Abstract
Even though it is estimated that at least 300 000 people in Canada may be affected by chronic oedema/lymphoedema, recognition of the seriousness of this chronic disease in health care is scarce. Lymphoedema affects up to 70% of breast and prostate cancer patients, substantially increasing their postoperative medical costs. Adding to this problem are the escalating rates of morbid obesity across North America and the fact that 80% of these individuals are thought to suffer with an element of lymphoedema. The costs related to these patient populations and their consumption of health care resources are alarming. Untreated chronic oedema/lymphoedema is progressive and leads to infection, disfigurement, disability and in some cases even death. Thus, prognosis for the patient is far worse and treatment is more costly when the disease is not identified and treated in the earlier stages. Although the number of individuals coping with chronic oedema/lymphoedema continues to increase, the disparity between diagnosis, treatment and funding across Canada endures. The reasons for this include a lack of public awareness of the condition, insufficient education and knowledge among health care providers regarding aetiology and management and limited financial coverage to support appropriate methods and materials.
Keywords: Chronic oedema, Compression, Erysipelas, Lymphoedema, Venous insufficiency
Introduction
Although the number of individuals coping with chronic oedema/lymphoedema continues to increase, the disparity between diagnosis, treatment and funding across Canada endures. Consensus documents outlining evidence‐based, best practice guidelines 1, 2 have been published, yet recognition of the seriousness of chronic oedema/lymphoedema in health care is scarce. According to Stout et al., the reasons for this include a lack of public awareness of the condition, insufficient education and knowledge among health care providers regarding aetiology and management and limited financial coverage to support appropriate methods and materials 3.
Primary lymphoedema occurs in 1 in 6000 at birth. Secondary lymphoedema is most commonly caused by lymphatic filariasis and is estimated to affect more than 120 million individuals worldwide (International Lymphoedema Framework, ILF). However, it is uncommon in the western hemisphere being endemic in only two locations, Leogone Haiti and Recife Brazil. Approximately 40 million individuals are disfigured and incapacitated by lymphoedema. Although there are few Canadian statistics documenting the numbers of people suffering with secondary lymphoedema, it is estimated that 3–5 million people in the USA are affected by secondary lymphoedema. Canadian statistics extrapolated from a recent study in the USA estimates that at least 300 000 people in Canada may be affected by lymphoedema. It is suggested that the incidence of breast‐cancer‐related lymphoedema ranges from 6% to 70%; lymphoedema may be a common and under‐reported morbidity 4. Up to 70% of men with prostate cancer may develop lymphoedema. A study by Ya‐Chen et al. found that lymphoedema boosted 2‐year, postoperative medical costs of breast‐cancer‐related lymphoedema by $14 877–$23 167, the additional cost came from office visits, treatments for infections and mental health services, including prescriptions for antidepressants 5. Extremity oedema has substantial symptom load and infectious complications, but compared with upper extremity oedema, those with lower extremity oedema have more severe symptoms and more infections 6. The rates of morbid obesity across North America continue to increase, and 80% of these individuals are thought to suffer with an element of lymphoedema 1, 2. The costs related to both these patient populations and their consumption of health care resources are alarming. Despite this prevalence, explicit assessment methodologies, effective means of treatment and comprehensive management strategies remain largely inadequate (ILF). Untreated chronic oedema/lymphoedema is progressive and leads to infection, disfigurement, disability and in some cases even death. Thus, prognosis for the patient is far worse and treatment is more costly when the disease is not identified and treated in the earlier stages. A study by Moffat et al. 7 estimated that for every £1 spent on lymphoedema treatments, £100 in hospital admission costs was saved. Chronic oedema/lymphoedema has been described as the hidden epidemic.
Definition of chronic oedema/lymphoedema
Chronic oedema is defined as swelling lasting for more than 3 months. Lymphoedema is defined as a gradual abnormal swelling of a limb and/or the related quadrant of the trunk due to the accumulation of protein‐rich fluid in the tissue spaces of the skin 8. It is minimally responsive to overnight leg elevation or diuretics and is accompanied by skin changes such as thickened/fibrotic skin, hyperkeratosis and papillomatosis 9. Lymphoedema may be primary or secondary. Primary lymphoedema is related to congenital absence or malformation of lymphatics and may appear at birth or later in life. Secondary lymphoedema results from damage to lymphatics. Common causes of secondary lymphoedema are venous insufficiency, obesity, recurrent infections, surgeries and radiation treatments and it is a common side effect of cancer treatments that remove or damage lymph nodes or vessels. Lymphoedema is a chronic condition that cannot be cured but can be managed.
Pathophysiology of chronic oedema/lymphoedema
Lymphatic fluid (also known as lymph) primarily consists of water and protein filtrate. Lymph fluid contains large quantities of macromolecules, which are not absorbed into the arteriovenous capillary system but are transported by the lymphatic system into the venous system. The lymphatic system also transports fat (chyle) and waste products of metabolism. Normal functioning depends on the load and transport capacity of this system, which consists of the Incredible World series of videos fluid volume (including lymphatic proteins, cells, fats and hyaluronan) 10 and the maximum quantity of volume that can be functionally transported in a given period of time. The functional reserve is the difference between transport capacity and lymphatic load. The lymphatic system becomes overworked when load exceeds maximum transport capacity, resulting in insufficiency or failure 8.
Lymphatic failure is best discussed in the context of overall tissue fluid dynamics. The cells are nested in an extracellular matrix and are bathed in a constant flow of tissue fluid that nourishes and supports the cells as well as carrying away the products of metabolism. Fluid moves under the influence of the push of hydrostatic pressure within the capillaries and in the extracellular compartment and the pull of osmotic force where fluid moves across a semi‐permeable membrane from areas of low concentration of dissolved proteins to a region of higher concentration. When filtration and re‐absorption are in balance, no excess fluid accumulates in the extracellular matrix.
Recent research has shown that capillary hydrostatic pressure is never less than the tissue hydrostatic pressure in the extremities and that under normal circumstances there is no net re‐absorption of fluid into the capillary bed. To further complicate the equation, the movement of proteins and other larger molecules across the semi‐permeable membrane of the capillary walls is much more dynamic than previously thought. The newer models of tissue fluid balance suggest that in fact 100% of excess tissue fluid is handled by the lymphatics 11. In other words, there is a net filtration of fluid out of the capillary bed with the vast majority of fluid being re‐absorbed by the lymphatic system.
There are three methods by which the lymphatic system may fail: (i) dynamic insufficiency (otherwise known as high‐output insufficiency), (ii) mechanical insufficiency (also known as low‐output failure) or (iii) or a combination of the two. When the lymphatic system becomes overwhelmed, the tissue spaces become saturated with protein‐rich fluid and thus swelling of the affected area occurs. If left untreated, the presence of macromolecules, such as growth factors, proteases and pro‐inflammatory molecules, may lead to chronic inflammation and hardening of the skin 8, 12, 13. Additionally, the accumulation of cellular debris and blocking of the lymphatic vessels impede transportation of macrophages and lymphocytes, thus limbs affected by lymphoedema are more prone to infections 8.
Examples of high‐output failure (when normal transport capacity of an intact lymphatic system is overwhelmed by excessive burden of blood capillary filtrate) include hepatic cirrhosis (ascites), nephrotic syndrome (anasarca) and venous insufficiency of the leg (peripheral oedema) 14. These instigators of oedema should be distinguished from lymphoedema, which is characterised by decreased lymphatic transport. Patients in whom high‐output transport failure is long‐standing, functional deterioration of the current lymphatics system is inevitable and results in a reduction of overall transport capacity. Low‐output failure may relate to tissue damage, obstruction of the lymph seen lymphatics or immobility and dependency of the limb. Examples such as recurrent infection, thermal burns and repeated allergic reactions are known as ‘safety valve insufficiencies’ of the lymphatic system and are considered a mixed form of oedema/lymphoedema. These are particularly difficult to treat 14, 15.
Although the pathophysiology of chronic oedema/lymphoedema may differ, the clinical presentations are similar. The challenge for clinicians is that when the clinical signs of lymphoedema are obvious, the condition has been present for a long time. The resultant damage is not easily managed and the requisite health care resources increase exponentially.
The cost of chronic oedema/lymphoedema
Early intervention and management of chronic oedema/lymphoedema is a key factor in reducing the risks to overall patient health, recovery, lifestyle and work. These risks become unnecessary costs to our health care system, as hospitalisation/intravenous treatment may be needed. These are avoidable costs that will only multiply because of the increase in number of patients with obesity, complex medical conditions and cancer.
A prevalence study carried out in the UK by Moffat 7 reported that 823 patients in 619 000 had chronic oedema. Of these:
27% were admitted to hospital for antibiotic treatment.
32% received some form of compression bandaging.
29% had an infection in the past 12 months.
The mean length of stay was 12 days at a mean cost of £2300.
80% had taken time off work.
8% had to give up work.
In 2012, the challenges of chronic oedema/lymphoedema were compared between the UK and Canada. It was noted that both had a lack of public awareness, poor professional knowledge, inadequate information, delayed diagnosis and inappropriate treatment, poor understanding of treatment options, a lack of evidence‐based guidance and difficulties ensuring concordance with treatment 16. Participants in this study believed that appropriate compression therapy was rarely used to manage chronic oedema/lymphoedema in Canada. In the collective view of the participants, this was because of a fundamental lack of awareness that compression therapy should be the primary treatment. It was believed that this was symptomatic of the deficit in knowledge and skills. The reimbursement system was a major frustration as many of the products required were unavailable from both government and insurance companies in Canada 17. Modification of products led to suboptimal treatment and poor outcomes exacerbating frustration for professionals and patients. Unique to Canada were the geographical issues patients faced and the fact that many of these patients deliver their own care.
Best practice
The most comprehensive document on Best Practice is the document from the International Lymphoedema Framework. (Lymphoedema Framework. Best Practice for the Management of Lymphoedema. International consensus. London: MEP Ltd, 2006.) When combined with the recent Position Statement on Compression Therapy, the recommended best practice for care and treatment of lymphoedema and chronic oedema include:
Compression bandaging.
Meticulous skin care.
Education.
Manual lymphatic drainage.
Exercise.
Compression garments for maintenance.
Of these, compression therapy is regarded as the most essential. Compression therapy works to enhance both venous and lymphatic drainage from the extremity. Short‐stretch, more rigid bandage systems have proven to be more effective than elastic systems in promoting mobilisation of tissue fluid (IFL).
Compression therapy has several effects on venous return:
Improved valvular function reducing reflux and decreasing hydrostatic pressure in the veins;
Increased venous flow velocity through enhancement of calf muscle pumping activity;
Reduction of the cross section of the veins reducing the blood volume in the legs;
Increased tissue hydrostatic pressure leading to decreased net filtration.
There are also beneficial effects on the lymphatic system:
Reduction of venous congestion decreases net filtration and fluid load on the lymphatic capillaries;
Increased interstitial tissue fluid hydrostatic pressure increases tension on the anchoring filaments, which will increases the ingress of lymph fluid and macromolecules into the lymphatic capillaries;
Enhanced muscle pump activity will improve lymph propulsion through the lymph vessels;
Down‐regulation of inflammatory cytokines leads to breakdown of fibrosclerotic tissue.
Clinical presentations
Skin changes
Once fluid starts to accumulate in the tissues, a number of changes take place (Figure 1). The epidermis becomes stretched, there is a proliferation of fibroblasts and collagen fibres in the local tissues, dilation of local lymphatics and increased production of inflammatory agents. In the early stages, oedema is usually pitting and reduces with elevation. In time, as fluid and waste products accumulate, the tissues become hard, fibrosed, non‐pitting and does not respond to elevation. Skin and tissue changes transpire and, as the lymphatic system has an important immunological function, an increased risk of bacterial and fungal infection is common. Positive Stemmer's sign (inability to pick up a fold of skin at the base of the second toe, because of thickening of the tissues/fibrosis) is part of a clinical diagnostic assessment for lymphoedema 18. MacDonald and Geyer suggest that oxygen‐free radicals, fibrin cuffing and white cell sequestration, contribute to the repair of tissue in a chronically inflamed environment, thus increasing protein permeability 19. It is this process that in turn damages the lymphatic system. Oedema, being rich in protein, develops into the principal pathology contributing to the development of ulceration.
Figure 1.
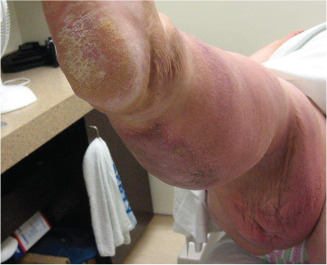
Skin changes.
Cellulitis/erysipelas
Patients with chronic oedema are at increased risk of recurrent episodes of acute cellulitis/erysipelas, an infection of the skin and subcutaneous tissues. The cause of most episodes is believed to be Group A β‐haemolytic Streptococci. It may also be caused by Staphylococci or other bacteria. The impetus may be tinea pedis (athlete's foot), venous eczema, ulceration, ingrown toe nails, scratches from plants or pets or insect bites. Good skin care reduces the likelihood of cellulitis/erysipelas and consequently the need for antibiotics. Symptoms are variable. Episodes may become evident in minutes, develop over several weeks or be preceded by systemic upset. Symptoms include pain, swelling, warmth, redness, lymphangitis, lymphadenitis and sometimes blistering of the affected part. Severe cases have a greater degree of systemic upset, for example chills, rigor, high fever, headache and vomiting that can develop quickly requiring hospitalisation. In rare cases, these symptoms may be indicative of necrotising fasciitis.
Folliculitis
Folliculitis is the result of inflammation of the hair follicles. It causes a red rash with pimples or pustules and is most commonly seen on hairy limbs. The cause is usually Staphylococcus aureus; however, it may also be caused by daily wrapping with compression bandages (ILF). This can also cause impetigo and it may precede cellulitis/erysipelas.
Fungal infection
Fungal infection occurs in skin creases and on skin surfaces that touch. It results in moist, whitish scaling and itching and is particularly common between the toes. It can cause a portal of entry for the development of cellulitis/erysipelas.
Ulceration
It is important to establish the underlying cause of ulcer because the cause determines treatment and whether compression is appropriate.
Venous eczema
Venous eczema (also known as varicose eczema or stasis dermatitis) usually occurs on the lower legs, particularly around the ankles, and is associated with varicose veins. The skin becomes pigmented, inflamed, scaly and itchy.
Contact dermatitis
Contact dermatitis is the result of an allergic or irritant reaction. It usually starts at the site of contact with the causative material, but may spread. The skin becomes red, itchy and scaly and may weep or crust.
Lymphangiosarcoma
Patients with chronic lymphoedema for 10 years have a 10% risk of developing lymphangiosarcoma, the most dreaded complication of this disease. In the most severe cases of lymphoedema, lymphangiosarcoma, a rare form of lymphatic cancer (Stewart–Treves syndrome), can develop. It occurs most often in patients who have been treated for breast cancer with mastectomy and/or radiotherapy. The sarcoma first appears as a reddish or purplish discolouration or as a bruised area that does not change colour. It progresses to an ulcer with crusting and eventually to extensive necrosis of the skin and subcutaneous tissue. Patients with suspected lymphangiosarcoma require urgent referral to an oncologist because it could widely metastasise. This tumour is highly aggressive, requires radical amputation of the involved extremity and has a very poor prognosis. Other neoplasms identified in areas of chronic lymphoedema are squamous cell carcinoma, Kaposi sarcoma, B‐cell lymphoma and malignant fibrous histiocytoma. Health care professionals who determine a history of cancer in patients presenting with lymphoedema should be alert to this risk and continue to monitor closely.
Postoperative oedema
Oedema is sometimes not identified in the early subclinical stage, where tissue density increases before swelling becomes apparent by an increase in limb circumstance. Patients sometimes believe oedema is to be expected and do not report any problems. Postoperative oedema occurs in up to 100% of patients undergoing infrainguinal arterial reconstruction for chronic ischemia 20, 21, 22. As patients start ambulating, oedema becomes more prominent lasting from weeks to months after surgery. This swelling can impair normal ambulation and cause discomfort and delay in wound healing despite a successful arterial reconstruction. Oedema is pitting in character, most prominent over the ankle and the foot, although the calf and the thigh are occasionally involved. Its occurrence does require skin care and compression, as it may compromise the healing of ischemic ulcers 22 and it frequently delays the resumption of normal activities. Occasionally, the oedema has been described as persisting permanently 23. For these patients and others who are unable or unwilling to wear compression stockings and avoid long periods of leg dependency, oedema constitutes a significant cause of postoperative morbidity 21. Successful revascularisation increases the lymphatic load and lymphatic injury decreases its drainage (obstruction of the deep and superficial lymph channels during dissection of the groin, the popliteal space and along the greater saphenous vein). If left unmanaged, this can progress to lymphovenous oedema, which can lead to more severe forms of lymphoedema with problematic skin challenges 24.
There have been no large quality studies on the effect of compression stockings for the treatment and prevention of post reconstructive oedema. However, the study by Kolh showed that for the prevention and treatment of oedema, class I stocking (defined here as 18 mmHg, used day and night for 1 week and then daily until 8 weeks after surgery) proved superior to Intermittent Pneumatic Compression (IPC) (used nightly only for 7 days in hospital and then class I compression stocking until 8 weeks after surgery) 25. The working mechanism of the compression stocking is to increase the pressure on the interstitial space 26 and to augment the peripheral circulation by lowering flow resistance at the arteriolar level 21.
Pharmacological therapy
Currently, there are no medications proven to be effective in the treatment of lymphoedema.
Surgical intervention
Based on the review performed by the American Lymphedema Framework Project that was launched at the ILF conference in 2011 (Montpellier), it was concluded that there is no evidence supporting the use of surgery in the treatment of lymphoedema. While microsurgical approaches are being developed, further work needs to be undertaken to effectively define indications for such surgery 27. Even though some surgical techniques have shown promise, the need for continued use of conventional therapies including compression for long‐term maintenance cannot be underestimated.
Conclusion
Much like the recognition of chronic oedema/lymphoedema often being too little too late, many professionals fear that action taken to manage the escalating numbers of individuals with this chronic condition may also be too little too late. In a Canadian study carried out by Hodgson et al. 17, many patients with lymphoedema, especially those with non‐cancer‐related lymphoedema and children with lymphoedema, had limited or no access to treatment. The article states that the compromised access stems from a lack of hospital‐based services and an insufficient number of private clinics or from the cost of treatment. Patients in rural/suburban areas may not have access to trained therapists or physicians who can properly diagnose and treat chronic oedema/lymphoedema. Although there is excellent information accessible in the IFL documents 1, 2 for diagnosis and appropriate treatment, inconsistencies in diagnosis, treatment and funding persist across Canada. Thus, there is an urgent need for professionals to enhance their practice with the latest evidence‐based recommendations, for patients and families to be better informed and educated, and for changes to provincial reimbursement policies. Individuals with this condition often are socially stigmatised, they have their lifestyle and economic means impeded and if the condition is not managed their physical and mental health will deteriorate. Chronic oedema/lymphoedema demands an interprofessional approach, as this condition affects multiple aspects of health care including the physical, mental, social and financial health of individuals and families. Successful models of care delivery for chronic oedema/lymphoedema have been implemented in other countries, so there is opportunity to replicate and improve the care delivery model in Canada. The challenge that remains is for Canadians to take action and be proactive, not reactive, in order to provide quality, evidence‐based cost‐effective care.
References
- 1. The International Lymphoedema Framework . Best practice for the management of lymphoedema – 2nd edn. Compression therapy: a position document compression bandaging, 2012. http://www.lympho.org/mod_turbolead/upload//file/Resources/Compression%20bandaging%20‐%20final.pdf [accessed on 22 October 2012]
- 2. The Canadian Lymphedema Framework . Improving lymphedema education, research and management, 2010. http://www.bclymph.org/Resources/Documents/CLF;%20Education,%20Research,%20Management%20‐%20Dr.%20Anna%20Towers.pdf [accessed on 22 October 2012]
- 3. Stout NL, Weiss R, Feldman JL, Stewart BR, Armer JM, Cormier JN, Shih Y‐CT. A systematic review of care delivery models and economic analyses in lymphedema: health policy impact (2004–2011). Lymphology 2013;46:27–41. [PubMed] [Google Scholar]
- 4. McLaughlin SA. Lymphedema: separating fact from fiction. Oncology 2012;26:242–9. [PubMed] [Google Scholar]
- 5. Ya‐Chen TS, Ying X, Cormier JN, Giordano S, Ridner SH, Buchholz TA, Perkins GH, Elting LS. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2‐year follow‐up study. J Clin Oncol 2009;27:2007–14. [DOI] [PubMed] [Google Scholar]
- 6. Ridner SH, Deng J, Fu MR, Radina E, Thiadens SR, Weiss J, Dietrich MS, Cormier JN, Tuppo CM, Armer JM. Symptom burden and infection occurrence among individuals with extremity lymphedema. Lymphology 2012;45:113–23. [PubMed] [Google Scholar]
- 7. Moffatt CJ, Franks PJ, Doherty D, Williams AF, Badger C, Jeffs E, Bosanquet N, Mortimer PS. Lymphoedema: an underestimated health problem. QJM 2003;96:731–8. [DOI] [PubMed] [Google Scholar]
- 8. Lawenda BD, Mondry TE, Johnstone PAS. Lymphedema: a primer on the identification of a chronic condition in oncologic treatment. CA Cancer J Clin 2009;59:8–24. [DOI] [PubMed] [Google Scholar]
- 9. Kubik S, Kretz O. Anatomy of the lymphatic system. In: Foldi M, Foldi E, editors. Foldi's textbook of lymphology. Munich: Elsevier, 2006:1–50. [Google Scholar]
- 10. Zuther E. Lymphedema management: the comprehensive guide for practitioners. New York: Thieme Medical Publishers, 2005. [Google Scholar]
- 11. Sheffield PJ, Smith APS, Fife CE. Wound care practice. Flagstaff: Best Publishing Company, 2004. ISBN: 1‐930536‐16‐X. [Google Scholar]
- 12. Foldi E, Junger M, Partsch H. The science of lymphoedema bandaging. In: European Wound Management Association (EWMA). Focus Document: Lymphoedema bandaging in practice. London: Medical Education Partnership Ltd, 2005:2–4. [Google Scholar]
- 13. MacLaren J. Skin changes in lymphoedema: pathophysiology and management options. Int J Palliat Nurs 2001;7:381–8. [DOI] [PubMed] [Google Scholar]
- 14. The diagnosis and treatment of peripheral lymphedema: 2009 Consensus Document of the International Society of Lymphology. Lymphology 2009;42:51–60. [PubMed] [Google Scholar]
- 15. Keast DH. Lymphoedema. In: Flanagan M, editor. Wound healing and skin integrity, principles and practice. Chichester: John Wiley and Sons Ltd, 2013:175–92. [Google Scholar]
- 16. Stout NL, Brantusb P, Moffatt C. Lymphoedema management: an international intersect between developed and developing countries. Similarities, differences and challenges. Glob Public Health 2012;7:107–23. [DOI] [PubMed] [Google Scholar]
- 17. Hodgson P, Towers A, Keast D, Kennedy A, Pritzker R, Allen J. Lymphedema in Canada: a qualitative study to help develop a clinical, research, and education strategy. Curr Oncol 2011;18:e260–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. AF Williams. An overview of non‐cancer‐related chronic oedema. A UK perspective. World Wide Wounds 2005. http://www.worldwidewounds.com [accessed on 29 May 2013]
- 19. JM MacDonald, MJ Geyer. World Health Organization (WHO) paper. Wound and lymphoedema management, 2010. http://www.wawlc.org/ [accessed on 29 May 2013]
- 20. Porter JM, Lindell TD, Lakin PC. Leg edema following femoropopliteal autogenous vein bypass. Arch Surg 1972;105:883. [DOI] [PubMed] [Google Scholar]
- 21. Schubart PJ, Porter JM. Leg edema following femorodistal bypass. In: Bergan JJ, Yao JST, editors. Reoperative arterial surgery. Orlando: Grune & Stratton, 1986:311. [Google Scholar]
- 22. AbuRahma AF, Woodruff BA, Lucente FC. Edema after femoropopliteal bypass surgery: lymphatic and venous theories of causation. J Vasc Surg 1990;11:461. [PubMed] [Google Scholar]
- 23. Vaughan BF, Slavotinek AH, Jepson RP. Edema of the lower limb after vascular operations. Surg Gynecol Obstet 1970;133:282. [PubMed] [Google Scholar]
- 24. Muldoon J. Managing chronic oedema and lymphoedema. J Community Nurs 2011;25:3. [Google Scholar]
- 25. Kolh P. Reducing leg oedema after femoro‐popliteal bypass surgery: a challenge. Eur J Vasc Endovasc Surg 2010;40:643–4. [DOI] [PubMed] [Google Scholar]
- 26. Pflug JJ, Calnan JS. The relationship between the blood, lymphatics and interstitial circulation in the leg. Vasa 1973;2:75–80. [PubMed] [Google Scholar]
- 27. International Lymphoedema Framework . Best practice for the management of lymphoedema, 2nd edn: surgical intervention – a position document on surgery for lymphoedema, 2012. http://www.lympho.org/mod_turbolead/upload//file/Resources/Surgery%20‐%20final.pdf [accessed on 24 September 2013]


