Abstract
Foot ulcers are major sources of morbidity in individuals with diabetes mellitus. As royal jelly (RJ, a worker honey bee product) contains enzymatic, antibacterial and vasodilative properties, it can potentially help in healing of diabetic foot ulcers (DFUs). This study aimed to evaluate the efficacy of topical RJ on healing of DFUs. Diabetic patients with foot ulcers who were referred to us at Khorshid Hospital, Isfahan, Iran, were managed by offloading, infection control, vascular improvement and debridement (if required). Then, all ulcers were randomly selected to receive either 5% sterile topical RJ or placebo on their total surface area. Patients were followed for 3 months or until complete healing. Twenty‐five patients (6 females and 19 males) and a total of 64 ulcers were included and randomly allocated to case or control group (32 per group). Four ulcers were excluded and 60 ulcers included in the final analysis. Healing parameters including depth, length and width reduction rate, duration of complete healing and incidence of complete healing did not show any significant difference (P = 0·69, 0·95, 0·7, 0·74 and 0·6, respectively) between groups. We did not observe any side effect of topical RJ application. This study could not confirm any significant superiority of 5% topical RJ over placebo for the treatment of DFUs.
Keywords: Debridement, Diabetic foot ulcer, Offloading, Royal Jelly, Wound healing
Introduction
Diabetic foot ulceration is full‐thickness penetration of the dermis of the foot in a person with diabetes 1. Foot ulcers and infections are the major sources of morbidity in individuals with diabetes mellitus (DM). Approximately 15% of individuals with DM develop a foot ulcer and a significant subset will ultimately undergo amputation (14–24% risk with that ulcer or subsequent ulceration) 2. It has been estimated worldwide that every 30 seconds, a lower limb is lost because of DM, and its incidence will increase because of the expected rise in type 2 DM in future 3. Diabetic foot ulcers (DFUs) also have a significant negative impact on health‐related quality of life 4. Standard management strategies include offloading, improvement in vascular supply, infection control and preparation of the wound bed (e.g. by debridement). Despite the use of these strategies, healing rates of DFUs remain low. It is increasingly important to identify and use low‐cost, effective dressings for managing DFUs, as medical costs and rates of diabetes continue to rise.
Royal jelly (RJ), the exclusive food of queen honey bee (Apis mellifera) larva, is secreted from the hypopharyngeal and mandibular glands of the worker honey bee 5. It has been demonstrated to possess several pharmacological activities including immunomodulatory, antibacterial, anti‐inflammatory and vasodilative activities 6, 7, 8, 9, 10. It also increases high‐density lipoprotein levels and ameliorates insulin resistance 11, 12. Research studies on animal models have shown that RJ can dilate the vascular system, so it can facilitate blood flow in the vascular beds of lower limbs 10. This honey bee product can increase the growth rate of cells, facilitate the cell differentiation and at the same time has antitumour activity 13, 14, 15. The sum of these activities exerted by RJ may promote wound healing. The side effects of RJ use are limited to some case reports, which have been caused by oral ingestion 16, 17, 18, 19.
Considering the pathogenesis of DFUs and the mechanisms based on which the current management has been designed, the application of RJ dressing on DFUs might be an effective adjuvant therapy. Some case series and experimental studies have mentioned this idea regarding the efficacy and safety of RJ for adjuvant therapy of DFUs. Recent studies including the pilot study by Abdelatif et al. and our previous pilot study showed promising effects of topical RJ on healing of DFUs 20, 21. However, as no randomised placebo‐controlled clinical trial has been performed till now, we conducted the present study. Evaluation of efficacy and safety of RJ for healing of DFUs is the main purpose of this study.
Patients and methods
This was a randomised double‐blind placebo‐controlled clinical trial on patients with DFUs who were referred to the endocrinology clinic of Khorshid Hospital, Isfahan, Iran, from October 2010 to June 2011. Patients suffering from type 2 DM with one or multiple ulcers on their feet participated in this study. Patients with foot gangrene, osteomyelitis, severe sepsis, history of alcohol and drug abuse, cancer, congestive heart failure, end‐stage renal disease, liver failure, use of drugs that may interact with wound healing (glucocorticoids, immunosuppressive drugs and cytotoxic drugs) and those who preferred to receive the treatment out of the study were not included. The side effects of drug (hypersensitivity to RJ), inappropriate follow‐up by the patient, the patient's desire to withdraw in each phase of the study and being non responder were considered as exclusion criteria. The ulcers that did not show any signs of healing by the end of 12th week of the study were considered as non‐responders.
The study was explained to all the patients and they were assured about receiving the standard treatments even if they would not accept to enrol into the study. After filling and signing the consent form by the patients or their family members, they were educated on wound care and management of the ulcer. Complete information including age, sex, duration of being affected by DM and ulcer, risk factors for DFUs, history of recurrent ulceration, previous wound healing problems, prior therapies and response, functional impact of wound on the patient, sufficient social history to define potential adverse impact on diabetic foot care, condition of glycaemic control and current antidiabetic therapy (oral hypoglycaemic agents and/or insulin usage) and their dosages were obtained, and the patient's general health condition was examined systematically by an endocrinologist. The trial is registered at Iranian Registry of Clinical Trials, number IRCT201009263278N2.
The DFUs were assessed with regard to number, position of the ulcer, length, width (measured by digital calliper), depth (measured by means of a sterile probe placed in the ulcer) and the presence of infection, callus, necrotic tissue or ischaemia. Dorsalis pedis and tibialis posterior pulses were evaluated by palpation. Ankle brachial index (ABI) was determined as a comprehensive physical examination for assessing the vascular status of the patients. Normal values of ABI ranging from 0·91 to 1·30, and ratio <0·91 or >1·30, could be indicative of peripheral arterial disease 22. The ulcer condition was categorised according to the Texas University Wound Classification System (Table 1).
Table 1.
Texas University Wound Classification System for diabetic foot ulcers
| Stage | Grade 0 | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|
| A | Preulcer or postulcer lesion | Superficial ulcer | Deep ulcer to tendon or capsule | Wound penetrating bone or joint |
| No skin break | ||||
| B | +Infection | +Infection | +Infection | +Infection |
| C | +Ischaemia | +Ischaemia | +Ischaemia | +Ischaemia |
| D | +Infection and ischaemia | +Infection and ischaemia | +Infection and ischaemia | +Infection and ischaemia |
Standard treatments (i.e. offloading, infection control, vascular improvement and debridement, if required) were applied for all patients. The glycaemic values in this patient population were evaluated at the beginning of the study and, then, at regular periods of time. We advised special fitting shoes and/or total contact cast for each patient based on the position of the ulcer for offloading the wounds. Antibiotics were used when needed. Everyone with suspected arterial insufficiency on physical examination (lack of tibialis posterior and dorsalis pedis pulses, ABI <0·91 or >1·30) was referred for further evaluation (e.g. Colour Doppler or angiography if needed) and treatment (e.g. angioplasty or bypass grafting based on the condition). Wound debridement has been proposed to be performed as often as needed based on the presence of necrotic or fibrinous tissue.
Every patient was visited three times a week. In each visit, photography of the ulcers was taken at a distance of 20–30 cm and in the same light; then the wound condition, length, width, depth, healing progression, presence of infection and the need for debridement were assessed and all these data were recorded in patient's information card. The total area of the wound was washed and cleaned with normal saline without the use of chlorhexidine on its surface, treated with sterile 5% RJ or placebo (depending on the group to which the patients belonged) and finally was covered with layers of sterile gauze. As mentioned before, all other routine treatments and evaluations were applied for all the patients and they did not use any other drugs on their wound during the study. The primary endpoint of this study was the proportion of ulcers achieving improvement from baseline to 12 weeks after treatment.
Based on 0·84 statistical power to detect a significant difference and confidence coefficient 0·95%, 32 patients were required for each study group (P = 0·8 and d = 0·2).
At the time of randomisation, each patient who qualified to receive double‐blind treatment was assigned a randomisation number and given RJ or placebo accordingly. Random allocation of patients to their treatment groups was concealed via the use of numbered containers, and investigators were blinded to the treatment assignment for each patient. RJ and placebo were supplied as gel tubes that were identical in appearance packaged in individually labelled tubes. RJ gel was composed of sterile natural RJ in a sterile base, which was pharmacologically ineffective (95%) and was prepared in the Department of Pharmaceutics, School of Pharmacy and Pharmaceutical Sciences of Isfahan. All patients and investigators were blinded to the application RJ or placebo in the period of study.
Each patient was followed up for 3 months or until complete healing, whichever occurred first. Complete ulcer healing was considered as complete epithelialisation of the wound so that it would not need cleaning and dressing in any part.
After completion of data collection, five parameters were defined and calculated for each participant to evaluate the healing process of the wounds:
- Depth reduction rate (mm/day), which was defined as:
- Length reduction rate (mm/day), which was defined as:
- Width reduction rate (mm/day), which was computed using the following formula:
Mean duration of complete healing (days).
Incidence of complete healing in each group.
The t‐test was used to compare the mean of depth, length and width reduction rate and the mean duration of complete healing. The χ 2 test was used to compare the incidence of complete healing between the groups. General linear model was applied to control confounding variables. All data were analysed using the 16.0 version of SPSS software, SPSS Inc: 2007, Chicago, IL.
Results
Eligible participants were recruited from October 2010 to June 2011. Twenty‐five patients (6 females and 19 males) were enrolled in this study. A large number of patients had multiple ulcers or have been included twice or more at different times through the recruitment period of the study, so a total of 64 ulcers were included, each one considered as a separate participant and randomly allocated to case or control group (32 per group). The mean (SD) age of participants was 60 years (7·3).
Four ulcers were excluded from the study because either they were lost to follow‐up (n = 2) or were non‐responders (n = 2) and 60 ulcers were included in the final analysis. The flow diagram of participant recruitment is demonstrated in Figure 1. The participants in both groups had similar baseline characteristics (Table 2). There was no significant difference between them considering the wound type and classification (Table 3), history of smoking, hypertension, retinopathy and nephropathy.
Figure 1.
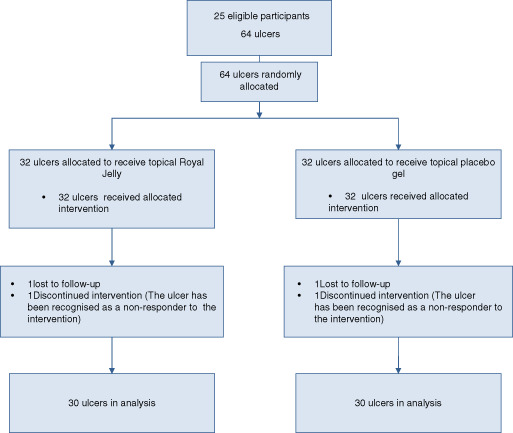
Flow diagram of the study.
Table 2.
Baseline data of the participants
| Group (n) | Female/Male | Age (years) mean (SD) | BMI (kg/m2) | DM duration (years) | History of previous wound, n/total (%) | History of previous amputation, n/total (%) |
|---|---|---|---|---|---|---|
| Placebo (32) | 7/25 | 60·6 (7) | 27·44 | 16 | 21/32 (65·6%) | 3 (9·4%) |
| RJ (32) | 11/21 | 60·0 (7) | 26·92 | 17 | 22/32 (68·8%) | 4 (12·5%) |
| Total (64) | 18/46 | 60·3 (7) | 27·21 | 16 | 43/64 (67·2%) | 7 (10·9%) |
| P value | 0·26 | 0·73 | 0·57 | 0·69 | 0·79 | 0·68 |
BMI, body mass index; DM, diabetes mellitus.
Table 3.
Wound type and classification, and ABI of participants
| Texas wound classification | Wound type | ABI (SD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I‐C | I‐A | I‐D | II‐D | II‐B | Ischaemic | Neuropathic | Neuroischaemic | Total | |||
| Gel | Placebo | 21 | 9 | 2 | 0 | 0 | 22 | 9 | 1 | 32 | 0·86 (0·33) |
| RJ | 15 | 12 | 2 | 2 | 1 | 15 | 13 | 4 | 32 | 1·05 (0·37) | |
| Total | 36 | 21 | 4 | 2 | 1 | 37 | 22 | 5 | 64 | 0·95 (0·36) | |
| P value | 0·351 | 0·146 | 0·043 | ||||||||
ABI, ankle brachial index.
ABI, which was measured in the first visit, was different between the groups (P = 0·043) (Table 3); healing parameters of the wounds are as following (Table 4). In placebo and RJ groups, mean (SD) of depth reduction rate was 0·009 (0·04) and 0·01 (0·04) mm/day, respectively (P = 0·69), length reduction rate was 0·59 (0·5) and 0·58 (0·49) mm/day, respectively (P = 0·95) and width reduction rate was 0·32 (0·2) and 0·35 (0·2) mm/day, respectively (P = 0·7), which were not statistically significant. Mean (SD) duration of complete healing was 36 days in the placebo group and 38 days in the RJ group, which was not statistically significant (P = 0·74). Twenty‐nine ulcers in the placebo group (90·6%) and 30 in the RJ group (93·8%) completely healed, which was not significantly different (P = 0·6).
Table 4.
Comparison of healing parameters of the wounds between groups
| Healing parameters | Placebo | RJ | P value |
|---|---|---|---|
| Mean of depth reduction rate (SD) (mm/day) | 0·008 (0·04) | 0·01 (0·04) | 0·69 |
| Mean of length reduction rate (SD) (mm/day) | 0·59 (0·5) | 0·58 (0·4) | 0·95 |
| Mean of width reduction rate (SD) (mm/day) | 0·32 (0·2) | 0·35 (0·2) | 0·7 |
| Mean duration of complete healing (SD) (days) | 36 (23) | 38 (33) | 0·74 |
| Incidence of complete healing, n/total (%) | 29/32 (90·6%) | 30/32 (93·8%) | 0·6 |
Discussion
This study could not confirm any significant superiority of 5% topical RJ over placebo for the treatment of DFUs. Healing parameters including mean of length, depth and width reduction rate, duration of complete healing and incidence of complete healing were not significantly different between RJ and placebo groups. On the other hand, we did not observe any adverse effects of topical RJ application on DFUs.
RJ consists of water (50–60%), proteins (18%), carbohydrates (15%), lipids (3–6%), mineral salts (1·5%) and vitamins 23 together with a large number of bioactive substances such as 10‐hydroxydecenoic acid (10HDA) (1·7%) 24 and several insulin‐like peptides 25. RJ contains a potent antibacterial protein, antibiotic residues 26, 27, 28, 29, 30 and strong antibacterial properties of 10HDA 6, 31. The primary bioactive component contained in RJ, 10HDA, has been shown to have various pharmacological activities 7, 32, 33. This fatty acid induces the fibroblast cell line to produce transforming growth factor‐β1, which is an important factor for collagen production 34. It also inhibits matrix metalloproteinases (MMPs) 7. On the other hand, major RJ protein activates keratinocytes by increasing the mRNA level of selected cytokines and MMP‐9, which may accelerate wound healing process 35. RJ also contains phenolic compounds that have antioxidant activity and antimicrobial capacity 23, 36, 37, 38, 39, 40.
Fujii et al. studied the wound healing properties of RJ in diabetic rats. They showed that RJ possesses an anti‐inflammatory action and is able to augment wound healing by decreasing exudation and collagen formation in granulation tissues 8. Anti‐inflammatory properties are related to the presence of flavonoids that are capable of inhibiting cyclooxygenase 23, 41.
Temamogullari et al. showed that RJ application on full‐thickness ulcers in rabbits accelerated wound healing. They concluded that RJ is a non‐toxic, easily available and cheap antiseptic that improves the ulcer outcome 42.
There is limited number of human studies on the wound healing properties of RJ. Abdelatif et al. conducted a pilot study using PEDYPHAR ointment (RJ and panthenol in an ointment base) for dressing of DFUs in 60 patients. They believed that this drug can create alkaline environment in the wound, while the antimicrobial, immunomodulatory and nutritional properties of RJ can eradicate infection and promote healing. They concluded that RJ may be a promising and safe conservative topical treatment and recommended that further double‐blinded randomised controlled studies are needed to confirm this 20.
As mentioned before, our previous study on 10 DFUs also showed promising effects of RJ dressing for treating DFUs besides standard treatments 21.
Our findings in this study are not in agreement with previous studies. There are some reasons that may explain this difference; this study was conducted as a double‐blind placebo‐controlled clinical trial with a larger study population. All of the possible confounding factors were controlled between groups. The patients and investigators were blinded to the applied gel until the end of the study, whereas recent previous studies were conducted in a small study population without a control group.
In the study by Abdelatif et al., they used 5% RJ in combination with panthenol (1%). In their study, frequency of RJ application was variable, ranging from twice a week to daily, depending on case condition, whereas we applied 5% natural pure RJ without additional substances and the frequency of RJ application was three times a week for all the cases. Their study was not a randomised clinical trial, which was the biggest limitation.
Our study had some limitations. Although the length, width and depth of wounds were measured with digital calliper, it may still have some inaccuracies; however, this problem can be solved by using more precise methods and devices. Our protocol was to apply RJ three times in a week, not on a daily basis. We just prepared one concentration of RJ, although it was ideal to use different concentrations and compare the results between different concentrations.
In conclusion, the most effective way for the treatment of DFUs is a multimodality approach based on standard treatment (offloading, infection control, vascular improvement and debridement, if required). Optimal management of DFUs involves regular foot care, blood glucose control and early recognition of foot problems. Adding 5% RJ to the above‐mentioned protocols does not show any significant benefit. Considering the high incidence of complete ulcer healing in both groups (i.e. 90·6% in placebo and 93·8% in RJ groups), it can be concluded that tight nursing care and regular visits of DFUs by care provider (e.g. three times a week) play an important role in DFU healing process. Further studies with increasing the frequency of RJ application (more than three times a week) and its concentration may show different results.
ACKNOWLEDGEMENTS
The authors thank the manager of Khorshid Clinic, Asghar Azimi, personnel of Khorshid Hospital and all patients who participated in this study. They also thank Manijeh Siavash for her kind cooperation.
References
- 1. Wagner FW Jr. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle 1981;2:64–122. [DOI] [PubMed] [Google Scholar]
- 2. Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J. Harrison's principles of internal medicine, 17 edn. New york, Chicago, San Francisco: McGraw‐Hill Medical Publishing Division, 2008. [Google Scholar]
- 3. Boulton AJ, Vileikyte L, Ragnarson‐Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–24. [DOI] [PubMed] [Google Scholar]
- 4. Reiber GE, Lipsky BA, Gibbons GW. The burden of diabetic foot ulcers. Am J Surg 1998;176(2A Suppl):5S–10S. [DOI] [PubMed] [Google Scholar]
- 5. Haydak MH. Honey bee nutrition. Annu Rev Entomol 1970;15:143–56. [Google Scholar]
- 6. Fujiwara S, Imai J, Fujiwara M, Yaeshima T, Kawashima T, Kobayashi K. A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J Biol Chem 1990;265:11333–7. [PubMed] [Google Scholar]
- 7. Yang XY, Yang DS, Wei Z, Wang JM, Li CY, Hui Y, Lei KF, Chen XF, Shen NH, Jin LQ, Wang JG. 10‐Hydroxy‐2‐decenoic acid from royal jelly: a potential medicine for RA. J Ethnopharmacol 2010;128:314–21. [DOI] [PubMed] [Google Scholar]
- 8. Fujii A, Kobayashi S, Kuboyama N, Furukawa Y, Kaneko Y, Ishihama S, Yamamoto H, Tamura T. Augmentation of wound healing by royal jelly (RJ) in streptozotocin‐diabetic rats. Jpn J Pharmacol 1990;53:331–7. [DOI] [PubMed] [Google Scholar]
- 9. Viuda‐Martos M, Ruiz‐Navajas Y, Fernandez‐Lopez J, Perez‐Alvarez JA. Functional properties of honey, propolis, and royal jelly. J Food Sci 2008;73:R117–24. [DOI] [PubMed] [Google Scholar]
- 10. Shinoda M, Nakajin S, Oikawa T, Sato K, Kamogawa A, Akiyama Y. Biochemical studies on vasodilative factor in royal jelly (author's transl). Yakugaku Zasshi 1978;98:139–45. [DOI] [PubMed] [Google Scholar]
- 11. Munstedt K, Henschel M, Hauenschild A, von Georgi R. Royal jelly increases high density lipoprotein levels but in older patients only. J Altern Complement Med 2009;15:329–30. [DOI] [PubMed] [Google Scholar]
- 12. Zamami Y, Takatori S, Goda M, Koyama T, Iwatani Y, Jin X, Takai‐Doi S, Kawasaki H. Royal jelly ameliorates insulin resistance in fructose‐drinking rats. Biol Pharm Bull 2008;31:2103–7. [DOI] [PubMed] [Google Scholar]
- 13. Tamura T, Fujii A, Kuboyama N. Antitumor effects of royal jelly (RJ). Nihon Yakurigaku Zasshi 1987;89:73–80. [DOI] [PubMed] [Google Scholar]
- 14. Kawamura J. Influence of geleeroyale on embryos. J Showa Med Assoc 1961;20:1465–71. [Google Scholar]
- 15. Hattori N, Nomoto H, Fukumitsu H, Mishima S, Furukawa S. Royal jelly and its unique fatty acid, 10‐hydroxy‐trans‐2‐decenoic acid, promote neurogenesis by neural stem/progenitor cells in vitro. Biomed Res 2007;28:261–6. [DOI] [PubMed] [Google Scholar]
- 16. Yonei Y, Shibagaki K, Tsukada N, Nagasu N, Inagaki Y, Miyamoto K, Suzuki O, Kiryu Y. Case report: haemorrhagic colitis associated with royal jelly intake. J Gastroenterol Hepatol 1997;12:495–9. [DOI] [PubMed] [Google Scholar]
- 17. Lee NJ, Fermo JD. Warfarin and royal jelly interaction. Pharmacotherapy 2006;26:583–6. [DOI] [PubMed] [Google Scholar]
- 18. Laporte JR, Ibaanez L, Vendrell L, Ballarin E. Bronchospasm induced by royal jelly. Allergy 1996;51:440. [PubMed] [Google Scholar]
- 19. Roger A, Rubira N, Nogueiras C, Guspi R, Baltasar M, Cadahia A. Anaphylaxis caused by royal jelly. Allergol Immunopathol (Madr) 1995;23:133–5. [PubMed] [Google Scholar]
- 20. Abdelatif M, Yakoot M, Etmaan M. Safety and efficacy of a new honey ointment on diabetic foot ulcers: a prospective pilot study. J Wound Care 2008;17:108–10. [DOI] [PubMed] [Google Scholar]
- 21. Siavash M, Shokri S, Haghighi S, Mohammadi M, Shahtalebi MA, Farajzadehgan Z. The efficacy of topical royal jelly on diabetic foot ulcers healing: a case series. J Res Med Sci 2011;16:904–9. [PMC free article] [PubMed] [Google Scholar]
- 22. Nathaniel Clark. Peripheral arterial disease in people with diabetes. American Diabetes Association Diabetes Care 2003;26:3333–41. [DOI] [PubMed] [Google Scholar]
- 23. T N, Inoue R. Preparation and functional properties of water extract and alkaline extract of royal jelly. Food Chem 2003;84:181–6. [Google Scholar]
- 24. Caparica‐Santos C, Marcucci MC. Quantitative determination of trans‐10‐hydroxy‐2‐decenoic acid (10‐HDA) in Brazilian royal jelly and commercial products containing royal jelly. J Apicultural Res 2007;46:149–53. [Google Scholar]
- 25. Kramer KJ, Tager HS, Childs CN, Speirs RD. Insulin‐like hypoglycemic and immunological activities in honeybee royal jelly. J Insect Physiol 1977;23:293–5. [DOI] [PubMed] [Google Scholar]
- 26. Zhou J, Xue X, Chen F, Zhang J, Li Y, Wu L, Chen L, Zhao J. Simultaneous determination of seven fluoroquinolones in royal jelly by ultrasonic‐assisted extraction and liquid chromatography with fluorescence detection. J Sep Sci 2009;32:955–64. [DOI] [PubMed] [Google Scholar]
- 27. Giannetti L, Longo F, Buiarelli F, Russo MV, Neri B. Tetracycline residues in royal jelly and honey by liquid chromatography tandem mass spectrometry: validation study according to Commission Decision 2002/657/EC. Anal Bioanal Chem 2010;398:1017–23. [DOI] [PubMed] [Google Scholar]
- 28. Ishii R, Horie M, Murayama M, Maitani T. Analysis of tetracyclines in honey and royal jelly by LC/MS/MS. Shokuhin Eiseigaku Zasshi 2006;47:277–83. [DOI] [PubMed] [Google Scholar]
- 29. Ishii R, Horie M, Murayama M, Maitani T. Analysis of chloramphenicol in honey and royal jelly by LC/MS/MS. Shokuhin Eiseigaku Zasshi 2006;47:58–65. [DOI] [PubMed] [Google Scholar]
- 30. Zhang X, Xu J, Shen C, Chen H, Wu B. Determination of streptomycin residue in royal jelly by high performance liquid chromatography with post‐column derivatization. Se Pu 2008;26:395–7. [PubMed] [Google Scholar]
- 31. Blum MS, Novak AF, Taber S III. 10‐Hydroxy‐delta 2‐decenoic acid, an antibiotic found in royal jelly. Science 1959;130:452–3. [DOI] [PubMed] [Google Scholar]
- 32. Townsend GF, Morgan JF, Hazlett B. Activity of 10‐hydroxydecenoic acid from royal jelly against experimental leukaemia and ascitic tumours. Nature 1959;183:1270–1. [DOI] [PubMed] [Google Scholar]
- 33. Townsend GF, Morgan JF, Tolnai S, Hazlett B, Morton HJ, Shuel RW. Studies on the in vitro antitumor activity of fatty acids. I. 10‐Hydroxy‐2‐decenoic acid from royal jelly. Cancer Res 1960;20:503–10. [PubMed] [Google Scholar]
- 34. Koya‐Miyata S, Okamoto I, Ushio S, Iwaki K, Ikeda M, Kurimoto M. Identification of a collagen production‐promoting factor from an extract of royal jelly and its possible mechanism. Biosci Biotechnol Biochem 2004;68:767–73. [DOI] [PubMed] [Google Scholar]
- 35. Majtan J, Kumar P, Majtan T, Walls AF, Klaudiny J. Effect of honey and its major royal jelly protein 1 on cytokine and MMP‐9 mRNA transcripts in human keratinocytes. Exp Dermatol 2010;19:e73–9. [DOI] [PubMed] [Google Scholar]
- 36. Kerem Z, Chetrit D, Shoseyov O, Regev‐Shoshani G. Protection of lipids from oxidation by epicatechin, trans‐resveratrol, and gallic and caffeic acids in intestinal model systems. J Agric Food Chem 2006;54:10288–93. [DOI] [PubMed] [Google Scholar]
- 37. Hu J, Huang Y, Xiong M, Luo S, Chen Y, Li Y. The effects of natural flavonoids on lipoxygenase‐mediated oxidation of compounds with a benzene ring structure‐‐a new possible mechanism of flavonoid anti‐chemical carcinogenesis and other toxicities. Int J Toxicol 2006;25:295–301. [DOI] [PubMed] [Google Scholar]
- 38. Cai SQ, Wang R, Yang X, Shang M, Ma C, Shoyama Y. Antiviral flavonoid‐type C‐glycosides from the flowers of Trollius chinensis . Chem Biodivers 2006;3:343–8. [DOI] [PubMed] [Google Scholar]
- 39. Goncalves JL, Leitao SG, Monache FD, Miranda MM, Santos MG, Romanos MT, Wigg MD. In vitro antiviral effect of flavonoid‐rich extracts of Vitex polygama (Verbenaceae) against acyclovir‐resistant herpes simplex virus type 1. Phytomedicine 2001;8:477–80. [DOI] [PubMed] [Google Scholar]
- 40. El Gammal AA, Mansour RM. Antimicrobial activities of some flavonoid compounds. Zentralbl Mikrobiol 1986;141:561–5. [DOI] [PubMed] [Google Scholar]
- 41. Kim DW, Chi YS, Son KH, Chang HW, Kim JS, Kang SS, Kim HP. Effects of sophoraflavanone G, a prenylated flavonoid from Sophora flavescens, on cyclooxygenase‐2 and in vivo inflammatory response. Arch Pharm Res 2002;25:329–35. [DOI] [PubMed] [Google Scholar]
- 42. Temamogullari FK, Hayat A, Baba F. Comparison of the royal jelly and povidone iodine on wound healing in rabbits. J Anim Vet Adv 2007;6:203–5. [Google Scholar]


