Abstract
The purpose of this uncontrolled study was to evaluate the clinical effectiveness of ReCell ® system in the treatment of chronic ulcers. From October 2011 to July 2012, 20 patients, 8 men and 12 women with a mean age of 70 years, with chronic ulcers of different aetiology that were unresponsive to conventional therapies were recruited and treated using the ReCell ® system. Patient pain rate, scar aesthetics and patient satisfaction were assessed using a Visual Analogue Scale, Manchester Scar Scale and the Rosenberg Self‐Esteem Scale, respectively. Complete ulcer healing, defined as 100% reepithelialisation was observed between 40 and 60 days in 14 patients (70%) depending on the type of ulcer and comorbidity. At day 60 postprocedure, 80% reepithelialisation was present in five patients (25%), while one patient with concomitant psoriasis had 50% reepithelialisation. Pain scores improved by day 7 postprocedure. The function and aesthetics of the ReCell‐treated patients were good. It is concluded that the ReCell technique may have provided the regenerative tissue stimulation necessary for the rapid healing of chronic ulcers, including those not responsive to more traditional methods.
Keywords: Chronic ulcers, ReCell ® system technique, Regenerative surgery
Introduction
Chronic ulcers (ulcers that do not heal within approximately 60 days of onset) can be a result of a delay in (or failure of) all phases of the healing process. They are sequelae of the senescence of the fibroblasts, which no longer respond to growth factors, and therefore, have a decreased index of proliferation and altered morphology 1, 2. Local factors (such as ischaemia, altered tissue, necrosis, extracellular matrix (ECM) remodelling, reduced reepithelialisation, hydration status, infection, foreign bodies, lack of response to growth factors, repeated trauma, inflammation, oedema, ionising radiation) and systemic factors (such as poor nutrition, metabolic and/or autoimmune pathologies, connective tissue pathologies, immunosuppressants, anti‐inflammatory therapy, genetic alterations, smoking) play an aetiology role in ulcer chronicity. The normal healing process of ulcers is divided into multiple phases 3: elimination of the necrotic base by proteolytic and lipolytic enzymes of leukocytes, formation of granulation tissue resulting from the intense proliferative activity of the connective tissue, and lastly, epithelialisation. The healing of ulcers is largely determined by three factors: reduction or elimination of the injury, proper wound care, and an adequate supply and contribution of nutrients. By assuming the reduction or the elimination of the injury (e.g. by pressure, micro traumatic, vascular), the healing of the ulcer cannot occur without adequate care of the wound, which itself is subdivided into: cleansing, reduction of the necrotic tissue and appropriate medications 4. Conventionally used therapies for ulcer treatment include: advanced dressings (hydrogel, hydrocolloids, antiseptic enzymes, polyurethane, alginates, hydro fibres, biosynthetic cellulose and hyaluronic acid); advanced therapies (hyperbaric, vacuum‐assisted closure therapy); reconstructive surgery (patches or grafts) 5 and finally, regenerative surgery (tissue bioengineering, Platelet‐Rich Plasma, lipostructures) 3. In patients with significant comorbidities, optimising the management of the underlying medical condition can markedly improve a wound's healing potential. In such a situation, measuring and acting on the results of biochemical parameters such as blood glucose and renal and hepatic function is necessary. Monitoring inflammatory markers and wound culture (biopsy/swab) may also assist in diagnosis and allow targeted treatment. Using clinical criteria alone to accurately predict which wounds are unlikely to heal in timely manner is difficult 6. Accurate detection of elevated protease activity or other biomarkers would aid early diagnosis and allow appropriate use of treatments aimed at optimising the wound environment 7. However, diagnostic tests directing treatment and predicting outcomes are not yet widely available or of proven efficacy; they may, however, be the key to improvements in outcome for the slow or non healing wound. All the techniques mentioned are valid, and normally allow the improvement or complete healing of the ulcer. The ReCell® system is an autologous skin‐graft suspension. It is minimally invasive and can be combined with other surgical techniques such as mesh graft 8, 9. The advantage of using the ReCell is that it requires a small donor site, and can be applied in chronic ulcers of different aetiology. It allows immediate application of an autologous cell suspension without the need for prior isolation and cultivation in the laboratory, therefore patients can immediately benefit from the application without the delay required by other techniques such as bioengineered tissue that requires at least 21 days for growth and cell expansion. Currently, there is no treatment suitable for all types of injury, or for all stages of tissue repair. The use of advanced therapies may improve the cost–benefit ratio, decrease hospitalisation time and accelerate healing, thus improving the quality of life of the patient.
The aim of this study was to assess the clinical effectiveness of the ReCell technique in patients with chronic ulcers classified as venous, arterial, diabetic or traumatic that were unresponsive to conventional therapies for at least 6 months.
Materials and methods
Patient population
Ethics Committee approval was obtained for this study. All patients received appropriate information on the intervention and provided informed consent. From October 2011 to July 2012, 20 patients, 8 men and 12 women with a mean age of 70 years, with chronic ulcers of venous arterial, diabetic or traumatic origin were treated using the ReCell® technique at U.O.C. Plastic and Reconstructive Surgery Center, Policlinic Casilino, “Tor Vergata” University, Rome. All patients had been previously treated with conventional therapies without complete healing either prior to enrolment or as part of a 4‐week preoperative run‐in period. Patients were excluded from participation in the study if the depth of the ulcer exceeded 4 mm (without soft‐tissue exposure ex. tendon or fat). Each procedure was executed by a team composed of two surgeons.
Study periods
The protocol was divided into three separate intervals: preoperative (T0), operative (T1) and postoperative (T2).
Preoperative interval
The preoperative interval (T0) was a 4‐week period, prior to ReCell treatment, in which the wound bed of the ulcers were prepared for ReCell procedures by the application of a series of advanced dressings and swabs with antibiogram, or other surgical procedures such as dermal epidermal graft (Figures 1, 2, 3, 4, 5, 6).
Figure 1.
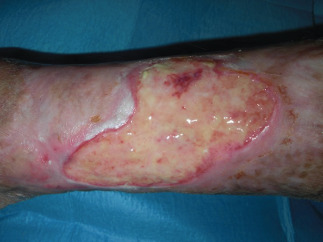
Preoperatory views at first surgical procedure.
Figure 2.
Intraoperatory views at first surgical procedure: autologous dermal–epidermal graft and dermal substitute.
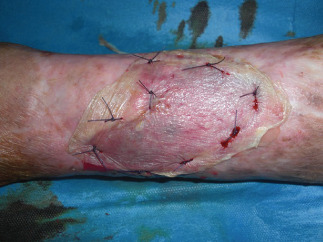
Figure 3.
Postoperatory views at 90 days from the first surgical procedure.
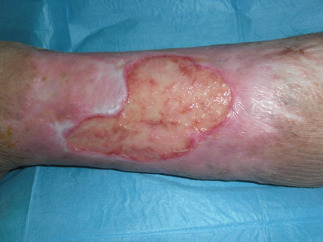
Figure 4.
Preoperatory views (120 days) from first surgical procedure.
Figure 5.
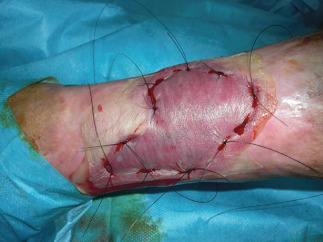
Intraoperatory views at second surgical procedure: autologous dermal epidermal graft and dermal substitute.
Figure 6.
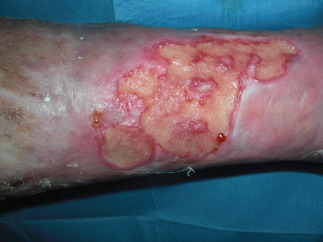
Postoperatory views at 90 days from the second surgical procedure and preoperatory views at ReCell procedure.
Operative (T1) interval
On the day of the surgery with routine aseptic precautions, the patient was sedated with a small dose of midazolam (1–2 mg) for pre‐medication (or induction of sedation), and propofol (0·5–0·75 mg/kg) followed by a variable‐rate infusion at 25–100 µg/kg/min. A local anaesthetic (1% lidocaine without adrenaline) was administered to the donor and ReCell recipient (ulcer) sites. A 1·5 × 1·0 cm, 0·25–0·3 mm thick donor sample was obtained using an electric dermatome from the patient's thigh. The ulcerate area was prepared by surgical debridement. The donor sample was obtained, processed and the resultant cell suspension (1·5–2·0 ml) was applied using the ReCell® system according to manufacturers' instructions with minor modifications (Table 2). Following application of the cellular suspension, the wound was covered with a primary, hyaluronic acid‐based dressing (fibres) 10 in order to protect and minimise the tangential forces, limit any infections, to enable adequate haemostasis and to serve as a growth scaffold (Figures 6, 7, 8). A non adherent dressing (Bio‐cellulose micro‐crystalline) was applied to the donor site.
Table 2.
Comparison of manufacturers' instructions to the protocol used in this patient population
| Manufacturer's instructions | Ulcer protocol | |
|---|---|---|
| Size of donor biopsy | 1·0 × 1·0 cm | 1·5 × 1·0 cm |
| Depth of donor biopsy | 0·2–0·3 mm | 0·25–0·3 mm |
| Donor site | Same aesthetic and functional characteristics as receiving site | No restrictions |
| Preparation of receiving site | Dermabrasion with rotating diamond burr | Surgical debridement and standard surgical hygiene on the day of procedure |
| Primary dressing | Non adherent | Hyaluronic acid* |
| Postoperative follow‐up | Identical | Identical |
Hyaluronic acid in fibres was applied to the recipient site following the application of the cell suspension as a growth scaffold and primary medication.
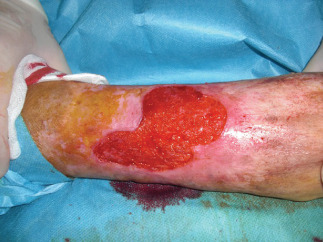
Figure 7.
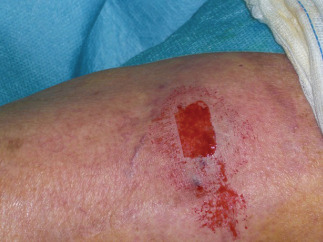
Intraoperatory biopsy site.
Figure 8.
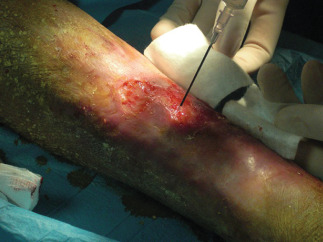
Intraoperatory ReCell procedure.
Postoperative (T2) interval
Patients were typically hospitalised for two nights. During the immediate postoperative period, particular attention was given to avoid direct injury to the wound by tangential forces (clothing, dressings). Patient mobilisation generally began within 24 hours of the procedure and was dependent on the size and location of the ulcer as well as general health of the individual. Patients were instructed to limit activity, protect the areas from further trauma or injury and to keep the area dry for 7 days. The use of protective sunscreen and the nightly application of a moisturising cream were advised to avoid photo damage and to improve appearance and texture of the wound. Compression bandages were applied as appropriate. The original hyaluronic acid dressing was removed at 7 days and replaced weakly since healing (on the top, the second dressing is sterile gauze). The patients were informed that healing process would be gradual to expect changes in the appearance (such as pigmentation and scarring) of the area; with final results being obtained between 3 and 6 months and up to a year postprocedure. Because patient compliance plays an important role in the successful outcome 11, each patient was well informed of the procedure and understood that adherence to the protocol was essential for optimal results (Figures 9 and 10).
Figure 9.
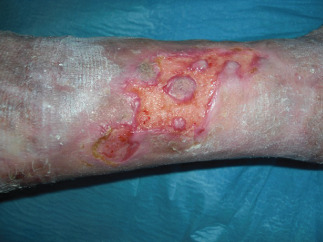
Postoperatory views at 20 days.
Figure 10.
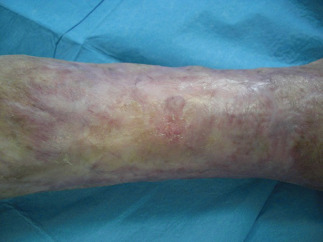
Healing, postoperatory view at 52 days post‐ReCell procedure.
Assessments
Images of the treated ulcer site were obtained preoperatively, intraoperatively as well as postoperatively. Healing, as complete reepithelialisation of the ulcer (and donor) sites and pain were assessed at approximately 7, 14 and 21, 30, 40, 50 and 60 days postoperatively. Wounds were swabbed and cultured at least 20 days after the procedure, and treated with antibiotics as appropriate. We used a Visual Analogue Scale (VAS) for the assessment of pain. The scale has a 100‐mm horizontal line, for which the left end was labelled ‘no pain’ and the right end was labelled 'the worst pain possible. Patients were asked to rate their current pain intensity. The VAS score is determined by measuring in millimetres from the left end of the line to the point that the patient marks 12. It was used for pain assessment in the biopsy site as well as the wound prior to and 7 days after ReCell procedure. The aesthetic appearance of the epithelialisation was evaluated when the wounds were considered healed (100% reepithelialisation) by an independent plastic surgeon unaware of the procedure, according to the Manchester Scar Scale (MSS) which uses a range of scores from 5 (best) to 18 (worst). MSS was used to assess the colour of the scars defined as perfect, slight, obvious or gross mismatch compared with the surrounding skin; to assess the appearance of the skin (matte or shiny); to assess the contours compared with the surrounding skin, slightly proud/indented, hypertrophic to keloid; to assess the texture from normal, just palpable, firm to hard; and to assess the margins, whether distinct or not 13, 14. Overall patient satisfaction was evaluated using the Rosenberg Self‐Esteem Scale: 1 = strongly agree, 2 = agree, 3 = disagree, 4 = strongly disagree. Patient compliance to the protocol was also evaluated.
Statistical analysis
Statistical analyses were conducted using SAS software (SAS Institute Inc., Cary, NC). Patient demographics and baseline characteristics were summarised and expressed as percentage of population and mean including range (Table 3). Patient pain ratings are expressed as mean of observed and change from baseline (Table 1). The Wilcoxon signed‐rank test was used assessing the null hypothesis that the median difference is equal to 0 to test the significance. Time to reepithelialisation was summarised and plotted. Complete ulcer healing and MSS components were summarised in figures and tables, with Kaplan–Meyer plot used for showing the reepithelialisation course. Under the hypothesis of a faster healing in the presence of an initially smaller ulcer, one‐tailed Spearman Rank Correlation Coefficient (R) was applied to compare reepithelialisation time with initial ulcer area. Statistically significant threshold was set at P < 0·05.
Table 3.
Demographic and baseline character
| Summary | All subjects |
|---|---|
| Number of subjects | 20 |
| Aetiology | |
| Arterial | 4 (20·0%) |
| Diabetic | 4 (20·0%) |
| Posttraumatic | 4 (20·0%) |
| Venous | 8 (40·0%) |
| Gender | |
| Male | 8 (40·0%) |
| Female | 12 (60·0%) |
| Age, mean (range) | 70·9 (48·83) |
| Ulcer age (months), mean (range) | 18·3 (6·36) |
| Ulcer depth (mm), mean (range) | 0·75 (0·2,1·2) |
| Ulcer area (cm2), mean (range) | 18·6 (4·51) |
| Previous treatment | |
| Advanced dressings | 9 (45·0%) |
| Dermal/epidermal graft | 9 (45·0%) |
| Comorbidities | |
| Arterial hypertension | 2 (10·0%) |
| Cardiopathy | 4 (20·0%) |
| Chronic renal insufficiency | 2 (10·0%) |
| Multidrug‐resistant pseudomonas | 2 (10·0%) |
| Psoriasis | 1 (5·0%) |
| Type 1 diabetes mellitus | 1 (5·0%) |
| Type 2 diabetes mellitus | 7 (35·0%) |
Table 1.
Pain ratings: Wilcoxon signed ranks test was used to assess the differences from Vaseline to 7 days post‐treatment
| Summary | Type of ulcer | All subjects | Signed‐rank test, P‐value 1 | |||
|---|---|---|---|---|---|---|
| Arterial | Diabetic | Posttraumatic | Venous | |||
| Number of subjects | 4 | 4 | 4 | 8 | 20 | |
| Baseline, mean (range) | 6·5 (5 to 8) | 3·5 (0 to 6) | 5·8 (5 to 7) | 3·5 (0 to 7) | 4·6 (0 to 8) | |
| 7 Days post‐treatment, mean (range) | 2·5 (1 to 4) | 1·0 (0 to 2) | 2·3 (0 to 4) | 0·5 (0 to 2) | 1·4 (0 to 4) | |
| Change from baseline, mean (range) | −4·0 (−6 to −3) | −2·5 (−4 to 0) | −3·5 (−5 to −2) | −3·0 (−5 to 0) | −3·2 (−6 to 0) | <0·0001 |
| Pain rating of biopsy site, mean (range) 2 | 2·5 (0–8) | 2·5 (0–6) | 2·5 (0–6) | 2·1 (0–7) | 2·4 (0–8) | |
Results
From October 2011 to July 2012, 20 patients, 8 men and 12 women with a mean age of 70 years (range 48–83) with chronic ulcers of venous (N = 8), arterial (N = 4), diabetic (N = 4) or traumatic (N = 4) origin were treated by a team of two surgeons using the ReCell® technique at U.O.C. Plastic and Reconstructive Surgery Center, Policlinic Casilino, “Tor Vergata” University, Rome (Table 3). The average age of the ulcer was 18·3 months (range 6–36 months), with an average depth of 0·8 mm (range 0·2–1·2 mm), ranging in size from 2·2 cm × 2·2 cm to 8·5 × 6·0 cm. Twelve patients (60%) had at least one comorbidity, with type 2 diabetes being the most frequent. MSS components were summarised for the enrolled subjects in Table 4. Eleven patients did not experience any pain associated with the donor biopsy site. Nine patients reported some degree of pain (range 2–8) at the biopsy site for the first 7 days, which resolved over the course of the next several days (Table 1). Seventeen patients reported pain associated with the ulcer prior to ReCell treatment (range 3–8). In each instance, there was an improvement in pain scores at the day 7 follow‐up assessment (range 0–4). The postprocedure pain was not severe enough to interfere with daily activities. There were no instances in which a patient's pain score worsened as a result of the ReCell procedure (Table 1).
Table 4.
Manchester scar scale and components
| Summary | Type of ulcer | All Subjects | |||
|---|---|---|---|---|---|
| Arterial | Diabetic | Posttraumatic | Venous | ||
| MSS total, mean (range) | 9·7 (9–10) | 10·0 (10–10) | 10·6 (8–15) | 10·6 (7–14) | 10·4 (7–15) |
| Texture, mean (range) | 8·5 (8–9) | 10·0 (10–10) | 9·0 (6–12) | 10·0 (7–13) | 9·6 (6–13) |
| Contour, mean (range) | 10·0 (8–12) | 10·7 (9–12) | 12·0 (5–17) | 9·7 (7–13) | 10·4 (5–17) |
| Colour, mean (range) | 10·0 (9–11) | 10·3 (8–14) | 10·7 (8–16) | 10·7 (6–15) | 10·5 (6–16) |
| Appearance, mean (range) | 10·0 (10–10) | 11·3 (8–13) | 11·3 (8–14) | 11·5 (8–16) | 11·2 (8–16) |
| Margins, mean (range) | 10·0 (8–12) | 7·7 (7–9) | 10·0 (7–14) | 11·3 (9–14) | 10·1 (7–14) |
There were no complications associated with the procedure with the exception of a local worsening of the wound observed in one patient with concomitant psoriasis. This patient had a pre‐existing infection that worsened after the surgery. In one case, we achieved a 100% reepithelialisation even if the patient was affected by essential thrombocytosis and in treatment with hydroxyurea. This case suggests us to improve and to broaden the research for such complex cases.
Complete ulcer healing, defined as 100% reepithelialisation was observed between 45 and 60 days post‐ReCell in 14 patients (70%) (Table 5, Figure 11). At day 60 postprocedure, 80% reepithelialisation was present in five patients (25%), whereas one patient with concomitant psoriasis had 50% reepithelialisation. Of the five patients with 80% reepithelialisation at day 60, two patients had concomitant multidrug‐resistant Pseudomonas infections, type 2 diabetes mellitus and cardiopathy, one patient had arterial hypertension and the other two patients had large ulcers (8·5 × 6·0 cm and 6·5 × 4·0 cm). Figure 12 shows the Kaplan–Meyer plot for the timing of healing. The healing time was similar for four types of ulcers, with a mean (range) of 46·5 (45–48) days for arterial ulcers, 49·3 (47–52) for diabetic ones, 49·3 (40–56) for posttraumatic ulcers and 47·5 (41–54) for venous ones. On the other hand, the healing time was found significantly correlated with the initial ulcer area (R = 0·397, P = 0·042)
Table 5.
Percentages of complete ulcer healing by day 60
| Summary | Type of ulcer | All subjects | |||
|---|---|---|---|---|---|
| Arterial | Diabetic | Posttraumatic | Venous | ||
| Success by day 60 | 2/4 (50·0%) | 3/4 (75·0%) | 3/4 (75·0%) | 6/8 (75·0%) | 14/20 (70·0%) |
| Exact 95% confidence interval | 6·8, 93·2 | 19·4, 99·4 | 19·4, 99·4 | 34·9, 96·8 | 45·7, 88·1 |
Figure 11.
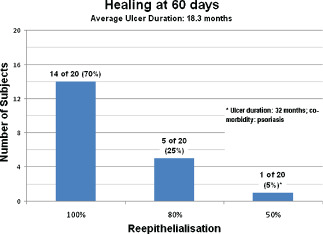
Healing at 60 days.
Figure 12.
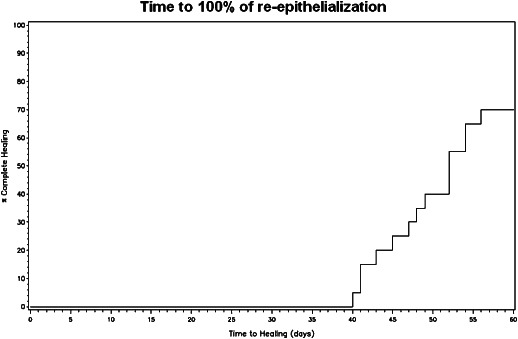
Time of 100% of reepithelialisation.
Discussion
A chronic wound can be described by different parameters which include: the age of the ulcer, inflammation, blood flow, pain and coexisting systemic factors. Wound healing is a complex process to restore the skin barrier that involves multiple cell types including: keratinocytes, fibroblasts, endothelial cells, macrophages and platelets. The migration, infiltration, proliferation and differentiation of these cells will culminate in an inflammatory response, the formation of new tissue and ultimately wound closure. The ReCell® technique, already used for the treatment of other pathologies, can provide a positive stimulation to assist and accelerate in the healing of chronic ulcers. It is based on traditional epidermal–dermal grafting methods; however, this technique uses an autologous cell suspension which is applied (sprayed or dripped) directly onto the wound rather than grafting of intact donor tissue. An advantage of using this method over traditional skin grafting is that only a small donor site is required; the cell suspension obtained from a small donor site split‐thickness biopsy can cover an acute wound with a much larger surface area (80 times larger than the donor site) 8, 15. The ReCell kit is a ready‐to use, disposable kit for autologous cell sampling, which includes a processing unit that, from a small partial thickness skin biopsy generates a cell suspension composed of keratinocytes, melanocytes, fibroblasts and Langerhans cells with a high proliferating capacity to be applied to the receiving site 9. The application of a healthy cell population onto a wounded area favours the migration and proliferation of healthy cells, resulting in quicker reepithelialisation of the wound, regeneration of the tissue and accurate repigmentation. The ReCell method, studied primarily for the treatment of burns, has additionally been used by our group (and others) for the treatment of scars and stable vitiligo lesions with favourable clinical outcomes 1, 8, 11, 16, 17. The protocol was modified slightly in this patient population to ensure that all necrotic and fibrinous tissue were removed prior to the application of the cell suspension, and to provide a growth scaffold for the newly applied cells.
ReCell, compared with traditional surgical treatments is easy to use and minimally invasive. It can be in complicated patients unresponsive to conventional treatments and also can improve the outcome when used in combination with other techniques.
Limitations of this study include a small patient population, ulcers of various aetiologies, concomitant diseases and lack of a control group for comparison. Despite these limitations, we believe that the positive results obtained in this patient population demonstrate the potential utility of this technique for the treatment of chronic ulcers.
Conclusion
Complete ulcer healing, defined as 100% reepithelialisation was observed between 40 and 60 days in 14 patients (70%) depending on the type of ulcer and comorbidity. Five patients achieved 80% reepithelialisation and one patient achieved 50% by day 60. All donor biopsy sites were healed without complications and were often indistinguishable from the surrounding skin. We found no worsening of infection. Colour, texture and aesthetic results were good. The pain was minimal and did not interfere with the ability to engage in normal daily activities. Compliance and patient satisfactions were very good. In this study, the healing was obtained by performing just one procedure with ReCell, but this does not exclude a possible reduction of healing period by performing more than one session with the same system.
The ReCell® technique is simple, minimally invasive, biocompatible and effective in the treatment of chronic ulcers. In chronic ulcers unresponsive to previous treatments, the ReCell system may give the stimulus necessary for tissue regeneration. It could potentially be used for the treatment of ulcers involving the tissues under the reticular dermis in combination with tissue substitutes.
References
- 1. Cervelli V, De Angelis B, Lucarini L, Spallone D, Balzani A, Palla L, Gentile P, Cerulli P. Tissue regeneration in loss of substance on the lower limbs through use of platelet‐rich plasma, stem cells from adipose tissue, and hyaluronic acid. Adv Skin Wound Care 2010;23:262–72. [DOI] [PubMed] [Google Scholar]
- 2. Jun JI, Lau L. Cellular senescence controls fibrosis in wound healing. Aging (Albany NY) 2010;2:627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cervelli V, Brinci L, Spallone D, Tati E, Palla L, Lucarini L, Angelis D. The use of Matriderm® and skin grafting in post‐traumatic wounds B. Int Wound J 2011. DOI: 10.1111/j.1742-481X.2011.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lionelli GT, Lawrence WT. Wound dressings. Surg Clin North Am 2003;83:617–38. [DOI] [PubMed] [Google Scholar]
- 5. Wicker J, Kamler K. Current concepts in limb regeneration: a hand surgeon's perspective. Ann N Y Acad Sci 2009;1172:95–109. [DOI] [PubMed] [Google Scholar]
- 6. Serena T, Sibbald G, Snyder R. Virtual conference: wound assessment and diagnosis made easy discussion. Wounds Int 2011;2:1–7. [Google Scholar]
- 7. Snyder RJ, Driver V, Fife CE, Lantis J, Peirce B, Serena T, Weir D. Using a diagnostic tool to identify elevated protease activity levels in chronic and stalled wounds: a consensus panel discussion. Ostomy Wound Manage 2011;57:36–46. [PubMed] [Google Scholar]
- 8. Gravante G, Di Fede MC, Araco A, Grimaldi M, DeAngelis B, Arpino A, Cervelli V, Montone A. A randomized trial comparing ReCell system of epidermal cells delivery versus classic skin grafts for the treatment of deep partial thickness burns. Burns 2007;33:966–72. [DOI] [PubMed] [Google Scholar]
- 9. Wood FM, Giles N, Stevenson A, Rea S, Fear M. Characterization of the cell suspension harvested from the dermal epidermal junction using a ReCell® kit. Burns 2012;38:44–51. [DOI] [PubMed] [Google Scholar]
- 10. Scuderi N, Onesti MG, Bistoni G, Ceccarelli S, Rotolo S, Angeloni A, Marchese C. The clinical application of autologous bioengineered skin based on a hyaluronic acid scaffold. Biomaterials 2008;29:1620–9. [DOI] [PubMed] [Google Scholar]
- 11. Cervelli V, De Angelis B, Balzani A, Colicchia G, Spallone D, Grimaldi M. Treatment of stable vitiligo by ReCell system. Acta Dermatovenerol Croat 2009;17:273–8. [PubMed] [Google Scholar]
- 12. Huskisson EC. Visual Analogue Scales. In: Melzack R, editor. Pain measurement and assessment. New York: Raven Press, 1983:33–7. [Google Scholar]
- 13. Bayat A, McGrouther DA, Ferguson MWJ. Skin scarring. BMJ 2003;326:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roques C, Teot L. A critical analysis of measurements used to assess and manage scars. Int J Low Extrem Wounds 2007;6:249–53. [DOI] [PubMed] [Google Scholar]
- 15. Wood FM. In: Hyakusoku H et al., editors. Color Atlas of burn reconstructive surgeryChapter 6. Berlin Heidelberg: Springer‐Verlag, 2010;6:50–4. [Google Scholar]
- 16. Wood FM. Clinical potential of autologous epithelial suspension. Wounds 2003;15:16–22. [Google Scholar]
- 17. Mulekar SV, Ghwish B, Al Issa A, et al. Treatment of vitiligo lesions by ReCell vs. conventional melanocyte‐keratinocyte transplantation: a pilot study. J Dermatol 2008;158:45–9. [DOI] [PubMed] [Google Scholar]


