Abstract
Hard‐to‐heal leg ulcers are a major cause of morbidity in the elderly population. Despite improvements in wound care, some wounds will not heal and they present a significant challenge for patients and health care providers. A multi‐centre cohort study was conducted to evaluate the effectiveness and safety of a synthetic, extracellular matrix protein as an adjunct to standard care in the treatment of hard‐to‐heal venous or mixed leg ulcers. Primary effectiveness criteria were (i) reduction in wound size evaluated by percentage change in wound area and (ii) healing assessed by number of patients healed by end of the 12 week study. Pain reduction was assessed as a secondary effectiveness criteria using VAS. A total of 45 patients completed the study and no difference was observed between cohorts for treatment frequency. Healing was achieved in 35·6% and wound size decreased in 93·3% of patients. Median wound area percentage reduction was 70·8%. Over 50% of patients reported pain on first visit and 87·0% of these reported no pain at the end of the study. Median time to first reporting of no pain was 14 days after treatment initiation. The authors consider the extracellular synthetic matrix protein an effective and safe adjunct to standard care in the treatment of hard‐to‐heal leg ulcers.
Keywords: Biomimetic scaffold, ECM protein, ECM replacement, Hard‐to‐heal wounds, Vitronectin
Introduction
All health care professionals treating wounds inevitably face the challenge of treating wounds recalcitrant to standard care and this can be a source of significant frustration for both patients and clinicians. Hard‐to‐heal venous leg ulcers (VLUs), or ulcers of a duration longer than 4 weeks and which fail to respond to standard of care (moist wound healing and compression), are a major cause of morbidity in the elderly population 1, 2, 3, 4, 5, 6. The annual number of patients with hard‐to‐heal wounds is conservatively estimated to be at 1% of the general population and the burden of hard‐to‐heal wounds, especially VLUs, is expected to increase in ageing populations 6, 7, 8, 9.
The failure of a hard‐to‐heal wound to close may be the result of a number of factors, including prolonged inflammation, inadequate fibroblast function, inadequate tissue oxygenation, excessive or uncontrolled protease activity, or a dysfunctional extracellular matrix (ECM) 1, 10, 11. Varied systemic factors such as advanced age, malnutrition, diabetes and renal disease may also impair healing 12. In addition, pain has been shown to delay healing and pain‐induced stress has been suggested as an obstacle to wound healing 13.
Use of a systematic clinical approach is important when hard‐to‐heal wounds present as the healing process needs to be managed to convert a non‐healing wound to a healing wound. It is important to first evaluate and control those reasons for the lack of healing progression related to chronic disease, and thereafter focus on the local condition of the wound itself 14. One common finding regardless of aetiology is high levels of degradative proteases leading to a hostile wound environment and a dysfunctional wound ECM 11, 15, 16.
The ECM constitutes most of the dermis and is integral to wound healing as it provides sites for attachment of skin cells. Importantly, critical cellular functions such as proliferation and migration and survival of skin cells are all dependent on cell adhesion 17, 18. Hard‐to‐heal wounds overexpress proteases, resulting in degradation of the ECM and growth factors, increased inflammatory response, reduced cell responsiveness and consequently delayed wound healing 19. An impaired ECM interferes with the normal interaction between cells and the ECM and may delay or ultimately stop healing. Effective clinical treatment therefore requires repair and restoration of the ECM.
The importance of the ECM for wound healing is well recognised and a number of ECM replacement products aimed at restoring the cellular milieu in hard‐to‐heal wounds have recently been made commercially available to health care providers 20. These have been derived from allogeneic or xenogenic sources and have been designed in various forms, including collagen‐based scaffolds, decellularised dermis, small intestinal submucosa and flowable ECM replacement proteins 20, 21, 22, 23.
The aim of treating hard‐to‐heal wounds with an ECM replacement is to change the non‐healing status of the wound by providing a temporary biomimetic ECM replacement that when placed in the wound bed offers a temporary support to the cells which can attach, migrate and proliferate in an organised manner, and which may therefore lead to tissue regeneration and ultimately to wound closure 1, 24.
The first international consensus on acellular matrices was recently published to help clinicians evaluate how to use these products in a variety of wound healing situations. Ideal products should achieve wound closure or reduce ulcer area, have no host immune response, reduce pain and lower complication rates 19. Because wounds require a functional matrix in order to heal, acellular matrices are likely to be a clinically relevant adjunct to standard of care in the treatment of all hard‐to‐heal wounds. Therefore, biomimetic scaffolds should be easy to use and compatible with commonly used non‐adherent, semi‐occlusive wound dressings that promote moist healing, as well as with compression therapy and pressure offloading.
A synthetic, biomimetic, acellular matrix has been developed as an ECM replacement. This synthetic ECM protein comprises portions of vitronectin and IGF‐1 (insulin‐like growth factor‐1) and is intended for the treatment of hard‐to‐heal ulcers, primarily leg ulcers. The pre‐clinical work providing evidence that this synthetic matrix protein has the ability to function as an acellular scaffold for wound healing purposes has been reported in this Journal by Shooter et al. 25. The primary objective of the study reported in this article was the evaluation of the effectiveness and safety of this synthetic ECM protein.
Methods
Study design and main inclusion/exclusion criteria
A prospective, multi‐centre, cohort study was conducted in four centres in the UK and Australia. The investigators were chosen for their expertise in conducting wound studies. The main inclusion criteria for the study were: (i) presence of a hard‐to‐heal leg ulcer; (ii) grade C6 venous disease (open ulcer) according to CEAP (Clinical—Etiology—Anatomy—Pathophysiology) classification 26; (iii) patient age ≥18 years; (iv) ulcer surface area ≥2 cm2 and ≤30 cm2 and the largest length <10 cm; and (v) an ankle brachial pressure index ≥0·7 to exclude patients not suitable for graduated compression therapy 27, 28. The main exclusion criteria were: (i) Patients who had not received standard compression therapy at the time of recruitment for 4 weeks; (ii) presence of clinical infection; (iii) poorly controlled diabetes (HbA1c ≥ 12%); (iv) treatment with corticosteroids, phlebotropic drugs, drugs with a vasorelaxing effect, antimicrobials or antiseptics; (v) inability to give informed consent; and (vi) participation in any other wound research trials in the previous month.
To mitigate the potential for treatment effects, all patients were maintained on wound dressings and compression bandaging in accordance with local practices. Hence, the only change introduced to a patient's treatment regime was the addition of treatment with the investigational product.
Ethical considerations
This study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki, ICH Good Clinical Practice Guidelines and BS EN ISO 14155:2009 regarding clinical investigations of medical devices for human patients. Prior to recruiting patients, approval was obtained from the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK and the Therapeutics Goods Administration (TGA) in Australia, as well as the local ethics committees for all the study sites.
Written informed consent was obtained from all patients and study records were de‐identified for confidentiality. Patients were free to withdraw from the study at any time without prejudice to their subsequent treatment.
Study cohorts
Patients were allocated to one of two cohorts: C1 to receive treatment with the investigational product once per week, and C2 to receive treatment with the investigational product twice per week. Each site allocated consecutively numbered patients to C1 and C2 in turn, starting with the first patient being allocated to C1.
Treatment protocol and follow‐up
Patients were treated with the investigational product in conjunction with standard wound care for 12 weeks, or until completely healed (defined as 100% epithelialisation). Standard wound care included wound cleansing with saline or water and gentle removal of sloughy or necrotic tissue. Sharp debridement was not used. The synthetic ECM protein was applied topically to the wound bed according to the manufacturer's instructions for use (Figure 1) and covered by a non‐adherent dressing followed by compression. Dressings were changed and the treatment procedure repeated once per week for C1 and twice per week for C2, at which time points the safety and effectiveness assessments were also performed. Duration of treatment and follow‐up was 12 weeks or healing, whichever came first.
Figure 1.
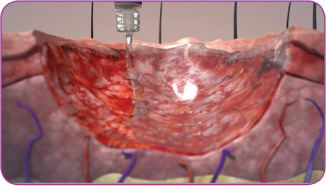
Illustration of the application of the flowable, synthetic extracellular matrix beginning at the wound edges in a slow, circular motion working towards the centre of the wound.
Endpoints
Effectiveness
Healing was assessed by the number and percentage of patients healed (i.e. 100% epithelialised) by the end of the 12‐week study period. Reduction in wound size was evaluated by the percentage change in wound area using computer planimetry assessments of ulcer size from wound tracings on gradated acetate sheets and by digital photography. Tracings and photography were carried out at entry to the study and at each planned study visit.
All patients were asked to evaluate their level of pain using a visual analogue scale (VAS), a validated instrument for measuring the severity of pain using a 100 mm scale 29. Patients were presented with a 100‐mm line where the 0 mm mark indicated no pain and 100 mm indicated the worst pain imaginable. To ensure the robustness of this subjective endpoint, pain was recorded on the case report form by the patient 30. The patient completed the assessment of overall ulcer pain in the week preceding the visit at baseline, and at each subsequent follow‐up assessment the patient assessed the overall ulcer pain since his or her previous visit.
Each patient's pain measurement was read centrally using a 100‐mm ruler and was then converted to a score out of 100.
The following interpretation of the VAS scores was used: 0–4 mm = no pain; 5–44 mm = mild pain; 45–74 mm = moderate pain; and 75–100 mm = severe pain, with a 33% reduction in pain representing a clinically meaningful pain reduction 31.
Safety
Patients were carefully monitored throughout the study for adverse events (AEs). An AE was defined as any unfavourable or unintended sign or symptom, or disease temporally associated with the use of the study product and/or cover dressing. At each follow‐up visit, patients were asked if they had experienced any AE since their last visit and spontaneously reported AEs were also captured. Details recorded included description, seriousness and relationship to the study product and/or cover dressing. The investigators determined the relationship using the following categories: none, improbable, possible, probable or definite.
An AE was classified as a serious adverse event (SAE) if it led to death or a serious deterioration in the health of a patient that resulted in a life threatening illness or injury, resulted in permanent impairment of body structure or a body function, required inpatient hospitalisation or prolongation of hospitalisation or resulted in medical or surgical intervention to prevent permanent impairment to a body structure or body function.
Statistical methods
In this observational, non‐comparative study a sample size of 58 patients based on feasibility was chosen to account for a dropout rate of approximately 30% providing a minimum of 40 evaluable patients completing the study.
Evaluation of the effectiveness and safety endpoints was descriptive in nature. Healing was assessed by the number and percentage of patients healed by the end of the study. Reduction in wound size was evaluated by percentage change in wound area from baseline and calculated for each assessment (the difference between baseline and post‐baseline assessment value divided by the baseline value and the result converted to a percentage). Time to first occurrence of at least 50%, 75% reduction and to healing were presented graphically using Kaplan–Meier product limit survival estimates.
Change in pain was evaluated by percentage change in pain from baseline and was calculated for each assessment (the difference between baseline and post‐baseline assessment value divided by the baseline value and the result converted to a percentage). The time to first occurrence of a clinically meaningful pain reduction (i.e. at least 33%) and to no pain were also presented graphically using Kaplan–Meier product limit survival estimates.
Safety was analysed using descriptive summaries of overall frequency and incidence of all AEs and AEs by relatedness to the study product and/or cover dressing.
Patients who were treated with the synthetic ECM protein comprised the intention‐to‐treat population, or in other words the full analysis set (FAS). Patients who completed the study (i.e. 12 weeks or complete healing) comprised the per protocol or the completers analysis set (CAS) population.
Results
Demographics
Fifty‐four patients with venous insufficiency or mixed leg ulcers were recruited. Following allocation to cohorts, one patient no longer satisfied the inclusion criteria and was withdrawn prior to treatment, while eight patients did not complete the study for various reasons. This resulted in 45 evaluable patients (CAS) and therefore the target of 40 evaluable patients was met. The flow of patients throughout the study and reasons for non‐completion are shown in Figure 2.
Figure 2.
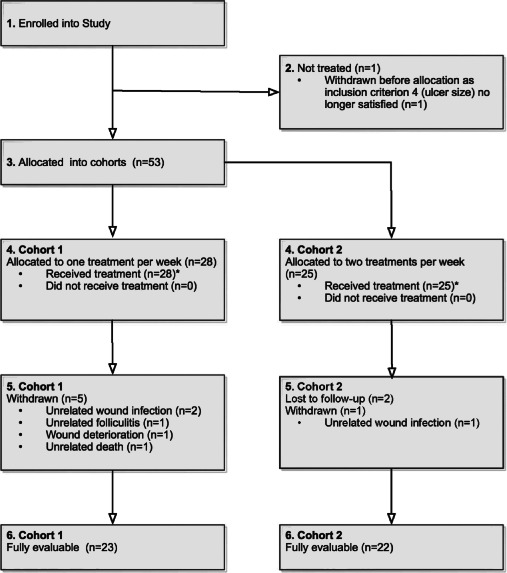
Patient flow diagram.*The final cohort (C) numbers of 28 and 25 patients result from three sites treating one more patient in C1 than in C2. All sites allocated alternately numbered subjects to C1 and C2 in turn, hence three more subjects were enrolled into C1 than into C2.
The mean patient age was 74·2 years. The mean ulcer duration was 37·1 months and the mean ulcer size was 7·36 cm2 at baseline. Three (6·7%) patients had an ABPI of <0·8 and 20 of 45 (44·4%) patients had an ABPI > 1·2 indicating a mixed arterial/venous ulcer aetiology in 23 of 45 (51·1%) of the patients 27, 32. Importantly however, it was determined by the clinicians responsible that compression was safe and beneficial to use in the treatment of these mixed ulcers. The demographics and baseline wound characteristics for both the FAS and the CAS populations are shown in Table 1.
Table 1.
Patient demographic information
| Baseline data | FAS (n = 53) | ||
|---|---|---|---|
| Age (years) | Wound duration (months) | Wound area (cm2) | |
| Mean | 74·0 | 33·4 | 7·38 |
| Maximum | 100 | 360 | 32·9 |
| Minimum | 49 | 1 | 1·8 |
| Median | 75·0 | 10·0 | 4·5 |
| SD (±) | 11·5 | 59·5 | 6·96 |
| Gender | Males | Females | Total |
| 29 (54·7%) | 24 (45·3%) | 53 | |
| ABPI a | <0·8 | ≥0·8–≤1·2 | >1·2 |
| 4 | 25 | 23 | |
| Baseline data | CAS (n = 45) | ||
|---|---|---|---|
| Age (years) | Wound duration (months) | Wound area (cm2) | |
| Mean | 74·2 | 37·0 | 7·36 |
| Maximum | 100 | 360 | 32·90 |
| Minimum | 49 | 1 | 1·80 |
| Median | 77·0 | 10·2 | 4·69 |
| SD (±) | 11·8 | 63·8 | 6·92 |
| Gender | Males | Females | Total |
| 26 | 19 | 45 | |
| ABPI a | <0·8 | ≥0·8–≤1·2 | >1·2 |
| 3 | 21 | 20 | |
ABPI, ankle brachial pressure index; FAS, full analysis set; CAS, completers analysis set.
Missing data for one patient.
Dressings used in the study
The compatibility of the synthetic ECM protein with dressings and compression bandages commonly used in the provision of standard leg ulcer care is demonstrated by the great variety of dressings and compression systems successfully used with the synthetic ECM protein in the study (Table 2).
Table 2.
Range of cover dressings and compression systems used in the study
| Cover dressing/Compression type used in >5% of treatments | Number of treatments (%) N = 713 | Number of patients (%)N = 53 |
|---|---|---|
| Non‐adherent dressing; High compression | 180 (25·2%) | 25 (47·2%) |
| Antimicrobial dressing; High compression | 132 (18·5%) | 13 (24·5%) |
| Absorbent dressing; Short stretch | 102 (14·3%) | 7 (13·2%) |
| Tubular paste bandage; Short stretch | 61 (8·6%) | 4 (7·5%) |
| Absorbent dressing; High compression | 37 (5·2%) | 4 (7·5%) |
Cohort analysis
Cohort analysis showed that the two study cohorts were similar and no statistical difference or clinically meaningful difference in effectiveness between cohorts was observed.
Healing and wound area reduction
The incidence of complete healing was 16 of 45 patients, that is 35·6% (Figure 3), and the median wound area percentage reduction was 70·8% (Table 3).
Figure 3.
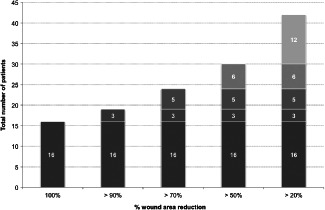
Percentage wound area reduction [completers analysis set (CAS)].
Table 3.
Healing and percentage wound area reduction at final visit
| Total CAS (n = 45) | |
|---|---|
| Completely healed n (%) | 16 (35·6%) |
| % Reduction in wound area at last assessment | |
| Mean | 54·05 |
| Maximum | 100·00 |
| Minimum | −120·00a |
| Median | 70·80 |
| SD | 57·28 |
A negative value indicates a percentage increase in wound area compared with study entry.
In a total of 30 of 45 (66·7%) patients, significant improvement, that is >50% wound area reduction, was observed and 42 of 45 patients (93·3%) improved, that is had over 20% wound area reduction (Figure 3). Time to healing/partial healing is shown in Figure 4.
Figure 4.
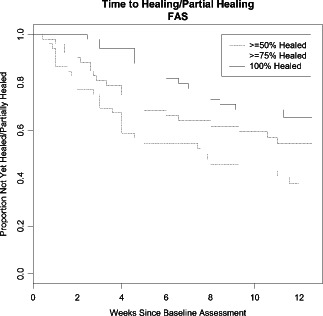
Time to healing/partial healing [completers analysis set (CAS)].
No formal statistical subgroup analysis was performed; however, as expected, there was a trend for healing outcome to be associated with smaller wound size at baseline and with shorter durations of wounds at baseline. Among healed patients, the majority of wounds were <3 cm2. More than 40% of those who healed within this 12‐week study (7/16) presented with a wound duration at baseline of 6 months or less, while among non‐healed patients approximately 28% (8/29) were in this category. Of the healed patients, 43·8% patients were <65 years and of the non‐healed patients 4/29 (13·8%) were <65 years (Table 4).
Table 4.
Final study outcome and (a) wound size, (b) wound duration and (c) age group at baseline
| Wound size at baseline | Healed patients (n = 16) | Non‐healed patients (n = 29) | All patients (n = 45) | |||
|---|---|---|---|---|---|---|
| <3 cm2 | 10 | 62·5% | 4 | 13·8% | 14 | 31·1% |
| >3 to ≤6 cm2 | 3 | 18·8% | 10 | 34·5% | 13 | 28·9% |
| >6 to ≤12 cm2 | 2 | 12·5% | 8 | 27·6% | 10 | 22·2% |
| >12 cm2 | 1 | 6·3% | 7 | 24·1% | 8 | 17·8% |
| Total | 16 | 100·0% | 29 | 100·0% | 45 | 100·0% |
| Wound duration | Healed patients (n = 16) | Non‐healed patients (n = 29) | All patients (n = 45) | |||
|---|---|---|---|---|---|---|
| ≤6 months | 7 | 43·8% | 8 | 27·6% | 15 | 33·3% |
| >6 to ≤12 months | 5 | 31·3% | 5 | 17·2% | 10 | 22·2% |
| >12 to ≤24 months | 0 | 0·0% | 3 | 10·3% | 3 | 6·7% |
| >24 months | 4 | 25·0% | 13 | 44·8% | 17 | 37·8% |
| Total | 16 | 100·0% | 29 | 100·0% | 45 | 100·0% |
| Age group at baseline | Healed patients (n = 16) | Non‐healed patients (n = 29) | All patients (n = 45) | |||
|---|---|---|---|---|---|---|
| <65 years | 7 | 43·8% | 4 | 13·8% | 11 | 24·4% |
| >65 to ≤75 years | 3 | 18·8% | 8 | 27·6% | 11 | 24·4% |
| >75 to ≤85 years | 4 | 25·0% | 12 | 41·4% | 16 | 35·6% |
| >85 years | 2 | 12·5% | 5 | 17·2% | 7 | 15·6% |
| Total | 16 | 100·0% | 29 | 100·0% | 45 | 100·0% |
Examples of outcomes when adding the synthetic ECM protein as an adjunct treatment are provided in Figures 5A, B, 6A and B.
Figure 5.
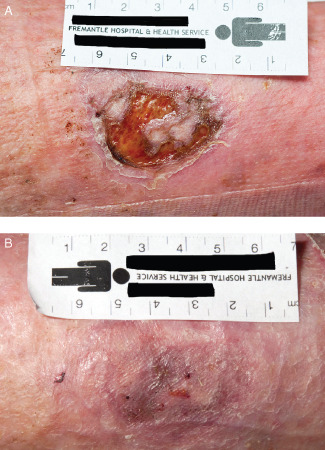
(A) Photograph prior to wound bed preparation of a recurrent ulcer, present for 3 months before study entry. (B) Re‐epithelialised ulcer after 6 weeks of weekly treatment with the synthetic extracellular matrix protein and standard care.
Figure 6.
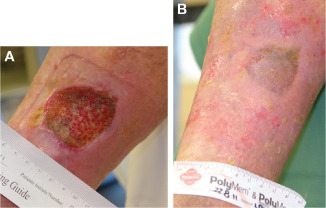
(A) Photograph prior to wound bed preparation of an ulcer, present for 5 years before study entry. (B) Re‐epithelialised ulcer after 7 weeks of bi‐weekly treatment with the synthetic extracellular matrix protein and standard care.
A small (2·29 cm2), recurrent venous ulcer had been present for 3 months on the left leg, medial calf area, in this 77‐year‐old woman with no response to standard care (Figure 5A). Following 6 weeks of standard care and the synthetic ECM protein, healing was achieved (Figure 5B).
In a woman aged 78 years, a venous ulcer (15·4 cm2 in size) had been present for 5 years on the right leg in the medial gaiter area (Figure 6A). The ulcer, which had not responded to standard care alone, healed within 7 weeks of bi‐weekly treatment with standard care and the synthetic ECM protein (Figure 6B).
Pain
At baseline, 23 of 45 (51·1%) patients presented with pain. Of these 6 had severe pain, 5 had moderate pain and 12 had mild pain. All patients reporting severe pain at baseline reported no pain at final visit. A clinically meaningful (33%) pain reduction was experienced by all patients and the median time to achieve such pain reduction was 7 days after treatment with the study product had been initiated. Of the 23 patients with pain, 20 of 23 (87·0%) reported reduction to no pain at the end of the study and the median time to the patients' first report of no pain was 14 days after treatment with the study product had been initiated (Figure 7).
Figure 7.
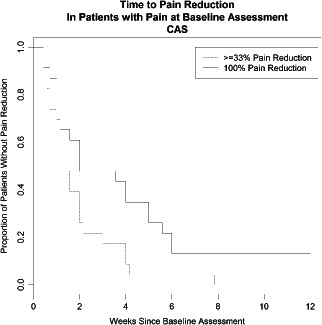
Time to pain reduction in patients with pain at study entry.
Safety
A variety of AEs were reported during the study (Table 5). Of a total of 105 AEs, 48·6% (51/105) were considered associated with the study wound (Table 6). However, no AEs were considered definitely or probably related to the synthetic ECM protein.
Table 5.
Adverse events reported in the study
| AEs considered to be study wound associated | Other AEs |
|---|---|
| Bleeding wound bed | Atrial fibrillation |
| Eczema | Chest infection |
| Folliculitis | Chesty cough |
| Hypergranulation tissue | Cold |
| Increased exudate | Conjunctivitis |
| Maceration to surrounding skin causing new ulcer | Death |
| New ulcer | Dizziness |
| New wound | Furring of tongue |
| Oedema | Haemorrhage behind left eye due to hypertension |
| Overgranulating tissue | Hay fever |
| Pain | Hives |
| Superficial maceration | Nausea |
| Unhealthy tissue | Rash |
| Worsening itch | Superficial foot cellulitis |
| Wound infection |
AEs, adverse events; ECM, extracellular matrix.
Table 6.
Relationship of wound associated AEs to the synthetic ECM protein and/or to the cover dressing
| AEs related to the synthetic ECM protein | AEs related to the cover dressing | |||
|---|---|---|---|---|
| None | 7 | 13·7% | 19 | 37·3% |
| Improbable | 31 | 60·8% | 16 | 31·4% |
| Possible | 13 | 25·5% | 13 | 25·5% |
| Probable | 0 | 0·0% | 2 | 3·9% |
| Definite | 0 | 0·0% | 1 | 2·0% |
| Total | 51 | 100·0% | 51 | 100·1%a |
Percentage is not equal to 100.0% due to rounding.
Thirteen of the study wound associated [13/51 (25·5%)] AEs were considered possibly related to the synthetic ECM protein. These comprised five AEs of hypergranulation, two AEs of maceration, two AEs of bleeding wound bed, and one AE each of increase in wound size, pain associated with study wound, erythema with excoriation of surrounding skin, and itching around the wound. Three AEs [3/13 (23·1%)] were considered moderate in severity (increase in wound size, pain, erythema with excoriation of skin) and all others [10/13 (76·9%)] were considered mild in severity.
The three most frequently reported AEs were study wound associated AEs and included maceration [19/51 (37·3%)], infection [8/51 (15·7%)] and hypergranulation [6/51 (11·8%)]. Importantly, none of the wound associated AEs were classified as severe by the investigators. The AEs reported in the study were all of the type which is commonly encountered in the treatment of patients with leg ulcers. There were 23 cover dressing related AEs in total, of which 16 were study wound associated (Table 6).
In the case of four patients, a wound associated AE led to withdrawal from the study. In three of the cases, the reason was that the protocol stipulated withdrawal owing to wound infection, and in one case the reason was increase in ulcer size. All four were classified as moderate in terms of severity.
There were two deaths reported in the study. Given the age and comorbidities of the study participants this was not unexpected and importantly neither of them was study wound associated or considered related to the synthetic ECM protein.
Discussion
This study included a number of subjective and objective endpoints providing a comprehensive evaluation of the effectiveness and safety of a synthetic ECM protein in the treatment of hard‐to‐heal leg ulcers.
There were no SAEs or severe AEs where causality was definitely, or even possibly, attributable to the synthetic ECM protein thereby further illustrating the low risk profile of this synthetic product. Safety data are surprisingly often missing in published wound care studies. However, based on the few publications providing such data we conclude that the AE profile of the patients in this study is similar to the safety data published for patients with hard‐to‐heal leg ulcers 33, 34, 35, and that treatment with the synthetic ECM protein is well tolerated by this particular patient population.
Leg ulcers are associated with disabling pain and chronicity 36, have a major impact on quality of life 37 and for patients with previously unsuccessful wound treatment, the condition can persist for years. ECM treatment of hard‐to‐heal wounds has been associated with greater pain reduction compared to control treatments 35, 38, 39, 40, 41, albeit that the instruments for measuring pain greatly vary. Nevertheless, the important pain reduction following treatment with the synthetic ECM protein compares favourably with that published for an animal‐derived protein, amelogenin (Xelma®, Mölnlycke, Sweden) in a reported 12 week study where pain reduction was significantly greater in the amelogenin treatment group compared to the control group 35, 40. Moreover, in the present synthetic ECM protein study, all patients with severe pain at baseline reported no pain at the final follow‐up visit, and 87% of all patients with pain reported no pain in a median time of 2 weeks after baseline treatment.
It has been concluded that amelogenin, in conjunction with compression, is beneficial in the treatment of hard‐to‐heal VLUs. The healing results of the present study also compare favourably with the clinical experience of Vowden et al. 35 and Romanelli et al. 40 using weekly applications of amelogenin in a similar 12‐week study to treat patients with hard‐to‐heal, mixed or venous ulcers 35, 40. Romanelli et al. 40 reported that 9 of 42 (21%) patients treated with amelogenin were fully healed at 24 weeks 40, while in this study, 16 of 53 (30%) patients treated with the synthetic matrix protein were fully healed by as early as 12 weeks (N = 53). Furthermore, given the percentage wound area reduction achieved at 12 weeks in this study it could be reasonably expected that an even greater percentage would have been fully healed at 24 weeks 42.
As previously noted, hard‐to‐heal wounds are a massive burden for patients and a significant challenge for health care providers as well as for the health care system. Some 20–30% of leg ulcers do not respond to compression therapy and the use of additional treatment options to improve outcomes may be needed 24. In this 12‐week study, the use of the synthetic ECM protein as an adjunct to standard care resulted in the per protocol population in an overall total healing rate of 35·6% for hard‐to‐heal ulcers (median duration at study entry 10 months). It may therefore be hypothesised that early referral and addition of the ECM protein as an adjunct to standard care may bring about a significant improvement in patient outcomes.
In addition, from an effectiveness, and therefore health economics point of view, it is important that the timing of the use of an adjunct therapy be sensibly chosen 43. Kantor and Margolis 3 demonstrated in a study aimed at helping health care providers decide when to use adjunct therapies in the treatment of wounds that the status of a wound following 4 weeks of treatment is a good predictor of final outcome. In light of the results of this study it would therefore follow that if there is no or poor response to standard care treatment alone after 4 weeks, patient suffering, use of resources and financial costs may be reduced by prompt addition of the synthetic ECM protein as an adjunct to standard care in order to improve outcomes.
A potential limitation of the study is the relatively short duration. Nevertheless, the study is a comprehensive evaluation of a total of 8·4 patient treatment years and the percentage wound area reduction achieved at 12 weeks in this study provides a useful indicator of further improved outcomes in the longer term 42.
Conclusion
This study demonstrates the effectiveness of the synthetic ECM protein in the treatment of hard‐to‐heal mixed and venous leg ulcers as measured by healing or reduction in wound size. The high level of performance in healing, wound area reduction and significant pain reduction, together with the robust safety profile, demonstrate the benefit of using synthetic ECM protein as an adjunct to standard care in the treatment of patients with hard‐to‐heal mixed or venous leg ulcers. Moreover, the results indicate an even greater benefit from an early use of this synthetic ECM protein when standard care alone does not provide results and suggest an important health economic benefit, especially in a community setting, following early referral and use.
Acknowledgements
The authors wish to thank Helen Amine‐Eddine, Msc, CStat, for her assistance with the statistical analysis and Dr Thanuja Athukoralalage and Alison Safstrom for their assistance during the conduct of the study. The study was supported by Tissue Therapies Ltd. KH is an Independent Clinical Advisor to Tissue Therapies Ltd. ZU is an inventor of the protein component of VitroGro® ECM and the consulting Chief Scientific Officer to Tissue Therapies Ltd. ZU and DVL are shareholders in Tissue Therapies Ltd. GS provides consulting services to Tissue Therapies Ltd. E‐LH is an employee of Tissue Therapies Europe Ltd (a wholly owned subsidiary of Tissue Therapies Ltd). The authors had full control over the contents of this article and approved its submission.
Harding K, Aldons P, Edwards H, Stacey M, Finlayson K, Gibb M, Jenkins L, Shooter G, Lonkhuyzen DV, Lynam E, Heinrichs E‐L, Upton Z. Effectiveness of an acellular synthetic matrix in the treatment of hard‐to‐heal leg ulcers.
References
- 1. Chadwick P, Acton C. The use of amelogenin protein in the treatment of hard‐to‐heal wounds. Br J Nurs 2009;18(Tissue Viability Suppl):S22–9. [DOI] [PubMed] [Google Scholar]
- 2. Phillips TJ, Machado F, Trout R, Porter J, Olin J, Falanga V. Prognostic indicators in venous ulcers. J Am Acad Dermatol 2000;43:627–30. [DOI] [PubMed] [Google Scholar]
- 3. Kantor J, Margolis DJ. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. Br J Dermatol 2000;142:960–4. [DOI] [PubMed] [Google Scholar]
- 4. Callam MJ, Ruckley CV, Harper DR, Dale JJ. Chronic ulceration of the leg: extent of the problem and provision of care. BMJ 1985;290:1855–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cornwall JV, Dore CJ, Lewis JD. Leg ulcers: epidemiology and aetiology. Br J Surg 1986;73:693–6. [DOI] [PubMed] [Google Scholar]
- 6. Hansson C, Andersson E, Swanbeck G. Leg ulcer epidemiology in Gothenburg. Acta Chir Scand Suppl 1988;544:12–6. [PubMed] [Google Scholar]
- 7. Skene AI, Smith JM, Dore CJ, Charlett A, Lewis JD. Venous leg ulcers: a prognostic index to predict time to healing. BMJ 1992;305:1119–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Collins L, Seraj S. Diagnosis and treatment of venous ulcers. Am Fam Physician 2010;81:989–96. [PubMed] [Google Scholar]
- 9. Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harding KG, Moore K, Phillips TJ. Wound chronicity and fibroblast senescence ‐ implications for treatment. Int Wound J 2005;2:364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hodde JP, Johnson CE. Extracellular matrix as a strategy for treating chronic wounds. Am J Clin Dermatol 2007;8:61–6. [DOI] [PubMed] [Google Scholar]
- 12. Harding KG, Morris HL, Patel GK. Healing chronic wounds. BMJ 2002;324:160–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soon K, Acton C. Pain‐induced stress: a barrier to wound healing. Wounds UK 2006;2:92–101. [Google Scholar]
- 14. Widgerow AD. Deconstructing the stalled wound. Wounds UK 2012;24:58–66. [PubMed] [Google Scholar]
- 15. Gibson D, Cullen B, Legerstee R, Harding KG, Schultz G. MMPs made easy. Wounds Int 2009;1:1–6. [Google Scholar]
- 16. Enoch S, Grey JE, Harding KG. Recent advances and emerging treatments. BMJ 2006;332:962–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huttenlocher A, Sandborg RR, Horwitz AF. Adhesion in cell migration. Curr Opin Cell Biol 1995;7:697–706. [DOI] [PubMed] [Google Scholar]
- 18. Kroening S, Goppelt‐Struebe M. Analysis of matrix‐dependent cell migration with a barrier migration assay. Sci Signal 2010;3:pl1. [DOI] [PubMed] [Google Scholar]
- 19. International consensus . Acellular matrices for the treatment of wounds. An expert working group review. Wounds Int 2010;1–16. [Google Scholar]
- 20. Agren M, Werthen M. The extracellular matrix in wound healing: a closer look at therapeutics for chronic wounds. Int J Low Extrem Wounds 2007;6:82–97. [DOI] [PubMed] [Google Scholar]
- 21. Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials 2007;28:3587–93. [DOI] [PubMed] [Google Scholar]
- 22. Vowden P, Romanelli M, Peter R, Bostrom A, Josefsson A, Stege H. The effect of amelogenins (Xelma) on hard‐to‐heal venous leg ulcers. Wound Repair Regen 2006;14:240–6. [DOI] [PubMed] [Google Scholar]
- 23. Kirsner RS, Marston WA, Snyder RJ, Lee TD, Cargill DI, Slade HB. Spray‐applied cell therapy with human allogeneic fibroblasts and keratinocytes for the treatment of chronic venous leg ulcers: a phase 2, multicentre, double‐blind, randomised, placebo‐controlled trial. Lancet 2012;380:977–85[See comments Lancet 2012;380:953‐4]. [DOI] [PubMed] [Google Scholar]
- 24. White R. Hard‐to‐heal wounds: results of an international survey. Wounds UK 2011;7:22–31. [Google Scholar]
- 25. Shooter GK, Van Lonkhuyzen DR, Croll TIU, Cao Y, Xie Y, Broadbent JA, Stupar D, Lynam EC, Upton Z. A pre‐clinical functional assessment of an acellular scaffold intended for the treatment of hard‐to‐heal wounds. Int Wound J 2013. DOI: 10.1111/iwj.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Porter JM, Moneta GL. An international consensus committee on chronic venous disease. Reporting standards in venous disease: an update. J Vasc Surg 1995;21:635–45. [DOI] [PubMed] [Google Scholar]
- 27. EWMA . Position document: understanding compression therapy; 2003.
- 28. O'Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers (Review). Cochrane Database Syst Rev 2012;1–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 1983;17:45–56. [DOI] [PubMed] [Google Scholar]
- 30. World Union of Wound Healing Societies . Principles of best practice. Minimising pain at wound dressing‐related procedures. A consensus document; 2004.
- 31. Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain 2003;4:407–14. [DOI] [PubMed] [Google Scholar]
- 32. Vowden P. Understanding the ankle brachial pressure index to treat venous ulceration. Wounds UK 2012;8:S10–5. [Google Scholar]
- 33. Huldt‐Nystrom T, Meuleneire F, Acton C. Xelma, an advanced wound treatment for venous ulcers: a European perspective. Wounds UK 2008;4:1–5. [Google Scholar]
- 34. Vanscheidt W, Ukat A, Horak V, Bruning H, Hunyadi J, Pavlicek R, Emter M, Hartmann A, Bende J, Zwingers T, Ermuth T, Eberhardt R. Treatment of recalcitrant venous leg ulcers with autologous keratinocytes in fibrin sealant: a multinational randomized controlled clinical trial. Wound Repair Regen 2007;15:308–15. [DOI] [PubMed] [Google Scholar]
- 35. Vowden P, Romanelli M, Price P. Effect of amelogenin extracellular matrix protein and compression on hard‐to‐heal venous leg ulcers. J Wound Care 2007;16:189–95. [DOI] [PubMed] [Google Scholar]
- 36. O'Donnell TF Jr, Lau J. A systematic review of randomized controlled trials of wound dressings for chronic venous ulcers. J Vasc Surg 2006;44:1118–25. [DOI] [PubMed] [Google Scholar]
- 37. Briggs M, Nelson EA, Martyn‐St James M. Topical agents or dressings for pain in venous leg ulcers. Cochrane Database Syst Rev 2012;1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Romanelli M, Dini V, Bertone M, Barbanera S, Brilli C. OASIS wound matrix versus Hyaloskin in the treatment of difficult‐to‐heal wounds of mixed arterial/venous aetiology. Int Wound J 2007;4:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Romanelli M, Dini V, Vowden P, Agren MS. Amelogenin, an extracellular matrix protein, in the treatment of venous leg ulcers and other hard‐to‐heal wounds: experimental and clinical evidence. Clin Interv Aging 2008;3:263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Romanelli M, Kaha E, Stege H, Wnorowski JW, Vowden P, Majamaa H, Lazaro JL. Effect of amelogenin extracellular matrix protein and compression on hard‐to‐heal venous leg ulcers: follow‐up data. J Wound Care 2008;17:17–23. [DOI] [PubMed] [Google Scholar]
- 41. Wollina U, Schmidt W‐D, Krönert C, Nelskamp C, Scheibe A, Fassler D. Some effects of a topical collagen‐based matrix on the microcirculation and wound healing in patients with chronic venous leg ulcers: preliminary observations. Int J Low Extrem Wounds 2005;4:214–24. [DOI] [PubMed] [Google Scholar]
- 42. Gelfand JM, Hoffstad O, Margolis DJ. Surrogate endpoints for the treatment of venous leg ulcers. J Invest Dermatol 2002;119:1420–5. [DOI] [PubMed] [Google Scholar]
- 43. Augustin M, Vanscheidt W. Chronic venous leg ulcers: the future of cell‐based therapies [comment]. Lancet 2012;380:953–4[Comment on Lancet 2012:380:977–85]. [DOI] [PubMed] [Google Scholar]


