Unfortunately, healing rates are poor with standard treatment. Prolonged healing times increase the risk for morbidities, hospitalisations and amputations, therefore prompt wound closure should be the primary goal in diabetic foot ulcer treatment (10). Chronic wounds differ from acute ones and are commonly complicated by local ischaemia, necrotic tissue and high bacterial loads that can each impede healing. Prolonged recruitment of macrophages and neutrophils to a wound can elicit a continued inflammatory response leading to the production of excessive inflammatory cytokines and matrix metalloproteinases (MMPs) (17). This wound environment harbours senescent cells leading to growth factor deficiencies, faulty receptor site functions and poor cell proliferation (17). Debridement, infection and inflammation control, moisture control and excision of non viable wound edges are modalities utilised to address this abnormal wound environment (10). It has been recommended that wounds should be re‐evaluated at 4 weeks after administering standard wound care, because failure to reduce the size of an ulcer after 4 weeks should prompt consideration of an advanced therapy 9, 10. Advanced modalities include DRT, negative pressure therapies, electrical stimulation, staged surgical intervention and the use of skin grafts. In the case study discussed multiple incision and drainages were performed, NPWT, dermal replacement therapy, PRFE and an autogenous split thickness skin graft were all used adjunctively to achieve definitive wound closure.
Dermagraft® is an allogeneic, neonatal‐derived bioengineered tissue comprised of dermal fibroblasts seeded onto an absorbable mesh substrate (11). The fibroblasts secrete extra‐cellular matrix components including collagen and glycosaminoglycans. The scaffold that the dermal fibroblasts grows over during the production process biodegrades over a 1‐ to 2‐week time period leaving only cellular components and proteins in the wound bed (11). The fibroblasts produce a diverse array of cytokines involved in the wound healing process including growth factors, interleukins and angiogenic factors (17).
Negative pressure wound therapy utilising reticulated open cell foam (NPWT/ROCF) is generally indicated for the management of chronic, acute, traumatic pressure and diabetic ulcers, partial‐thickness burns, grafts and flaps 12, 16. NPWT/ROCF unique mechanisms of action provides a closed, moist wound environment while removing fluids and infectious materials to reduce oedema and decrease the bioburden. It also promotes granulation tissue formation through cell migration and proliferation and the mechanical forces draw the wound edges together 13, 14. Clinical studies have shown that NPWT stimulates angiogenesis and a threefold to fivefold increase in cutaneous blood flow adjacent to the wound edges; this increases the availability of oxygen and vital nutrients needed for tissue regeneration (14). Healthcare professionals have been advised to select patients for NPWT carefully, recognising that not all wounds are appropriate for such treatment. Patients should be monitored frequently in an appropriate care setting by a trained practitioner and practitioners should be vigilant for potentially life‐threatening complications such as bleeding and be prepared to take prompt action if they occur (18).
As described by George et al. (19), PRFE provides a novel biophysical stimulus that involves the use of a low‐level, confined, radiofrequency signal to promote wound treatment via fibroblast and epithelial cell migration and proliferation. This novel bioactive technology is based on the concept of endogenously stimulating the cellular processes that initiate proliferation of fibroblasts and keratinocytes with subsequent induction of granulation and epithelialisation leading to wound closure. The technology is based on the mitogenic (i.e. cell‐cycle stimulating) properties of a low‐level, confined, high‐frequency, electromagnetic field. PRFE has recently been shown to be effective in the treatment of recalcitrant pressure ulcers as well as diabetic foot ulcers 19, 20. The effect of this treatment has been shown in a number of in vitro studies 21, 22, 23. More recently, the PRFE technology has been shown to induce a number of genes associated with normal wound healing 24, 25.
The Provant® Therapy System (Regenesis Biomedical, Inc.) is indicated for adjunctive use in the palliative treatment of postoperative pain and oedema in superficial soft tissue. While there have not yet been any randomised controlled trials involving the use of this therapy on chronic ulcers, our own off‐label clinical results in this setting have been favourable (21).
Poor glucose control as evidenced by haemoglobin A1c above 8% is an independent predictor of poor wound healing (26). The patient described in this report presented with a haemoglobin A1c of 9·5 as well as an extensive necrotising infection with septicaemia and acute renal insufficiency. Fortunately, there was no accompanying peripheral arterial insufficiency. Wounds in this type of patient often require additional surgery as well as extended hospital stays, with limb salvage by no means a certainty. In the case reported here, we were able to salvage the limb of a diabetic patient recovering from NF without additional amputation by using outpatient multimodal therapies. Specifically, we showed a novel PRFE treatment in addition to established treatment modalities that included NPWT and the use of a dermal tissue substitute.
CONCLUSION
A case of limb‐threatening necrotising infection in a diabetic patient has been presented. Multimodal advanced wound therapies appear to have resulted in progressive healing with limb salvage. The addition of PRFE, a technology known to stimulate genes associated with wound healing, ostensibly acted synergistically with the neonatal fibroblasts in the dermal replacement product as well as with the NPWT. Further study is warranted to investigate this possibility.
REFERENCES
- 1. Dworkin MS, Westercamp MD, Park L, McIntyre A. The epidemiology of necrotizing fasciitis including factors associated with death and amputation. Epidemiol Infect 2009;137:1609–14. [DOI] [PubMed] [Google Scholar]
- 2. Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, LeFrock JL, Lew DP, Mader JT, Norden C, Tan JS. Infectious Diseases Society of America Diagnosis and treatment of diabetic foot infections. Plast Reconstr Surg 2006;117(7 Suppl):212S–38S. [DOI] [PubMed] [Google Scholar]
- 3. Zgonis T, Stapleton JJ, Girard‐Powell VA, Hagino RT. Surgical management of diabetic foot infections and amputations. AORN J 2008;87:935–46; quiz 947–50. [DOI] [PubMed] [Google Scholar]
- 4. Liu Y, Chi C, Ho M, Chen C. Microbiology and factors affecting mortality in necrotizing fasciits. J Microbiol Immunol Infect 2005;38:430–5. [PubMed] [Google Scholar]
- 5. Jain A, Varma A, Mangalanandan Kumar PH, Bal A. Surgical outcome of necrotizing fasciitis in diabetic lower limbs. J Diabetic Foot Comp 2009;1:80–84 [Google Scholar]
- 6. Bahebeck J, Sobgui E, Loic F, Nonga BN, Mbanya JC, Sosso M. Limb‐threatening and life‐threatening diabetic extremities: clinical patterns and outcomes in 56 patients. J Foot Ankle Surg 2010;49:43–6. [DOI] [PubMed] [Google Scholar]
- 7. Wang K‐C, Shih CH. Necrotizing fasciitis of the extremities. J Trauma 1992;32:259–64. [DOI] [PubMed] [Google Scholar]
- 8. Voros D, Pissiotis C, Georgantas D, Katsaragakis S, Antoniou S, Papadimitriou J. Role of early and aggressive surgery in the treatment of severe necrotizing soft tissue infections. Br J Surg 1993;80:1190–1. [DOI] [PubMed] [Google Scholar]
- 9. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4‐week period is a robust predictor of complete healing in a 12‐week prospective trial. Diabetes Care 2003;26:1879–82. [DOI] [PubMed] [Google Scholar]
- 10. Snyder R, Kirsner R, Warriner R, Lavery LA, Hanft J, Sheehan P. Consensus recommendations on advancing the standard of care for treating neuropathic foot ulcers in patients with diabetes. Ostomy Wound Manage 2010; 56(Suppl 4):S1–S24. [PubMed] [Google Scholar]
- 11. Marston WA. Dermagraft, a bioengineered human dermal equivalent for the treatment of chronic nonhealing diabetic foot ulcer. Expert Rev Med Devices 2004;1:21–31. [DOI] [PubMed] [Google Scholar]
- 12. Sumio B, Driver V, Gibbons G, Hollowat G, Joseph W, Lavery L, McGuigan F, Steinberg J, Andersen C, Blume P, Attinger C. A multidisciplinary approach to limb preservation: the role of V.A.C. therapy. Wounds 2009; (Suppl):1–20. 25904579 [Google Scholar]
- 13. Orgill DP, Manders EK, Sumpio BE, Lee RC, Attinger CE, Gurtner GC, Ehrlich HP. The mechanics of action of vacum assisted closure: more to learn. Surgery 2009;146:40–51. [DOI] [PubMed] [Google Scholar]
- 14. Bollero D, Driver V, Glat P, Gupta S, Lázaro‐Martínez JL, Lyder C, Ottonello M, Pelham F, Vig S, Woo K. Ostomy Wound Management 2010;56(5 Suppl):1–18. [Google Scholar]
- 15. Frykberg RG, Zgonis T, Armstrong DG, Driver VR, Giurini J, Kravitz S, Landsman AS, Lavery LA, Moore JC, Schuberth JM, Wukich DK, Andersen C, Vanore J. Diabetic foot disorders: a clinical practice guideline (2006 revision). J Foot Ankle Surg 2006;45(Suppl 5):S2–S66. [DOI] [PubMed] [Google Scholar]
- 16. Andros G, Armstrong DG, Attinger C, Boulton AJM, Frykberg RG, Joseph W, Lavery LA, Morbach S, Niezgoda JA, Toursarkissian B. Consensus statement on negative pressure wound therapy (V.A.C. therapy) for the management of diabetic foot wounds. Ostomy Wound Manage 2006; 52(6 Suppl):1–32. [PubMed] [Google Scholar]
- 17. Naughton G, Mansbridge J, Gentskow G. A metabolically active human dermal replacement for the treatment of diabetic foot ulcers. Artif Organs 21:1203–10. [DOI] [PubMed] [Google Scholar]
- 18. Negative Pressure Wound Therapy (NPWT) systems – Preliminary Public Health Notification. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm190704.htm [accessed 10 March 2010].
- 19. George FR, Lukas RJ, Moffett J, Ritz MC. In‐vitro mechanisms of cell proliferation induction: a novel bioactive treatment for accelerated wound healing. Wounds 2002;14:107–15. [Google Scholar]
- 20. Larsen JA, Overstreet J. Pulsed radio frequency energy in the treatment of complex diabetic foot wounds: two cases. J Wound Ostomy Continence Nurs 2008;35:523–7. [DOI] [PubMed] [Google Scholar]
- 21. Frykberg R, Tierney E, Tallis A, Klotzbach T. Cell proliferation induction: healing chronic wounds through low‐energy pulsed radiofrequency. Int J Low Extrem Wounds 2009;8:45–51. [DOI] [PubMed] [Google Scholar]
- 22. Cleary SF, Liu LM, Merchant RE. In vitro lymphocyte proliferation induced by radio‐frequency electromagnetic radiation under isothermal conditions. Bioelectromagnetics 1990;11:47–56. [DOI] [PubMed] [Google Scholar]
- 23. Gilbert TL, Griffin N, Moffett J, Ritz MC, George FR. The Provant Wound Closure System induces activation of p44/42 MAP kinase in normal cultured human fibroblasts. Ann N Y Acad Sci 2002;961:168–71. [DOI] [PubMed] [Google Scholar]
- 24. Moffett JGN, Ritz M, George F. The effects of a pulsed radio frequency electromagnetic field on selected genes. Symposium on Advances in Wound Care. San Diego, CA, 2008.
- 25. Moffett JGN, Ritz M, George F. Increased gene expression in cultured human dermal fibroblasts in response to a pulsed radio frequency electromagnetic field and implications in chronic wound healing. Symposium on Advances in Wound Care, San Diego, CA, 2008.
- 26. Younger AS, Awwad MA, Kalla TP, de Vries G. Risk factors for failure of transmetatarsal amputation in diabetic patients: a cohort study. Foot Ankle Int 2009;30:1177–82. [DOI] [PubMed] [Google Scholar]


