Abstract
A bipedicle ischaemic rat skin flap model was used to study the effects of daily topical applications of platelet‐derived growth factor (PDGF) on the healing of ischaemic wounds. Levels of tumour necrosis factor‐alpha (TNFA), interleukin 1‐beta (IL1B) and both the latent and active forms of matrix metalloproteinase 2 (MMP2) and 9 (MMP9) were measured. Full‐thickness wounds were made on a total of 72 adult male Sprague–Dawley rats. Each group of 18 rats with normal and ischaemic wounds received either vehicle or 0·01% recombinant PDGF‐BB. Additional applications were made on the wounds on a daily basis. Wound areas were measured at 0, 1, 3, 5, 7 9 and 13 days after wounding. Ischaemia caused a delay in wound healing as well as an increase in TNFA, IL1B and both the pro and active forms of MMP2 and MMP9. PDGF accelerated the rate of wound healing in both normal and ischaemic wounds and negated the effect of ischaemia. PDGF reduced the TNFA concentration in both normal and ischaemic wounds, and the rate of wound healing closely resembled the pattern of TNFA protein expression. PDGF also reduced both the magnitude and duration of the increases in IL1B and both the pro and active forms of MMP2 and MMP9 induced by ischaemia.
Keywords: Matrix metalloproteinases, Platelet‐derived growth factor, Rat ischaemic skin model, Tumour necrosis factor‐alpha, Wound healing
Introduction
Wound healing is a complex process that integrates multiple metabolic pathways and cell types, and therefore, successful wound healing depends on precise spatiotemporal gene regulation 1. Perturbations that lead to dysregulation of this process disrupt normal wound healing, resulting in pathogenesis 2, 3. One such example, the chronic non‐healing wound, is an important cause of morbidity and mortality in surgical and medical patients 4, 5, 6. Chronic wounds are generally associated with other underlying medical conditions, including diabetes, trauma, peripheral vascular insufficiency, vasculitis, radiation injury, neuropathy, venous stasis, and most commonly, hypoxia 7.
Chronic non‐healing wounds of different aetiologies are characterised by having elevated levels of pro‐inflammatory cytokines and matrix metalloproteinases (MMPs) in both wound fluid and tissue 8. The release of pro‐inflammatory cytokines, particularly, tumour necrosis factor‐alpha (TNFA) and interleukin 1‐beta (IL1B) is an early response to tissue injury 1, 9. TNFA and IL1B exert their effect by stimulating the production of MMPs in fibroblasts. During wound healing MMPs play two critical roles. First, they degrade damaged components of the extracellular matrix (ECM), which ultimately allows for cellular adherence and lying down of the basement membrane; second, they are responsible for remodelling of the new ECM 8. However, for wound healing to be successful, the levels and duration of protease expression must be tightly controlled. Elevated levels of MMP2 and MMP9 have been observed in chronic wounds, leading to the speculation that overproduction of MMPs results in degradation of components necessary for normal wound healing 10, 11, 12.
Until recently, treatment strategies for chronic wounds were limited to preparation of the wound bed and included removing necrotic tissue by debridement, controlling infection and inflammation, maintaining optimal moisture balance and evaluating the edge of wounds 13. While these strategies are of benefit, they are only partially successful because they fail to target the pathophysiologic and molecular cause of most chronic wounding 14, 15, 16. Recently, animal models and human clinical studies have confirmed that exogenous application of platelet‐derived growth factor (PDGF) accelerates wound healing. As a result, topical application of recombinant human PDGF (PDGF‐BB) is now approved as a treatment for diabetic foot ulcers 17.
Studying the factors that regulate wound healing is key to understanding and maintaining health. Therefore, the purpose of this study was to test PDGF as a potential therapeutic agent for the healing of chronic ischaemic wounds and to monitor levels of TNFA, IL1B, MMP2 and MMP9 in order to develop a more mechanistic understanding of the effects of PDGF on wound healing.
Methods
Rat ischaemic skin wound model
For this procedure, we used our previously developed ischaemic bipedicle rat skin flap model 18. All the animal surgeries were conducted using isoflurane as an inhalation anaesthetic. Rats were initially anaesthetised with a higher induction dose in an induction chamber followed by a lower maintenance dose administered via a nose cone. All animal experiments were conducted under a protocol approved by the University of Florida Animal Care and Use Committee.
The dorsal aspect of 72 adult male Sprague–Dawley rats, obtained from Charles River Laboratories International, Inc., weighing on average 250–300 g, was shaved. An 11 × 2·5 cm rectangular template was centred on the spine and positioned between the base of the scapula and the iliac crest of each rat. The template contained six circular holes arranged with two adjacent holes in each of three rows. The holes were located at 2·5, 5·2 and 7·8 cm from the longitudinal end of the template and 3 mm from the lateral edges. Location of the holes was traced on the dorsal rat skin. The skin was folded to superimpose the two circles of each row and a wooden dowel was placed against one side of the skin. Under strict aseptic conditions, three pairs of full‐thickness wounds were created using a 6‐mm punch biopsy fitted to an electric drill. These normal skin punch biopsies, which served as day 0 samples, were immediately frozen in liquid nitrogen and then transferred to a −80°C freezer for future biochemical analyses.
Two parallel linear incisions were made along the long side of the template and extended to just above but not including the panniculus carnosus muscle layer. The skin flap was then elevated and the underlying vasculature was severed between the panniculus carnosus and the deep fascia of the skin. The skin flap was then repositioned and stapled on each lateral side. In so doing, this procedure created a condition of transient ischaemia.
Treatment application and sample collection
The area of each wound was traced on a transparent acetate sheet and was considered as the initial wound size. All of the wounds in each group of rats received one of two treatments, Regranex gel (Healthpoint Biotherapeutics, Forth Worth, TX) that contained 0·01% recombinant human PDGF‐BB in carboxymethylcellulose gel or 50 µl of 1% carboxymethylcellulose gel (Johnson and Johnson, New Brunswick, NJ; vehicle for Regranex that served as the control). In group 1, rats with ischaemic wounds were all treated with vehicle (1% carboxymethylcellulose gel); in group 2, rats with ischaemic wounds were all treated with Regranex gel (Healthpoint Biotherapeutics); in group 3, rats with normal wounds were all treated with vehicle; and in group 4, rats with normal wounds were all treated with Regranex gel. Treatments were topically applied to each of the six full‐thickness punch wounds. Following treatment, rats were housed in the animal care facility, assigned two per cage and given unrestricted food and water. Post‐surgery follow‐up was done on a daily basis and the respective groups were given an additional dose of either the control or PDGF‐BB. On post‐surgery days 1, 3, 5, 7, 9 and 13, three rats from each of the four treatment groups were anaesthetised. The size of each wound was traced and photographed. Tissue biopsies were collected from each of the original wounds using an 8‐mm punch biopsy. Tissue samples were frozen immediately and stored in a −80°C freezer for future biochemical analyses. After tissue collection, animals were euthanised by injecting 0·3 ml or >150 mg/kg of Beuthanasia‐D (sodium pentabarbitol 390 mg/ml, sodium phenytoin 50 mg/ml) intraperitoneally.
Analysis of wound size
Acetate traces of each wound were enlarged 200% using a photocopier, and the paper images were digitised using a high‐resolution HP Scan Jet 3C Scanner (Hewlett‐Packard, Palo Alto, CA). The size of each wound was calculated using the NIH ImageJ Program (http://rsbweb.nih.gov/ij/). Mean wound areas and standard error of the mean were expressed as mm2. The time required for each treatment to obtain 50% wound closure was interpolated from graphs of mean wound size (Figure 1).
Figure 1.
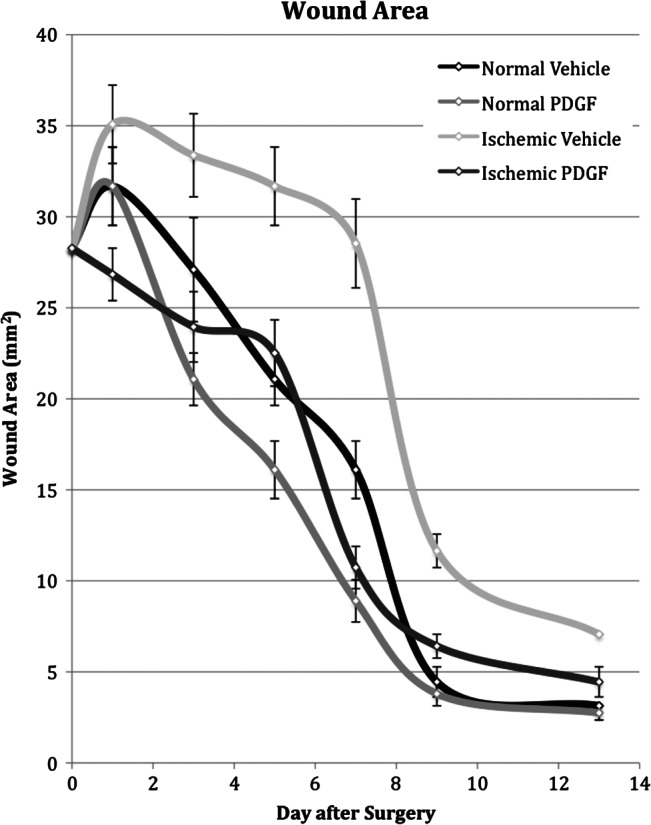
Measurement of wound areas in normal and ischaemic wounds treated with vehicle or platelet‐derived growth factor (PDGF). Wounds were treated with daily topical application of placebo (n = 18) or PDGF (n = 18). The wound areas were then measured on days 1, 3, 5, 7, 9 and 13 after surgery.
Biochemical analyses of biopsy tissue
Four of six skin punch biopsies from each rat were removed from the freezer, weighed, cut into small pieces, pooled and homogenised with a 15 ml glass homogeniser in phosphate buffered saline (PBS) containing 0·1% Triton‐X‐100 at a ratio of 1 ml buffer per gram of tissue. Homogenates were transferred to 1·5 ml microcentrifuge tube and centrifuged at 1700 g for 10 minutes at 4°C. Following centrifugation, the clear supernatant was collected and stored at −80°C for future use. Aliquots of each sample were used in enzyme‐linked immunosorbent assays (ELISA) to determine the level of TNFA (Quantikine, R&D Systems, Minneapolis, MN) and IL1B (Biotrak, Amersham Pharmacia, UK). All samples were run in duplicate and the concentrations (pg/ug total protein) of TNFA and IL1B were determined from standard curves generated from recombinant rat TNFA and IL1B included with the respective ELISA kits.
Aliquots of each sample were also analysed for the latent and active form of both MMP2 and MMP9 by gelatin zymography following the manufacturer's protocol (Invitrogen, Carlsbad, CA) and as previously described by Ladwig et al. 19 The amount (ng of MMP/ml homogenate) of protease was calculated as nanogram of MMP per milliliter of homogenate.
Statistical analyses
A one‐way analysis of variance (ANOVA) was conducted to evaluate the impact of the four treatments (TNFA, IL1B, MMP‐2 and MMP‐9 protein) on wound size for all three replications for each sample day. Post‐hoc comparisons were then conducted using Tukey's honestly significant difference (HSD) test to determine if group differences were significant. All statistical tests were performed using STATISTICA/Mac (StatSoft™, 1995).
Results
Wound healing
Typical wound healing in this study was represented by the normal wounds that received only vehicle gel (Figure 1). Normal wounds increased slightly in size immediately after wounding and then steadily decreased until day 9, when there was approximately 85% closure of the wound. Over the remaining sampling days, the rate of healing slowed considerably and by day 13, the last sampling day, there was approximately 92% wound closure. Normal wounds reached 50% closure in slightly less than 7 days.
Both ischaemia and PDGF affected the rate of wound healing (Figure 1). Ischaemia slowed the rate of wound healing, particularly between days 5 and 13. On day 7, ischaemic wounds were still as large as the initial wounds. Ischaemic wounds required 9 days to reach 50% closure and after 13 days, were still significantly larger than normal wounds.
Daily topical application of PDGF had the opposite effect on wounds; it accelerated the rate of healing. In normal wounds, PDGF accelerated the rate of healing, which was statistically significant on day 7 (Figure 1), and reduced the time for 50% wound closure to just under 6 days, approximately 1 day sooner than in normal wounds (P < 0·005). Similarly, PDGF‐treated ischaemic wounds were significantly smaller than vehicle‐treated ischaemic wounds on days 3 through 13 and reached 50% wound closure 2 days sooner, on day 7 (P < 0·005). Thus, the net effect of PDGF was to increase the rate of wound healing in normal wounds and increase the rate of healing in ischaemic wounds to that of normal untreated wounds.
TNFA concentration in wound tissue
The TNFA protein concentration in unwounded tissue was very low and increased as a result of wounding (Figure 2). The TNFA concentration in wound tissue increased to a maximum 3 days after wounding, representing an approximate sevenfold increase above the concentration in unwounded tissue. After day 3, the TNFA concentration gradually decreased and returned to a level slightly above its original unwounded concentration on day 7.
Figure 2.
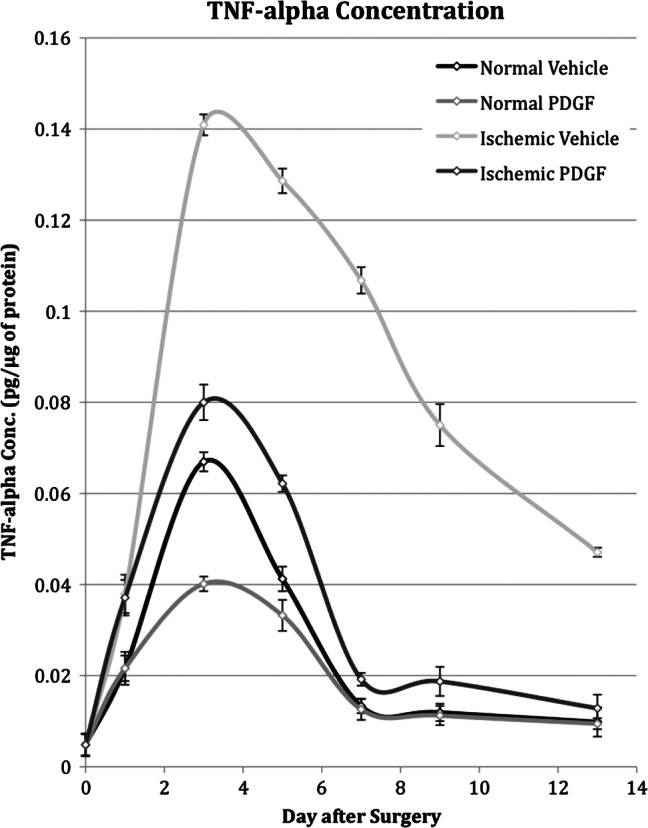
Tumour necrosis factor‐α (TNF‐α) concentration in normal and ischaemic wounds treated with platelet‐derived growth factor (PDGF) or placebo on days 0, 1, 3, 5, 7, 9 and 13 after surgery.
Both ischaemia and PDGF affected TNFA concentration in wound tissue. Ischaemia caused an increase in TNFA concentration and, similar to the expression pattern in normal wounds, it reached a maximum level 3 days after wounding. At its maximum, however, the TNFA concentration in ischaemic wounds was 14‐fold higher than the concentration in unwounded tissue and 2‐fold higher than the concentration in normal wounds. From its peak on day 3, the TNFA concentration in ischaemic wounds decreased slowly and on day 13 was still fivefold higher than the level observed in normal wounds. In contrast, daily topical application of PDGF decreased the TNFA concentration in both ischaemic and normal wounds (Figure 2). In ischaemic wounds treated with PDGF, there was a large (two‐ to five‐fold) reduction in the concentration on all sampling days except the first. By day 7, the TNFA concentration had essentially returned to normal wound levels. Thus, the net effect of PDGF was to lower the peak expression level in ischaemic wounds to near normal wound levels. In normal wounds, PDGF application also caused a substantial reduction in TNFA concentration on days 1, 3 and 5. At its maximum on day 3, the TNFA concentration of treated normal wounds was approximately half of that observed in normal wound tissue.
IL1B concentration in wound tissue
Relative to the concentration in unwounded tissue, wounding caused a twofold decrease in IL1B protein concentration in the first 24 hours (Figure 3). Over the remaining 12 days the concentration continued to decline but at a much slower rate.
Figure 3.
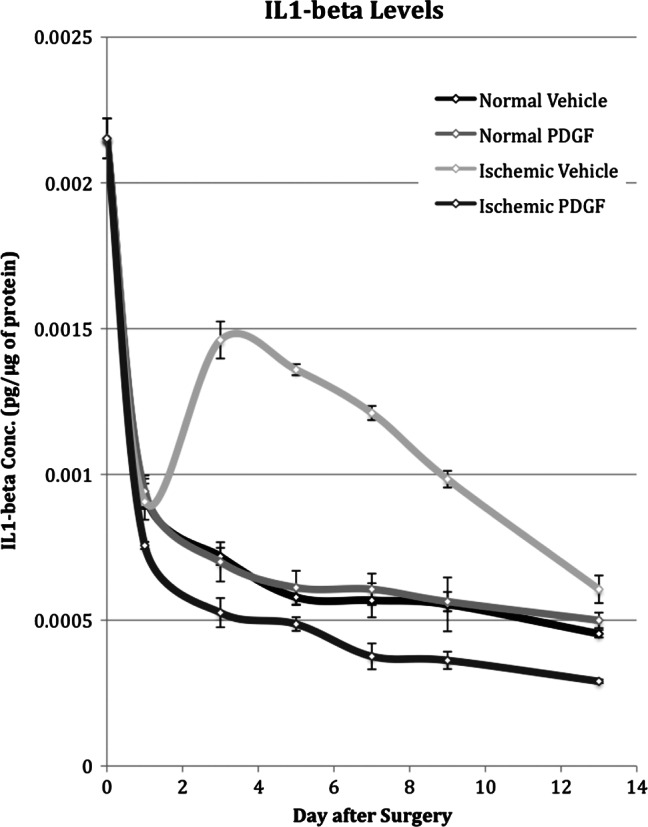
Interleukin‐1β (IL‐1β) concentration in normal and ischaemic wounds treated with platelet‐derived growth factor (PDGF) or placebo on days 0, 1, 3, 5, 7, 9 and 13 after surgery.
Between days 1 and 3, the IL1B concentration increased twofold relative to the concentration in normal wounds. From its peak on day 3, the concentration steadily decreased and eventually returned to the level in normal wound tissue on day 13.
PDGF had no effect on the concentration in normal wounds but did cause a reduction in ischaemic wounds. Although PDGF had no effect on the IL1B concentration in normal wounds, it was able to more than compensate for the effect of ischaemia and reduce the concentration to levels below that in normal wounds (Figure 3).
MMP2 concentration in wound tissue
During the first day after wounding, the concentration of pro‐MMP2 decreased between two‐ and three‐fold but remained stable thereafter (Figure 4). In contrast, the active form of MMP2 increased slightly during the first 24 hours after wounding. This increase was short lived and the activity returned to approximately normal wound levels between days 3 and 5 (Figure 5).
Figure 4.
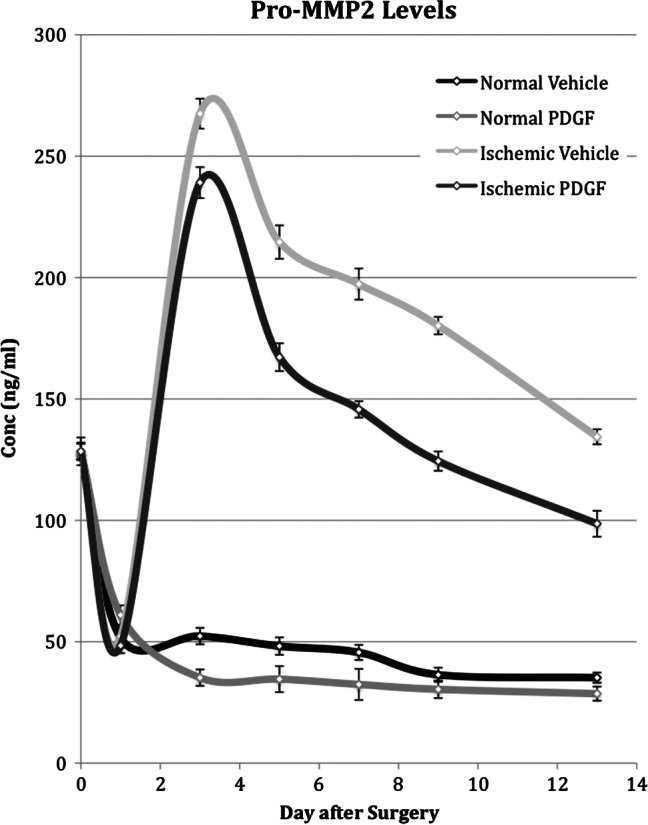
Time course of pro‐metalloproteinase 2 (pro‐MMP2) levels measured in both normal and ischaemic wounds treated with placebo or with platelet‐derived growth factor (PDGF). Pro‐MMP2 levels in normal and ischaemic wounds treated with placebo or PDGF were determined by gelatin zymography on days 1, 3, 5, 7, 9 and 13 after surgery.
Figure 5.
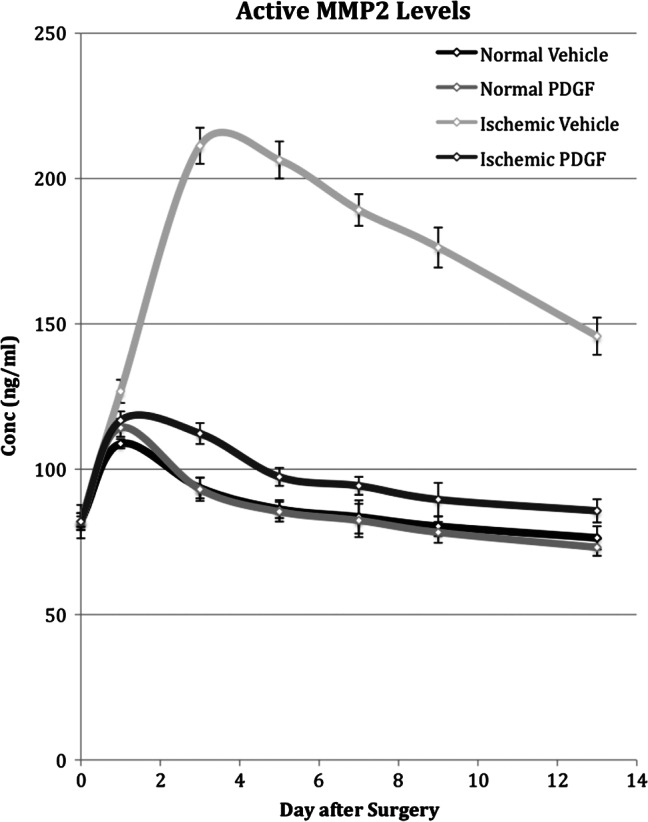
Time course of active metalloproteinase 2 (MMP2) levels measured in normal and ischaemic wounds treated with placebo or platelet‐derived growth factor (PDGF). Active MMP2 levels in normal and ischaemic wounds treated with placebo or PDGF was determined by gelatin zymography on days 1, 3, 5, 7, 9 and 13 after surgery.
Ischaemia caused a large and more long‐lasting increase in levels of both the pro and active forms of MMP2 (Figures 4 and 5). Peak activity was reached on day 3 and represented an approximate five‐ and two‐fold increase above the level in normal wounds for the pro and active forms of MMP2, respectively. Although activity steadily decreased after day 3, the concentration of both pro and active forms on day 13 still remained twofold higher than that in normal wound tissue.
PDGF application to normal wounds had little effect on pro‐MMP2 levels and no effect on active MMP2 levels. PDGF application did cause a decrease in the level of both pro‐ and active MMP2 in ischaemic wounds (Figures 4 and 5). In the case of the pro form, despite a decrease, PDGF application did not overcome the effect of ischaemia and the expression mimicked the pattern in ischaemic tissue, peaking on day 3 and remaining twofold higher than the level in normal wounds on day 13. For the active form, however, PDGF application reduced MMP2 levels close to those observed in normal wounds and caused the pattern of expression to mimic normal rather than ischaemic wounds.
MMP9 concentration in wound tissue
The level of pro‐MMP9 in unwounded tissue was low and unaffected by wounding (Figure 6). The level of active MMP9 also was low in unwounded tissue but increased approximately two‐ to three‐fold in the first 24 hours after wounding. After day 1 the active MMP9 level was stable (Figure 7).
Figure 6.
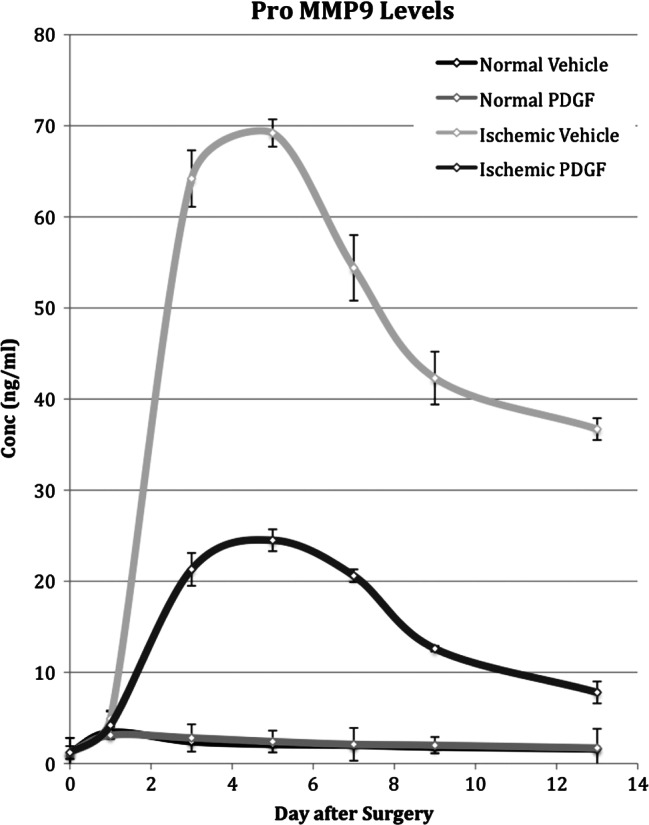
Time course of pro‐metalloproteinase 2 (pro‐MMP9) levels measured in normal and ischaemic wounds treated with placebo or platelet‐derived growth factor (PDGF). Pro‐MMP9 levels in normal and ischaemic wounds treated with placebo or PDGF was determined by gelatin zymography on days 1, 3, 5, 7, 9 and 13 after surgery.
Figure 7.
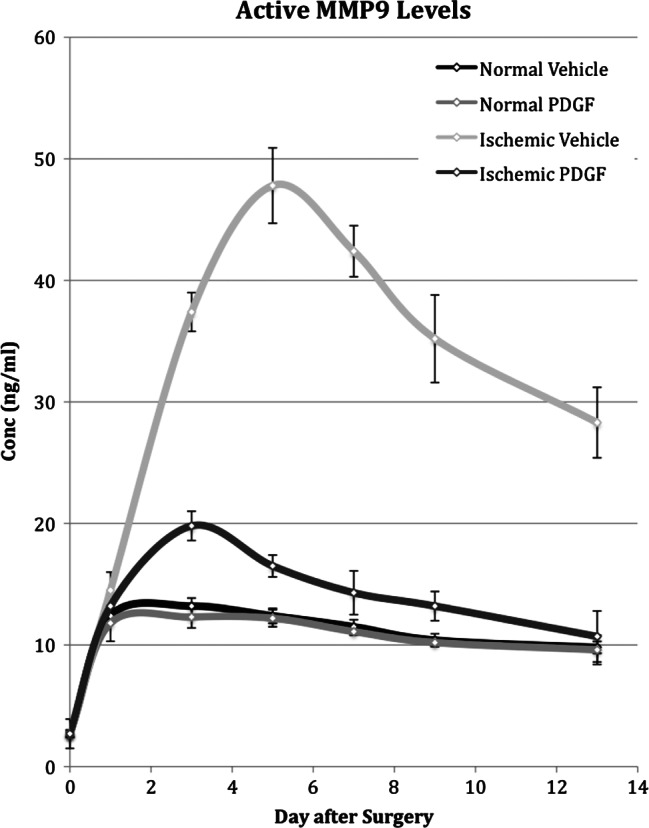
Time course of active metalloproteinase 9 (MMP9) levels measured in normal and ischaemic wounds treated with placebo or platelet‐derived growth factor (PDGF). Active MMP9 levels in normal and ischaemic wounds treated with placebo or PDGF was determined by gelatin zymography on days 1, 3, 5, 7, 9 and 13 after surgery.
Ischaemia caused a large and more long‐lasting increase in levels of both the pro and active forms of MMP9 (Figures 6 and 7). Peak activity of each was reached on day 5, representing an approximate 14‐ and 3‐fold increase above the level in normal wounds for the pro and active forms of MMP9, respectively. Although activity steadily decreased after day 5, the concentration of the pro and active forms on day 13 still remained eight‐ and three‐fold higher, respectively, than that in normal wound tissue.
PDGF application to normal wounds had no effect on either the pro or active form of MMP9. PDGF application did cause a decrease in the levels of pro‐ and active MMP9 in ischaemic wounds (Figures 6 and 7). In the case of the pro form, PDGF application did not overcome the effect of ischaemia and the pro MMP9 level was higher by three‐ to five‐fold than in normal wounds on all samples days. For active MMP9, however, PDGF application did reduce the level in ischaemic wounds to the level in normal wounds by day 7.
Discussion
Following wounding, chronic wound conditions are simulated by ischaemia. Normal wounds reached 50% wound closure in less than 7 days while ischaemic wounds required approximately 9 days (Figure 1). In this study, daily application of PDGF accelerated the rate of healing in both normal and ischaemic wounds. Levels of active forms of MMP2 and MMP9 were reduced to that of normal wounds.
The enhanced wound healing effect of PDGF has been observed in other chronic wound models. For example, Cheng et al. using a diabetic rat model found that PDGF enhanced cell proliferation 20. At the histological level, they also found that PDGF accelerated regeneration of epithelia and enhanced the formation of granulation tissue. Topical PDGF has already shown efficacy in treating chronic wounds, specifically diabetic foot ulcers in humans and is a recommended treatment modality 17.
Although the accelerated wound healing properties of PDGF are well documented, the exact mechanism is not. Recently it has been shown that PDGF may exert its effect through increasing cell proliferation and upregulation of the ERK pathway 20. Unwounded skin contains little PDGF 21. During the early stages of wound healing, platelets adhere to collagen fragments of the damaged ECM, become activated and secrete PDGF. PDGF stimulates chemotaxis of neutrophils, monocytes and fibroblasts. Neutrophils are the first to respond to injury and help destroy and remove bacteria, foreign material and devitalised tissue from the wound. Neutrophils also produce and release TNFA and IL‐1. Monocytes infiltrate the wound site and become activated macrophages 21. Activated macrophages have a dual role in wound healing. First, macrophages act as phagocytes ingesting bacteria, devitalised tissue and depleted neutrophils. Second, macrophages mediate the transition from the inflammatory phase to the proliferative phase of healing by releasing a wide variety of growth factors and cytokines including TNFA, PDGF and IL‐1 1, 22. These compounds recruit and activate fibroblasts, which are the main cell type involved in remodelling the new ECM 8. TNFA and IL1B stimulate the production of MMPs in human fibroblasts 23, 24. Various MMPs (MMP1, MMP2, MMP3 and MMP9) facilitate the migration and accumulation of fibroblasts at the wound site by clearing a path for their movement through the ECM 8. Once fibroblasts have migrated into the wound matrix, they begin to proliferate and to synthesise collagen, proteoglycans and other components that comprise granulation tissue 25.
In this study we monitored the levels of TNFA, IL1B, MMP2 and MMP9 during the course of wound healing. The concentration in normal wounds served as the standard for the effect of both ischaemia and PDGF application. Relative to normal wounds, ischaemia had two major effects. First, ischaemia caused a large and significant increase in the levels of TNFA, pro‐MMP2 (5‐fold), active MMP2 (2‐fold), pro‐MMP9 (14‐fold) and active MMP9 (4‐fold). Second, levels remained elevated for the remainder of the study in most cases. Levels of IL1B decreased rapidly after wounding and continued to decline but at a much slower rate. This may have been due to a decreased number of epithelial cells producing ILB.
Daily application of PDGF returned the pattern of TNFA protein expression in ischaemic wounds to near that of normal wounds. Not only was PDGF able to compensate for the effect of ischaemic on TNFA concentration, but in normal wounds PDGF application also caused a reduction in both peak concentration and duration. Thus, the level of wound healing and TNFA concentration are inversely related such that the fastest and slowest rates of wound healing are associated with lowest and highest TNFA concentrations, respectively.
Like TNFA, IL1B was elevated to higher levels and for a longer period of time in ischaemic wounds. IL1B, however, responded differently to PDGF. The IL1B concentration in normal wounds was not effected by PDGF but in ischaemic wounds was reduced to levels below that in both the treated and untreated normal wounds.
MMPs are initially synthesised as zymogens, inactive enzymes, such as pro‐MMP2 and pro‐MMP9. They are subsequently converted to active forms via cleavage of the pro‐peptide. PDGF had little or no effect on the pro‐MMP2 or pro‐MMP9 concentrations in normal wounds. Relative to normal wounds, ischaemia caused large and prolonged increases in the concentration of pro‐MMP2 and pro‐MMP9. Although PDGF application did cause a reduction in the level of both pro‐MMP2 and pro‐MMP9, the effect was not sufficient to compensate for the effect of ischaemia. Ischaemia also caused large and prolonged increases in the concentration of active MMP2 and active MMP9. Although PDGF had no effect on the MMP2 and MMP9 levels in normal tissue, it did reduce the active levels of MMP2 and MMP9. In the case of MMP2, the levels were reduced to near normal levels, and for MMP9, normal levels were returned to by day 7. It may be that PDGF reduces the synthesis of the precursor pro‐MMP2 and pro‐MMP9, perhaps at the level of transcription, in addition to reducing the concentration of active MMPs or even possibly increasing the synthesis of tissue inhibitors of metalloproteinases (TIMP).
As seen before, chronic wounds, simulated in this study by ischaemia, heal more slowly and have higher levels of pro‐inflammatory cytokines and proteases. As PDGF accelerates healing in ischaemic wounds, we attempted to determine if there were concomitant changes in the levels of pro‐inflammatory cytokines and proteases. For TNFA, PDGF did compensate for the increase caused by ischaemia and decreased the level in normal wounds; thus TNFA concentrations inversely correlated with wound healing. Although PDGF did reduce the level of IL1B and also both the pro and active forms of MMPs, these changes did not correlate well with changes in the rate of wound healing.
This study was limited in that samples from each group for each day used for measurement of ELISA were pooled and processed together such that statistical analysis could not be performed to assess for significant differences. Furthermore, of six wounds created, only four were randomly sampled from each of the three rats euthanized for biochemical analysis on each particular day of measurement. As ischaemia is not evenly distributed throughout the pedicle, some of the samples may have shown more or less of an effect of ischaemia. It should also be noted that a significant proportion of wound healing is accomplished via wound contraction. As this is the primary outcome, much of the observed differences may be secondary to modulation of wound contraction and not necessarily tissue regeneration.
Wound healing in ischaemic wounds was accelerated through the topical application of PDGF. As suggested by the findings of this murine study, the mechanism by which PDGF increased the rate of healing may be through attenuation of a counterproductive and exuberant inflammatory response and its inflammatory cytokine mediators, TNFA, ILB and MMPs or even modulation of wound contraction. Although this study improves our understanding of how PDGF augments the wound healing process and provides a rationale for its clinical utility in humans, research is needed to further clarify the differential action of PDGF on the various cells involved in wound healing.
Acknowledgements
None of the authors had any financial conflicts of interest. Ethicon, inc. (Somerville, New Jersey) provided the Regranex gel. Supplies and other animals were purchased with departmental discretionary funding.
References
- 1. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–46. [DOI] [PubMed] [Google Scholar]
- 2. Ihn H. Pathogenesis of fibrosis: role of TGF‐beta and CTGF. Curr Opin Rheumatol 2002;14:681–5. [DOI] [PubMed] [Google Scholar]
- 3. Weinstein DA, Kirsner RS. Refractory ulcers: the role of tumor necrosis factor‐alpha. J Am Acad Dermatol 2010;63:146–54. [DOI] [PubMed] [Google Scholar]
- 4. Cambou JP, Aboyans V, Constans J, Lacroix P, Dentans C, Bura A. Characteristics and outcome of patients hospitalised for lower extremity peripheral artery disease in France: the COPART Registry. Eur J Vasc Endovasc Surg 2010;39:577–85. [DOI] [PubMed] [Google Scholar]
- 5. Landi F, Onder G, Russo A, Bernabei R. Pressure ulcer and mortality in frail elderly people living in community. Arch Gerontol Geriatr 2007;44(1 Suppl):217–23. [DOI] [PubMed] [Google Scholar]
- 6. Iversen MM, Tell GS, Riise T, Hanestad BR, Ostbye T, Graue M, Midthjell K. History of foot ulcer increases mortality among individuals with diabetes: ten‐year follow‐up of the Nord‐Trondelag Health Study, Norway. Diabetes Care 2009;32:2193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Falanga V. Chronic wounds: pathophysiologic and experimental considerations. J Invest Dermatol 1993;100:721–5. [DOI] [PubMed] [Google Scholar]
- 8. Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, Murphy G, Schultz G. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 1999;7:442–52. [DOI] [PubMed] [Google Scholar]
- 9. Gabay C, Kushner I. Acute‐phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448–54. [DOI] [PubMed] [Google Scholar]
- 10. Wysocki AB, Staiano‐Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP‐2 and MMP‐9. J Invest Dermatol 1993;101:64–8. [DOI] [PubMed] [Google Scholar]
- 11. Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol 1996;107:743–8. [DOI] [PubMed] [Google Scholar]
- 12. Bullen EC, Longaker MT, Updike DL, Benton R, Ladin D, Hou Z, Howard EW. Tissue inhibitor of metalloproteinases‐1 is decreased and activated gelatinases are increased in chronic wounds. J Invest Dermatol 1995;104:236–40. [DOI] [PubMed] [Google Scholar]
- 13. Alterescu V, Alterescu KB. Pressure ulcers: assessment and treatment. Orthop Nurs 1992;11:37–49. [DOI] [PubMed] [Google Scholar]
- 14. Martin P. Wound healing‐‐aiming for perfect skin regeneration. Science 1997;276:75–81. [DOI] [PubMed] [Google Scholar]
- 15. Mustoe TA, O'Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies a unifying hypothesis. Plast Reconstr Surg 2006;117(7 Suppl):35S–41S. [DOI] [PubMed] [Google Scholar]
- 16. Brown DL, Kao WW, Greenhalgh DG. Apoptosis down‐regulates inflammation under the advancing epithelial wound edge: delayed patterns in diabetes and improvement with topical growth factors. Surgery 1997;121:372–80. [DOI] [PubMed] [Google Scholar]
- 17. Steed DL, Attinger C, Colaizzi T, Crossland M, Franz M, Harkless L, Johnson A, Moosa H, Robson M, Serena T, Sheehan P, Veves A, Wiersma‐Bryant L. Guidelines for the treatment of diabetic ulcers. Wound Repair Regen 2006;14:680–92. [DOI] [PubMed] [Google Scholar]
- 18. Chen C, Schultz GS, Bloch M, Edwards PD, Tebes S, Mast BA. Molecular and mechanistic validation of delayed healing rat wounds as a model for human chronic wounds. Wound Repair Regen 1999;7:486–94. [DOI] [PubMed] [Google Scholar]
- 19. Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase‐9 to tissue inhibitor of matrix metalloproteinase‐1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen 2002;10:26–37. [DOI] [PubMed] [Google Scholar]
- 20. Cheng B, Liu HW, Fu XB, Sun TZ, Sheng ZY. Recombinant human platelet‐derived growth factor enhanced dermal wound healing by a pathway involving ERK and c‐fos in diabetic rats. J Dermatol Sci 2007;45:193–201. [DOI] [PubMed] [Google Scholar]
- 21. Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet‐derived growth factor. Physiol Rev 1999;79:1283–316. [DOI] [PubMed] [Google Scholar]
- 22. Ruuls SR, Sedgwick JD. Unlinking tumor necrosis factor biology from the major histocompatibility complex: lessons from human genetics and animal models. Am J Hum Genet 1999;65:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murphy G, Willenbrock F, Crabbe T, O'Shea M, Ward R, Atkinson S, O'Connell J, Docherty A. Regulation of matrix metalloproteinase activity. Ann N Y Acad Sci 1994;732:31–41. [DOI] [PubMed] [Google Scholar]
- 24. Ito A, Sato T, Iga T, Mori Y. Tumor necrosis factor bifunctionally regulates matrix metalloproteinases and tissue inhibitor of metalloproteinases (TIMP) production by human fibroblasts. FEBS Lett 1990;269:93–5. [DOI] [PubMed] [Google Scholar]
- 25. Lobmann R, Pap T, Ambrosch A, Waldmann K, Konig W, Lehnert H. Differential effects of PDGF‐BB on matrix metalloproteases and cytokine release in fibroblasts of Type 2 diabetic patients and normal controls in vitro. J Diabetes Complications 2006;20:105–12. [DOI] [PubMed] [Google Scholar]


