Dear Editors,
Vitamin E, first described in 1922 by Evans and Bishop as an essential micronutrient for reproduction in rats 1, is a fat‐soluble vitamin with important functions of maintaining the integrity of the intracellular membrane by protecting its physical stability and providing defence against any tissue damage caused by oxidation 2. Molecular and cellular effects of vitamin E have been explained by regulating membranes and proteins activities by specific reactive oxygen species (ROS) and nitrous oxide systems (NOS) or by interacting and regulating specific enzymes and transcription factors and influencing cellular structures such as membranes and lipid domains 3.
Vitamin E is present in plants in eight different molecular forms with more or less equal antioxidant potential, nevertheless, in higher organisms only α‐tocopherol (α‐T) is preferentially retained suggesting a specific mechanism for the uptake for this analogue 4. As the natural vitamin E analogue is relatively unstable, several stabilised vitamin E derivatives [e.g. α‐tocopherol acetate (α‐TA)] have been synthesised for usage in supplements and cosmetics. These vitamin E derivatives are water‐soluble esters of α‐T that can be considered to be provitamins that are converted to their natural forms by epidermal esterases 5.
A common phenomenon in patients with disorders in wound healing is the acute phase response resulting in elevated levels of inflammatory markers. An imbalance between pro‐ and antioxidants is suggested to favour cell damage and to enhance inflammatory process 6. The antioxidant capacity of α‐TA and its effect on reprogramming of gene expression allow its topical use in skin diseases in which an inflammatory process is activated. Furthermore, it has been demonstrated that epidermal esterases remove the acetic acid from α‐TA that may act against microorganisms simply by lowering the pH and creating an anhydrous environment unsuitable for their growth and multiplication 7.
In the last 3 years, we have used α‐TA for treatment of superficial burns, post‐traumatic superficial ulcers and skin graft donor sites, even in presence of wound infection. The presence of exudate, pain and delayed reepithelialisation, absence of tissue necrosis and/or sepsis were criteria for topical use of α‐TA, avoiding conventional treatments such as local antibiotics, polyurethane foams and other occlusive dressings 8. Bacterial growth was assessed in 35 patients; wound tissue cultures were obtained, 20 (57·1%) of 35 patients had positive results for bacterial cultures: all of them had a single organism infection. The isolated organisms cultured from the wound tissue were Staphylococcus aureus (N = 6, 17·1%), Staphylococcus epidermidis (N = 6, 17·1%), Pseudomonas aeruginosa (N = 4, 11·4%), Proteus mirabilis (N = 2, 5·71%) and Escherichia coli (N = 2, 5·71%). Three of the six S. aureus isolated were multiple antibiotic resistant microorganisms.
Topical α‐TA was applied in the form of Vea Oil (Vea; Hulka s.r.l., Rovigo, Italy) on the wound every 24 hours after wound irrigations with saline solution; follow‐up visits were carried out every 7–10 days in outpatients. Home‐dressings were daily performed. Topical use of α‐TA is extremely easy, inexpensive, can be often performed by the patient himself leading to a total compliance of the patient and cost reduction.
Despite the presence of initial bacterial infection, a quicker reduction of exudates and pain and a progressive and faster wound healing was observed in all patients. Clinically several macroscopic changes of granulating tissue were observed. Initially the unhealthy granulation was dark red coloured, bleeding on contact, characterised by heavy exudate and bumpy irregular overgrowth and covered by white and yellow shiny fibrinous tissue. After treatment with topical α‐TA, the healthy granulation tissue became light red in colour, without exudate and was painless. Clinical data appear to confirm the bacteriostatic effect of α‐TA. In all treated patients no sepsis and tissue necrosis recurrence was observed (Figures 1A–C and 2A and B) until complete healing was achieved.
Figure 1.
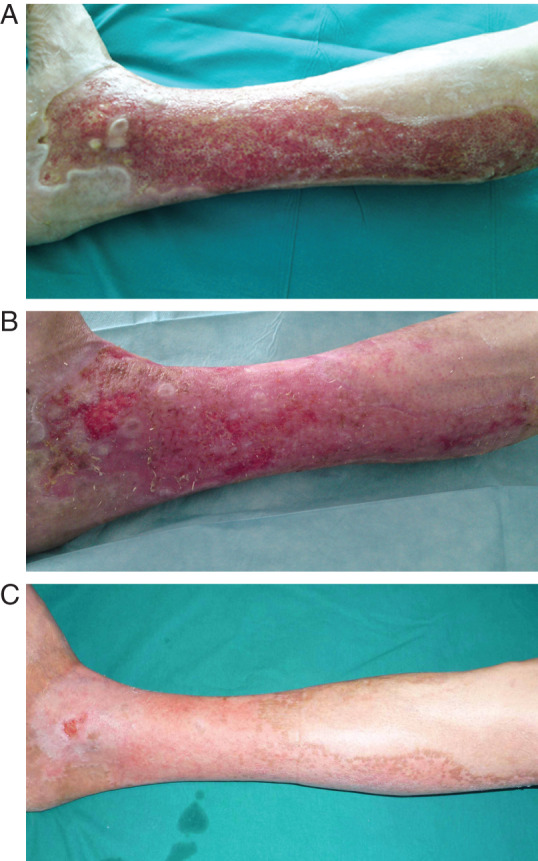
(A) Burn of right leg 1 month after trauma. Local infection by multiple antibiotic resistant Staphylococcus aureus. During this period the patient was treated (in another hospital) with topical and systemic antibiotics. (B) Progression of healing after 8 days of treatment only with topical α‐tocopherol acetate (α‐TA). (C) Complete wound resolution after 20 days of topical α‐TA treatment.
Figure 2.
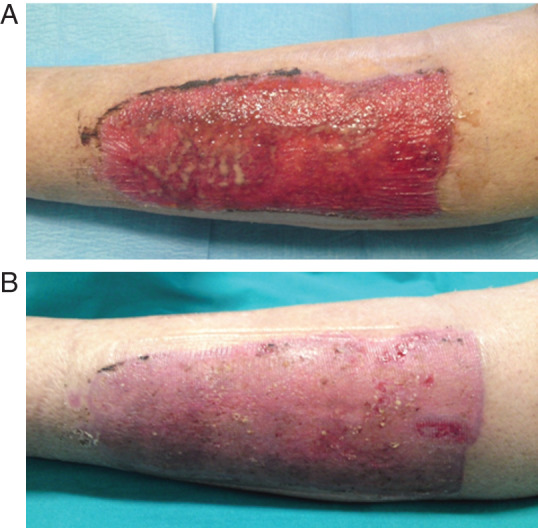
(A) Skin graft donor site with copius exudate and local infection treated for the previous 3 weeks with conventional dressing (polyurethane foams), without progression of healing. The isolated microorganism was Pseudomonas aeruginosa. (B) Complete reephitelialisation obtained after 7 days of topical α‐tocopherol acetate (α‐TA) treatment.
Clinical evidence was confirmed by instrumental examination; the growth of new capillary blood vessels was evaluated by intravital videocapillaroscopy analysis (IVCP), an in vivo imaging technique involving the direct visualisation of skin capillaries through an optic contact probe microscope attached to a computerised video microscope (FotofinderDermoscope at magnification 60×, 100×, 200×) 9. The IVCP showed both the early development of granulation tissue and the rapid resolution of newly formed blood vessels in regenerating tissues before the reepithelialisation phase (Figure 3A and B). These effects of vitamin E have been observed in several studies, possibly involving the stimulation of vascular endothelial growth factor (VEGF) 10.
Figure 3.
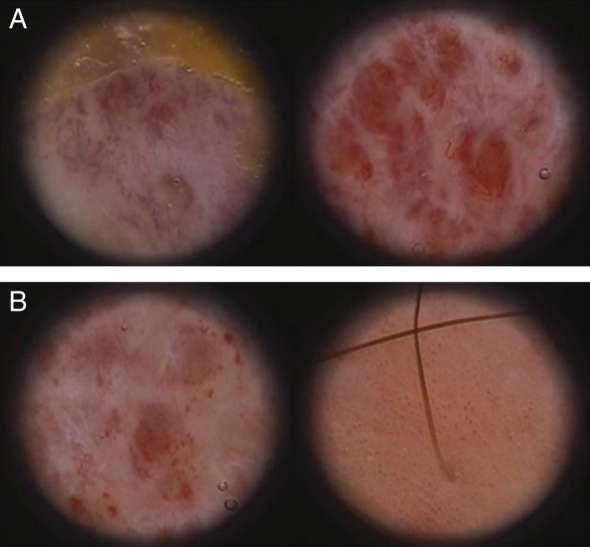
(A) Limit between necrotic eschar and granulation tissue (left); initial neoangiogenesis with visible capillaries in connective tissue observed after 5 days (right). (B) After 15 days, note the reduction of neoangiogenesis and granulation tissue with the progression of reepithelialisation (left). Complete reepithelialisation with residual neoangiogenesis and typical convoluted capillaries that are not detectable in normal or non‐treated skin (right).
In delayed wound healing treatment, we observed that molecular and cellular effects of α‐TA are confirmed by clinical outcomes and IVCP. As α‐TA stimulates granulation tissue, seems to reduce bacterial growth, modulates angiogenesis and improves epithelialisation, it may represent a safe, simple and inexpensive method for improving the healing of difficult wounds with local infection.
Our study is only a preliminary report and further studies will be necessary to confirm our findings.
Antonio Stanizzi, MD1, Manuela Bottoni, MD1, Matteo
Torresetti, MD1, Anna Campanati, MD2 & Giovanni Di
Benedetto, MD, PhD1
1Department of Plastic and Reconstructive Surgery, Marche
Polytechnic University Medical School
Regional Hospital
Ancona, Italy
2Department of Dermatology, Marche Polytechnic University
Medical School
Regional Hospital
Ancona, Italy
astanizzi@tiscali.it
References
- 1. Evans HM, Bishop KS. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 1922;56:650–1. [DOI] [PubMed] [Google Scholar]
- 2. Zampieri N, Zuin V, Burro R, Ottolenghi A, Camoglio FS. A prospective study in children: pre‐ and post‐surgery use of vitamin E in surgical incisions. J Plast Reconstr Aesthet Surg 2010;63:1474–8. [DOI] [PubMed] [Google Scholar]
- 3. Zingg JM. Vitamin E: an overview of major research directions. Mol Aspects Med 2007;28:400–22. [DOI] [PubMed] [Google Scholar]
- 4. Zingg JM, Azzi A. Non‐antioxidant activities of vitamin E. Curr Med Chem 2004;11:1113–33. [DOI] [PubMed] [Google Scholar]
- 5. Kalay Z, Cevher SC. Oxidant and antioxidant events during epidermal growth factor therapy to cutaneous wound healing in rats. Int Wound J 2012;9:362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blass SC, Goost H, Tolba RH, Stoffel‐Wagner B, Kabir K, Burger C, Stehle P, Ellinger S. Time to wound closure in trauma patients with disorders in wound healing is shortened by supplements containing antioxidant micronutrients and glutamine: a PRCT. Clin Nutr 2012;31:469–75. [DOI] [PubMed] [Google Scholar]
- 7. Basavraj N, Bharat W, Prabhakar K, Shirish K. Acetic acid treatment of Pseudomonal wound infections. Eur J Gen Med 2008;5:104–6. [Google Scholar]
- 8. Di Benedetto G, Pierangeli M, Scalise A, Andriessen A, Rowan S, Bertani A. An improbe tie‐over dressing technique for skin grafts using a hydrocellular dressing. Plast Reconstr Surg 2000;106:507–9. [DOI] [PubMed] [Google Scholar]
- 9. Ganzetti G, Campanati A, Offidani A. Alopecia areata: a possible extraintestinal manifestation of Crohn's disease. J Crohns Colitis 2012;6:962–3. [DOI] [PubMed] [Google Scholar]
- 10. Zingg JM, Meydani M, Azzi A. α‐Tocopheryl phosphate—an activated form of vitamin E important for angiogenesis and vasculogenesis. Biofactors 2012;38:24–33. [DOI] [PubMed] [Google Scholar]


