Abstract
Delayed wound healing in elderly males is a complex process in which the factors responsible are not fully understood. This study investigated the hormonal, oxidative and angiogenic factors affecting wound healing in aged rats. Two groups consisting of eight healthy male Wistar Albino rats [young (30 ± 7 days) and aged (360 ± 30 days)], and a cutaneous incision wound healing model were used. Scar tissue samples from wounds on the 7th, 14th and 21st days of healing were evaluated for hydroxyproline and vascular endothelial growth factor content. Macrophage, lymphocyte, fibroblast and polymorphonuclear cell infiltration; collagen formation and vascularization were assessed by light and electron microscopy. The free oxygen radical content of the wounds was measured by a chemiluminescence method. Blood sample analysis showed that the hydroxyproline and total testosterone levels were significantly higher, and the oxygen radical content was significantly lower in young rats. Histopathological, immunohistochemical and ultrastructural evaluations revealed higher amounts of fibroblasts and collagen fibers, and more vascularization in young rats. These results are indicative of the delayed wound healing in aged rats. A combination of multiple factors including hormonal regulation, free oxygen radicals and impaired angiogenesis appears to be the cause of delayed cutaneous healing.
Keywords: Free oxygen radicals, Male aged rats, Testosterone, Vascular endothelial growth factor, Wound healing
INTRODUCTION
Wound healing is often delayed in the elderly. In recent years, the increasing size of the geriatric population has increased the interest in delayed wound healing. An inverse correlation between age and wound healing has been reported previously. Aging is associated with a gradual decrease in healing capacity, which results in significant morbidity and mortality in the elderly 1, 2, 3, 4.
Impaired wound healing in the elderly is associated with enhanced proteolysis and degradation of matrix constituents due to excessive leukocytes and inflammation. Delayed reepithelialisation and neovascularisation, altered ratio of mature to immature macrophage populations, and impaired fibroblast migration are other characteristics of wound healing in elderly subjects 1, 2, 3, 5, 6, 7.
Gender differences in wound healing have also been reported in clinical studies. Wound healing was found to be significantly faster in women 1, 8. However, the mechanisms underlying altered inflammatory response and different healing rates between sexes have not been clearly understood yet 2, 4, 6, 8, 9. The difference between sexes may be because of the modulatory functions of sex hormones in wound healing. Reduced estrogen level associated with increased age has been identified as a significant risk factor for delayed cutaneous healing (10). Evidence shows that estrogen improves wound healing in humans by altering cytokine profiles, inducing a proinflammatory state and modulating the balance between matrix synthesis and degradation (6).
Ashcroft and others have previously showed that exogenous estrogen can reverse age‐related impaired healing in females by reducing wound size, stimulating wound contraction and increasing the rate of epithelialisation 1, 8, 10. However, the findings that wound healing is slower among elderly males than elderly females and that the response to exogenous estrogen is significantly reduced in males compared to females, strongly suggest the involvement of other unknown factors, apart from estrogen, in the wound‐healing process in elderly males (6). According to the studies demonstrating the effects of dominant male sex hormones on wound healing, androgens appear to have deleterious effects by directly enhancing inflammatory response and reducing matrix deposition, in contrast to estrogen 4, 6, 9. A positive correlation has been observed between increased testosterone levels and delayed healing 4, 6. However, testosterone levels gradually decline with increasing age; therefore, the slower wound‐healing process of elderly males compared with that of young males suggests the existence of factors other than testosterone.
Toxic oxygen radicals generated by neutrophils and macrophages are also important mediators of inflammatory response in wound healing 11, 12, 13, 14. Migration of neutrophils and macrophages to a particular tissue site initiates inflammation‐induced radical‐derived tissue damage. The products of activated neutrophils, superoxide and hydrogen peroxide, can degrade and destroy normal biomolecules such as lipids, proteins, nucleic acids and all components of the connective tissue compartment and collagen 14, 15, 16. In contrast, antioxidants can modulate this damage by scavenging free radicals and lipid peroxyl radicals (17). Our previous studies have shown that the application of a potent radical scavenger agent, desferrioxamine, resulted in significant decrease in oedema, mean epidermal thickness, mean number of cell layers and mixed inflammation in mouse skin (18). The well‐known phenomenon of decreased radical scavenging capacity of tissues on aging is also suggested to be one of the mechanisms underlying delayed wound healing in the elderly 14, 19, 20. To our knowledge, this study is the first to examine the impact of aging on oxidative stress during the wound‐healing process.
Angiogenesis is another complex process that could play a role in wound healing (21). Aging‐induced decrease in tissue perfusion and impairment of angiogenesis is known to affect wound healing (1). Although the decrease in the number of angiogenic cells in circulation has been shown to indicate delayed wound healing, the effects of many factors on angiogenesis are yet to be clarified (22).
The causative factors of delayed wound healing in elderly males are still under debate in the literature. The goal of this experimental study was to investigate the hormonal, oxidative and angiogenic factors affecting wound healing in aged male rats.
MATERIALS AND METHODS
This project was approved by the Istanbul University Local Ethical Committee for Animal Experiments (No.: 200, 12.30.2010).
Animal housing
Sixteen healthy male Wistar Albino Rats (Group A: 30 days old, 130 ± 18 g, 8 rats; Group B: 12 months old, 380 ± 22 g, 8 rats) were used in the study. Rats were housed in regular polypropylene cages (four rats per cage) on a 12‐h light, 12‐h dark cycle in a temperature‐controlled room and fed a standard diet (24% protein, 1% lysine, 0.6% methionine, 0.4% cystine, 7% cellulose, 1% NaCl, 1%–2% Ca and 2650 kcal/kg) and given water ad libitum during the experiment. The back hair of all the rats was shaved off with an electric razor before each surgical procedure.
Surgical procedures
Three separate incisions, 4 cm in length and including skin and subcutaneous tissue, were made in the back region of each rat under general anaesthesia, which was achieved by intramuscular injection of 50 mg/kg ketamine HCl into the back thigh. The first two incisions were on either lateral side 2 cm from the midline, and the third incision was on the midline of the caudal region beginning at the end of the first 2 incisions (Figure 1).
Figure 1.
Incisional wounds on the backs of rats.
All the incisions were closed instantly with a 9‐mm skin stapler. Rats were taken to their cages after the operation, and a topical anesthetic agent (2% lidocaine hydrochloride spray) was applied to their back region every 3 h for 12 h. All the metal sutures were removed on the fifth day after the operation. No wound or anaesthetic complications or deaths occurred during the experiment.
The scar tissue on the backs of the rats was excised on the 7th, 14th and 21st day after the first operation (left upper scars at the 7th day, right upper scars at the 14th day and lower midline scars at the 2lst day). The scar tissue excisions were 4 cm in length, 6 mm in width, and included skin and subcutaneous tissue, and were again carried out under anaesthesia induced by ketamine followed by topical anaesthesia. The wounds were immediately closed again with a skin stapler after the excisions, and the metal sutures were removed on the fifth day after the second operation. The scar excisions were divided into six subgroups of tissue in the two groups (A7, A14, A21, B7, B14 and B21) (Table 1).
Table 1.
Groups used in the study
| Group A young rats (n = 8) | Group B aged rats (n = 8) | |
|---|---|---|
| 7th Day | A7 (1 scar tissue/rat) | B7 (1 scar tissue/rat) |
| 14th Day | A14 (1 scar tissue/rat) | B14 (1 scar tissue/rat) |
| 21st Day | A21 (1 scar tissue/rat) | B21 (1 scar tissue/rat) |
Each of the excised scar tissues from all subgroups was divided transversely to four equal pieces (1 × 0·6 cm). Two excised tissue pieces were instantly stored in ice bags and sent to the Biochemistry Departments of Marmara and Namık Kemal Universities for measurement of free oxygen radicals by chemiluminescence and hydroxyproline levels. One excised tissue piece from each excised scar was stored in 10% formaldehyde solution and sent to Taksim Hospital, Pathology Department for vascular endothelial growth factor (VEGF) analysis and histopathology evaluation. The final excised tissue piece from each scar was stored in 2·5% glutaraldehyde solution and sent to Istanbul University, Histology Department for electron microscopy evaluation of the healing tissue.
All rats were euthanised by cardiac puncture after collecting blood samples for biochemical analysis following the last scar excisions at the 21st day of the experiment. Blood samples were sent to the Biochemistry Department of Haseki Hospital for analysis of plasma estrogen and total testosterone levels in the rats.
Blood sample analysis
Following centrifugation of the blood samples, plasma samples were collected. For total testosterone and estrogen measurement, chemiluminescence techniques were used with a Siemens Advia Centaur XP (Siemens Healthcare Diagnostics, Deerfield, IL) immunoassay system and the IMMULITE®2000 (Siemens Healthcare Diagnostics, Deerfield, IL) total testosterone (L2KTW2) and IMMULITE®2000 estrogen (L2KE22) kit, respectively.
Tissue free oxygen radical measurements
Reactive oxygen species were determined by a chemiluminescence technique. Measurements were made at room temperature using a Mini Lumat Junior LB 9509 luminometer (EG&G Berthold, Hamburg, Germany). Specimens were put in vials containing PBS‐HEPES buffer. Reactive oxygen species were quantified after the addition of lucigenin or luminol at a final concentration of 0.2 mM. Luminol detects mainly hydrogen peroxide, hydroxyl, hypochlorite, peroxynitrite and lipid peroxy radicals, whereas lucigenin is particularly selective for the superoxide radical. Counts were obtained at 1‐minintervals, and the results were given as the area under curve (AUC) for a counting period of 5 minand corrected for wet tissue weight (rlu = relative light unit/mg tissue) 23, 24.
Histopathologic evaluations
All tissues to be examined by light microscopy were immediately placed in 10% formalin and then dyed using the Masson trichrome technique. Five random consecutive regions were selected, and polymorphonuclear cells (PMNs), lymphocytes, macrophages, epithelisation and fibroblasts in each region were detected and recorded under 40× amplification. The Verhofstadt scoring system was used to evaluate histological parameters. Each parameter was scored from (−) to (+ + +): −, absent; +, slight increase; ++, moderate infiltration and + + +, massive infiltration (25).
VEGF analysis
Immunohistochemistry was undertaken to determine the index of vascularisation as previously described by Gun et al. (26). Sections of 4‐mm thickness were selected from paraffin blocks and processed using an automatic immunohistochemistry device (Ventana Benchmark XT, Ventana, AZ). The sections were stained automatically using VEGF Ab–1 (Rabbit PAb; Neomarkers, Fremont, CA) and the XT ABC 3 protocol (AEC Basic Detection Kit, Ventana), which employed the streptavidin–biotin amplification technique. Angiosarcoma tissue was used as the positive control, and internal controls were used as the negative control. VEGF immunreactivity was considered positive when the cytoplasm was stained. The slides were examined by light microscopy. Epithelial and endothelial cells were assessed separately. The staining was graded as no staining (−), faint staining (+), evident staining (++) and intense staining (+ + +).
Ultrastructure by electron microscopy
Tissues were fixed with 2·5% glutaraldehyde in 0.1 M sodium cacodylate buffer and post/fixed in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer for 1 h at 4°C. Tissues were incubated in 1% uranyl acetate for 1 h at 4°C, dehydrated in a graded acetone series and embedded in Epon 812. Samples were cut using a rotating blade microtome (Leica, Heerbrugg, Switzerland) and 70‐nm‐thick sections were mounted on copper grids. Sections were subsequently stained with 5% uranyl acetate and counterstained with Reynold's lead citrate. Sections were examined with a Jeol‐Jem 1011 (PT. Teknolabindo Penta Perkasa, Jakarta, Indonesia) transmission electron microscope for macrophage and fibroblast infiltration, collagen formation and vascularisation. Photographs were taken at several magnifications (27).
Hydroxyproline analysis
A simplified microplate assay was used for the determination of hydroxyproline. The assay was adapted from a segmented‐flow auto‐analysis procedure (28) based on a method originally described by Bergman and Loxley (29). Skin tissues were hydrolysed in 500 ml HCl and 500 ml distilled water in screw‐capped 6‐ml polypropylene tubes at 108°C for 8–12 h. On cooling, the hydrolysates were partially neutralised with 4 ml sodium hydroxide and centrifuged to sediment particulate debris. All tissue hydrolysates were diluted to 1:20 before the assay. Calibrators, unknown samples and blanks were pipetted in 50‐ml aliquots onto a 96‐well plate. An oxidising solution containing chloramine T (100 ml) was added to each well except those designated as blanks. The same amount of oxidation buffer without chloramine T was added to each blank well. The plate was mixed by gentle shaking and left for 5 min. Ehrlich's reagent (100 ml) was added to each well and mixed thoroughly. The plate was covered with an adhesive plate seal and incubated in a water bath at 60°C for 45 min. Absorbance was read at 550 nm using an ELx800 absorbance microplate reader (Biotech Instruments Inc., Vernon Hills, IL). A calibration curve was constructed, and the hydroxyproline concentration of the unknown samples was calculated from the ‘best fit' equation of the line 28, 29.
Statistical analysis
All data from the groups and subgroups were recorded using a computer‐based statistical program, Statistical Package for the Social Sciences (SPSS) 16.0. The groups were compared by t‐test, because the variances of the values in the groups were homogenous, and the distribution of variances was equal. The differences were considered significant at probability values of P < 0·05.
RESULTS
No rats died, and no wound complications were observed during the experiment. The mean hydroxyproline, luminol and lucigenin levels of the subgroups and the mean values of total testosterone and estrogen levels of the groups are shown in 2, 3, 4, 5
Figure 2.

Hydroxyproline levels of the groups. (*P < 0·001, t‐test, error bars: 95% confidence interval).
Figure 3.

Luminol levels of the groups. (*P < 0·05, **P < 0·01, t‐test, error bars: 95% confidence interval).
Figure 4.
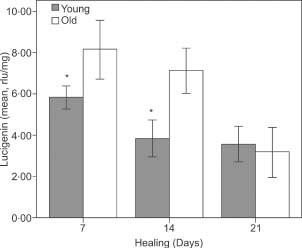
Lucigenin levels of the groups. (*P < 0·05, **P < 0·01, t‐test, error bars: 95% confidence interval).
Figure 5.
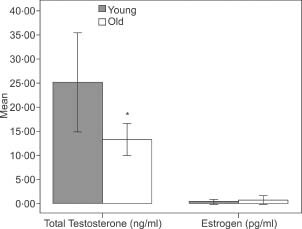
Total testosterone and estrogen levels of the groups. (*P < 0·05, t‐test, error bars: 95% confidence interval).
The mean hydroxyproline levels of the young rats were significantly higher than those of the aged rats on the 7th and 14th days of the experiment. As indicated by the levels of luminol and lucigenin, the older rats maintained higher levels of oxidative stress in the scar tissue than the young animals on days 7 and 14.
The mean total testosterone levels of the young rats were significantly higher than those of the aged rats (Figure 5). The histopathological, immunohistochemical and ultrastructural evaluations of the groups by light and electron microscopy are given in 2, 3 and Figure 6.
Table 2.
Histopathological evaluations of the groups by light microscopy, vascular endothelial growth factor expressions
| Days | Groups | PMNs | Lymphocytes | Macrophages | Epithelialisation | Fibroblasts | VEGF in epithelia | VEGF in endothelia |
|---|---|---|---|---|---|---|---|---|
| 7 | Young | ++ | ++ | + | + | ++ | + | + |
| Aged | + + + | ++ | + + + | + | + | – | – | |
| 14 | Young | − | − | + | ++ | + + + | ++ | + + + |
| Aged | + | + | ++ | + | + | + | + | |
| 21 | Young | − | − | + | + + + | + + + | ++ | ++ |
| Aged | − | + | + | ++ | ++ | + | + |
PMNs, polymorphonuclear cells; VEGF, vascular endothelial growth factor.
+, slight; ++, moderate; + + +, intense; −, absent. (Evaluation of eight samples together for each group).
Table 3.
| Days | Groups | Fibroblasts | Macrophages | Collagen fibers | Vascularisation |
|---|---|---|---|---|---|
| 7 | Young rats | ++ | ++ | + + + | + + + |
| Aged rats | + | + + + | + | + | |
| 21 | Young rats | + + + | + | + + + | + + + |
| Aged rats | + | ++ | + | + |
+, slight; ++, moderate; + + +, intense. (Evaluation of eight samples together for each group).
Figure 6.
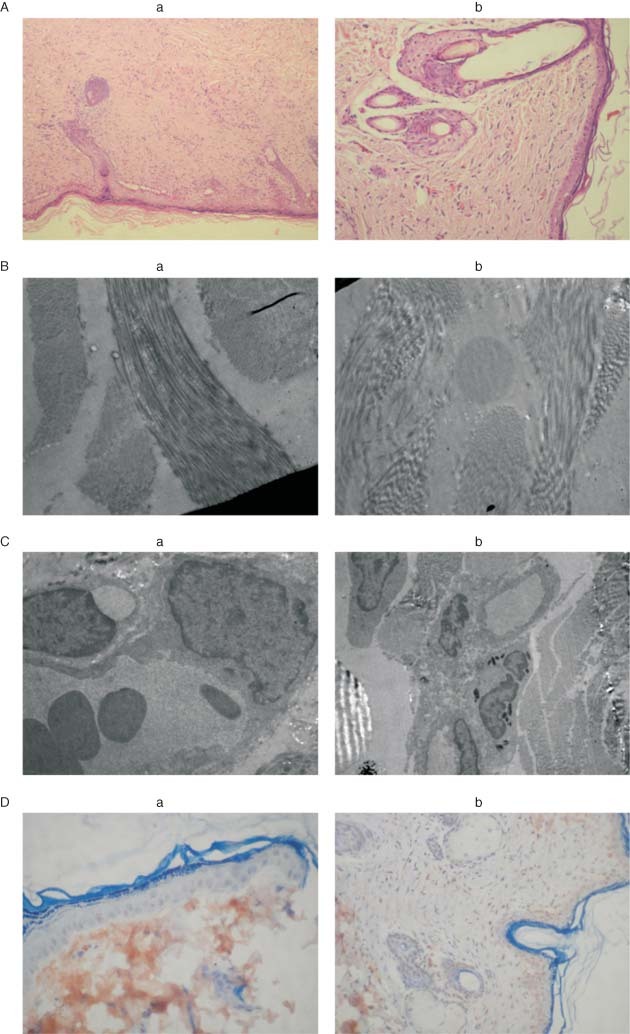
Increased collagen fibers and vascularisation shown by light microscopy (A) and electron microscopy (B), increased vascularisation shown by electron microscopy (C) and increased vascular endothelial growth factor in endothelium (D) in young rats (a) compared with aged rats (b) on the 21st day.
DISCUSSION
A wound is defined as a disruption in the continuity of cells, and wound healing is a complex and dynamic process involving restoration of the integrity of traumatised cellular structures and tissue layers. Normal wound healing follows a predictable pattern that can be divided into overlapping phases defined by characteristic cellular populations and biochemical activities 30, 31. All wounds need to progress through certain biologic stages including hemostasis and inflammation, proliferation and maturation and remodelling to successfully reestablish tissue integrity 1, 7, 30. Hemostasis initiates inflammation, which ensures neutrophil infiltration of the wound that peaks at 48 h. The second population of inflammatory cells are macrophages, which achieve significant numbers in the wound by 96 h 1, 7. The proliferative phase is the second phase of wound healing and spans days 4 through 14. Fibroblasts and lymphocytes and the endothelial cells are the cell populations that infiltrate the healing wound in the proliferative phase 1, 7. The maturation and remodelling phase, during which collagen is synthesised, degraded, reorganised and stabilised, begins through day 8 and continues for months, finally leading to scar formation 1, 6, 7, 30.
Delayed wound healing, as a consequence of age‐related structural and functional changes in the skin, is characterised by excessive inflammatory response and matrix degradation (1). Delayed reepithelialisation and neovascularisation, decline in dermal thickness and in the number of cell layers, and impaired fibroblast migration and proliferation are indicative of the disrupted healing process in elderly subjects 1, 5, 6, 7. Additionally, decreased hydroxyproline levels, which is an indicator of the total collagen amount of wound tissue, is the other marker of impaired strength and integrity and also retardation in wound healing (32).
In this study, fibroblasts, collagen fibers and epithelialisation were observed to a lesser extent in the wounds of aged rats (2, 3; Figure 6). The hydroxyproline levels of the aged rats were also significantly lower than those of young rats on the 7th, 14th and 21st days of healing (Figure 2). While the significant increase in hydroxyproline levels occurred in young rats between the 7th and 14th days, it was observed between the 14th and 21st days in aged rats. Furthermore, PMNs, lymphocytes and macrophages were higher in number in aged rats, from both light and electron microscopic evaluations, which suggested enhanced inflammation (2, 3; Figure 6). All these results are indicative of delayed wound healing in aged rats.
Recent studies have showed that male sex, with its altered inflammatory response, is a strong positive risk factor for delayed wound healing 2, 6, 8, 9. Therefore, hormonal regulation associated with impaired healing and particularly reduced estrogen levels are clearly significant risk factors for the non healing of skin wounds 2, 7, 10. Furthermore, estrogen hormone has been showed not only to accelerate wound repair in both human and animal models, but also to reverse age‐related impaired healing in elderly females and in males when used exogenously 1, 4, 6, 8, 33. However, the significantly reduced response to estrogen and slower healing in males compared with females suggest alternative mechanisms inhibiting wound healing in elderly males 4, 6, 8. In this study, minimal (near zero) mean plasma estrogen levels in both the aged and young groups of male rats led us to consider the involvement of mechanisms other than estrogen in impaired wound healing.
In contrast to estrogen, androgens have detrimental effects on wound healing by enhancing the inflammatory response and reducing matrix deposition 4, 6, 34. A positive correlation between increased levels of testosterone and delayed wound healing has been reported. Furthermore, improved wound healing was observed after castration of male mice (6). The maintenance of testosterone levels in elderly males (6) may explain their impaired wound healing when compared with elderly females. However, the significant impairment in wound healing in elderly males compared with young males (34) indicates that additional factors other than estrogen and androgens are also likely to affect wound healing. Although testosterone remains significantly bioactive throughout the life span (6), the gradual reduction of testosterone level with increasing age suggests that multiple mechanisms are involved in the delayed wound healing of elderly males. Reduction in testosterone levels in aged rats similar to that reported in the literature (6) was also observed in our study. The total testosterone levels of aged rats were significantly lower than those of young male rats (Figure 5). In contrast, skin is a steroidogenic tissue and contains the full cytochrome p450 system, responsible for producing sex steroids from cholesterol 4, 6, 35, 36, 37. This raises the possibility that testosterone locally synthesised within the wound microenvironment may also be important in the wound‐healing process. The fact that local levels of bioavailable estrogen and testosterone hormones were not measured is a limitation of this study.
Free oxygen radicals are important mediators in a number of biological processes including wound healing. They may be responsible for inflammation and tissue damage 12, 14, 15, 16. The superoxide‐dependent chemotactic factor appears to play a critical role in the development of neutrophil‐mediated inflammatory response 13, 14, 15, 16. For the secretion of these factors, oxidase systems in the neutrophil membrane related to NADPH are activated by cytokines to transform oxygen into toxic metabolites such as superoxide, peroxide and hydroxy radicals 14, 15, 16. Unstable and highly reactive toxic metabolites of molecular oxygen have extensive effects on tissues, resulting in lipid peroxidation and denaturation of enzymes and proteins 16, 38. While the production of oxygen radicals increases, the scavenging capacity of tissues is well known to decrease with aging 14, 19, 20. High levels of highly toxic oxygen radical production overcoming the intrinsic scavenger capacity of tissues is probably a critical factor in the mechanism underlying delayed wound healing in the elderly. The chemiluminescence‐based of peroxide, hydroxyl, hypochlorite, peroxynitrite, lipid peroxy and superoxide radicals in healing wound tissues demonstrated a significant difference between aged and young rats in this study (3, 4). As excess oxidative stress is related to tissue damage, it could play a significant role in the delayed and weaker healing capacity of the older rats.
Although the study of angiogenic processes is impeded by the lack of a quantitative model to continuously measure vascularisation in the wound, the role of growth factors in neovascularisation during the wound‐healing process has been investigated in a number of recent studies 1, 39. Growth factor levels increased in the collagen at the early phase of wound healing and facilitated neovascularisation by inducing cells to migrate, accumulate and proliferate 39, 40. However, the angiogenic response of tissues differs with age (6). The rate of capillary growth has been shown to decrease in old rats compared to young rats (41). Similarly, a significant reduction in VEGF production and endothelial responsiveness has been reported, possibly accounting for the delayed wound angiogenesis in aged mice 3, 6. In this study, VEGF expression in the endothelium and epithelium was less intense in aged rats than in young rats (Figure 6). Less intense VEGF expression indicates decreased angiogenesis as a risk factor for delayed wound healing in aged rats.
This experimental study has some limitations. Owing to their skin elasticity, redundancy and lack of strong adherence to the underlying structures, rats are described as loose‐skinned animals (42). Therefore, rat skin wound healing may not perfectly mimic human skin wound healing because of the difference in skin morphology. In addition, multiple additional factors that were not assessed in this study may also be causes of delayed healing. The limited number of rats in each group can also be considered as a possible limitation of the study.
Thus, we found that the lower levels of collagen, VEGF and vascularisation detected in the older rats during wound healing are associated with a loss of healing ability on aging in the male subjects. The higher number of inflammatory cells and excessive oxidative stress also play a significant role in the weaker wound healing ability of older male rats.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Istanbul University Experimental Medicine Research Center (DETAM) where the study has been performed. Editage provided editorial and publication support.
REFERENCES
- 1. Ashcroft GS, Mills SJ, Ashworth JJ. Aging and wound healing. Biogerontology 2002;3:337–45. [DOI] [PubMed] [Google Scholar]
- 2. Ashcroft GS, Horan MA, Ferguson MW. Aging alters the inflammatory and endothelial cell adhesion molecule profiles during human cutaneous wound healing. Lab Invest 1998;78:47–58. [PubMed] [Google Scholar]
- 3. Swift ME, Burns AL, Gray KL, DiPietro LA. Age‐related alterations in the inflammatory response to dermal injury. J Invest Dermatol 2001;117:1027–35. [DOI] [PubMed] [Google Scholar]
- 4. Oh DM, Philips TJ. Sex hormones and wound healing. Wounds 2006;18:8–19. [Google Scholar]
- 5. Ashcroft GS, Horan MA, Ferguson MW. Aging is associated with reduced deposition of specific extracellular matrix components, an upregulation of angiogenesis, and an altered inflammatory response in a murine incisional wound healing model. J Invest Dermatol 1997;108:430–7. [DOI] [PubMed] [Google Scholar]
- 6. Ashcroft GS, Mills SJ. Androgen receptor‐mediated inhibition of cutaneous wound healing. J Clin Invest 2002;110:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ashcroft GS. Age‐related differences in the temporal and spatial regulation of matrix metalloproteinases (MMPs) in normal skin and acute cutaneous wounds of healthy humans. Cell Tissue Res 1997;290:581–91. [DOI] [PubMed] [Google Scholar]
- 8. Ashcroft GS, Greenwell‐Wild T, Horan MA, Wahl SM, Ferguson MW. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol 1999;155:1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taylor RJ, Taylor AD, Smyth JV. Using an artificial neural network to predict healing times and risk factors for venous leg ulcers. J Wound Care 2002;11:101–5. [DOI] [PubMed] [Google Scholar]
- 10. Margolis DJ, Knauss J, Bilker W. Hormone replacement therapy and prevention of pressure ulcers and venous leg ulcers. Lancet 2002;359:675–77. [DOI] [PubMed] [Google Scholar]
- 11. Bulkley GB. Pathophysiology of free radical mediated reperfusion injury. J Vasc Surg 1987;5:512–7. [PubMed] [Google Scholar]
- 12. Köksoy FN, Köse H, Soybir GR, Yalçın O Çokneşeli B. The prophylactic effects of superoxide dismutase, catalase, desferrioxamine, verapamin and sisulfiram in experimental colitis. J R Coll Surg Edinb 1997;42:27–30. [PubMed] [Google Scholar]
- 13. Soybir G, Köksoy F, Ekiz F, Yalçın O, Özşeker A, Çokneşeli B. Effects of mangan‐desferrioxamin in the prevention of peritoneal adhesions. J R Coll Surg Edinb 1998;43:26–8. [PubMed] [Google Scholar]
- 14. Chiu A, Kimball AB. Topical vitamins, minerals and botanical ingredients as modulators of environmental and chronological skin damage. Br J Dermatol 2003;149:681–91. [DOI] [PubMed] [Google Scholar]
- 15. Whitaker SH, Pierce JD. Oxygen free radicals and the disease rrocess. Nurse Pract 2003;28:53–4. [DOI] [PubMed] [Google Scholar]
- 16. Darr D, Fridovich I. Free radicals in cutaneous biology. J Invest Dermatol 1994;102:671–75. [DOI] [PubMed] [Google Scholar]
- 17. Shindo Y, Witt E, Han D. Enzymic and non‐enzymic antioxidants in epidermis and dermis of human skin. J Invest Dermatol 1994;102:122–24. [DOI] [PubMed] [Google Scholar]
- 18. Soybir GR, Koyuncu H, Köksoy F, Yalçın O, Özşeker A, Alatlı C, Topuzlu C. Protective effect of desferrioxamin against TPA caused inflammation in CD‐1 mouse skin. Surg Oncol 1996;5:253–58. [DOI] [PubMed] [Google Scholar]
- 19. Helfrich YR, Sachs DL, Voorhees JJ. Overview of skin aging and photoaging. Dermatol Nurs 2008;20:177–83. [PubMed] [Google Scholar]
- 20. Hensley K, Floyd RA. Reactive oxygen species and protein oxidation in aging: a look back, a look ahead. Arch Biochem Biophys 2002;397:377–83. [DOI] [PubMed] [Google Scholar]
- 21. Malcherek P, Schultz G, Wingren U, Franzen L. Formation of healing tissue and angiogenesis in repair of connective tissue stimulated by epidermal growth factor. Scand J Plast Reconst Surg Hand Surg 1994;28:1–7. [DOI] [PubMed] [Google Scholar]
- 22. Zhang X, Sarkar K, Andrikopoulou E, Xing D, Liu L, Reinblatt M, Laines RS, Marti GP, SEmenza GL, Harmon JW. Delayed wound healing in aged mice is associated with diminished mobilization of circulating angiogenic cells. J Am Coll Surg 2010;211:S78. [Google Scholar]
- 23. Boveris A, Cadenas E, Reiter R, Filipowsky M, Nakase Y, Chance B. Organ Chemiluminescence non‐invasive assay for oxidative radical reactions. Proc Natl Acad Sci U S A 1980;77:347–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haklar U, Yüksel M, Velioğlu A, Türkan M, Haklar G, Yalçın AS. Oxygen radicals and nitric oxide in chondral and meniscal lesions or both. Clin Orthop 2002;403:135–42. [PubMed] [Google Scholar]
- 25. Verhofstad MHJ, Lange WP, van der Laak JAWM, Verhofstad AAJ, Hendriks T. Microscopic analysis of anastomotic healing in the intestine of normal and diabetic rats. Dis Colon Rectum 2001;44:423–31. [DOI] [PubMed] [Google Scholar]
- 26. Gan L, Fagerholm P, Palmblad J. Vascular endothelial growth factor (VEGF) and its receptor VEGFR‐2 in the regulation of corneal neovascularization and wound healing. Acta Ophthalmol Scand 2004;82:557–63. [DOI] [PubMed] [Google Scholar]
- 27. Robinson G, Gray T. Electron microscopy‐2: practical procedures. In: Bancroft JD, editor. Theory and practice of histological techniques, 4th edn. Hong Kong: Churchill Livingstone,1996:598–600. [Google Scholar]
- 28. Bergman I, Loxley R. Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal Chem 1963;35:1961–5. [Google Scholar]
- 29. Worsfold M, Davie MWJ, Haddaway MJ. Age‐related changes in body composition, hydroxyproline, and creatinine excretion in normal women. Calcif Tissue Int 1999;64:40–4. [DOI] [PubMed] [Google Scholar]
- 30. Barbul A, Efran DT. Wound healing. In: Brunicardi FC, Anderson DK, Biliar TR, Dunn DL, Hunter JG, Matthews JB, Pollock RE, editors. Schwartz's principals of surgery, 9th edn. New York: McGraw‐Hill, 2010:209–33. [Google Scholar]
- 31. Zaja‐Milatovic S, Richmond A. CXC chemokines and their receptors: a case for a significant biological role in cutaneous wound healing. Histol Histopathol 2008;23:1399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kaplan B, Gönül B, Dinçer S, Kaya FND, Babül A. Relationships between tensile, ascorbic acid, hydroxyproline, and zinc levels of rabbit full‐thickness incision wound healing. Surg Today 2004;34:747–5. [DOI] [PubMed] [Google Scholar]
- 33. Ashcroft GS, Dodsworth J, van Boxtel E. Estrogen accelerates cutaneous wound healing associated with an increase in TGF‐β1 levels. Nat Med 1997;3:1209–15. [DOI] [PubMed] [Google Scholar]
- 34. Hardman MJ, Ashcroft GS. Hormonal influences on wound healing: a review of current experimental data. Wounds 2005;17:313–20. [Google Scholar]
- 35. Thiboutot D, Jabara S, McAllister JM, Sivarajah A, Gilliland K, Cong Z, Clawson G. Human skin is a steroidogenic tissue: steroidogenic enzymes and cofactors are expressed in epidermis, normal sebocytes, and an immortalized sebocyte cell line (SEB‐1). J Invest Dermatol 2003;120:905–14. [DOI] [PubMed] [Google Scholar]
- 36. Labrie F, Luu‐The V, Labrie C, Simard J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front Neuroendocrinol 2001;22:185–212. [DOI] [PubMed] [Google Scholar]
- 37. Van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab 2000;85:3276–82. [DOI] [PubMed] [Google Scholar]
- 38. Soybir G, Köksoy F, Ekiz F, Yalçın O, Fincan K, Haklar G, Yksel M. The effects of free oxygen radical scavenger and platelet‐activating factor antagonist agents in experimental acute pancreatitis. Pancreas 1999;19:143–49. [DOI] [PubMed] [Google Scholar]
- 39. Soybir G, Topuzlu C, Odabaş Ö, Dolay K, Bilir A, Köksoy A. The effects of melatonin on angiogenesis and wound healing. Surg Today 2003;33:896–901. [DOI] [PubMed] [Google Scholar]
- 40. Hosgoog G. Wound healing. The role of platelet‐derived growth factor and transforming growth factor beta. Vet Surg 1993;22:490–5. [DOI] [PubMed] [Google Scholar]
- 41. Yamaura H, Matsuzawa T. Decrease in capillary growth during aging. Exp Gerontol 1980;15:145–50. [DOI] [PubMed] [Google Scholar]
- 42. Dorset‐Martin WA. Rat models of skin wound healing: a review. Wound Rep Reg 2004;12:591–99. [DOI] [PubMed] [Google Scholar]


