Abstract
Treatment of an enterocutaneous fistula is complex and may require multidisciplinary management, especially when associated with a neoplastic process. Here, we describe the case of a 59‐year‐old patient with a squamous cell carcinoma that had invaded the abdominal wall through a chronic enterocutaneous fistula identified 30 years ago. We combined parietectomy with small intestine and colon resection and inguinal lymphadenectomy in order to obtain clear surgical margins. At the same time, plastic surgery involved the implementation of a large bioprosthesis and coverage with a vastus lateralis muscle free flap.
Keywords: Abdominal parietectomy; Bioprosthesis; Parietal squamous cell carcinoma
Introduction
The development of a parietal squamous cell carcinoma on a chronic enterocutaneous fistula is a rare, infrequently described entity. The diagnosis is often made late in the course of the disease, and so most patients are elderly at the time of diagnosis, making curative surgery no longer feasible (1).
Here, we report on a rare case of giant squamous cell carcinoma that had developed on a chronic enterocutaneous fistula identified 30 years ago. Surgical management was made possible by the patient's young age and the relative absence of comorbidities.
Case report
Mr H (aged 59) suffered damage to his left kidney after a work injury in 1972 and underwent emergency nephrectomy via a midline laparotomy. In 1978, a second midline laparotomy was performed in order to remove an abscess that had formed at the site of the damaged kidney. Soon afterward, the patient developed chronic discharge through the umbilicus but chose to treat himself (with regular disinfection) rather than consult a physician. In December 2010, an increase in the suppuration rate, a change in the aspect of the fluid (digestive juices) and the emergence of local trophic disorder prompted hospitalisation. A further laparotomy showed the presence of an enterocutaneous fistula. A 15‐cm intestinal loop resection with jejunojejunal anastomosis was performed, with drainage of the abdominal cavity through a vacuum drain placed on the left flank.
Analysis of the surgical specimen showed the presence of an infiltrating squamous cell carcinoma metastasis in the wall of the small intestine. Tumour marker assays (alpha fetoprotein, CA 19‐9, CA 125, prostate specific antigen and angiotensin‐converting enzyme) and other screening examinations for the suspected primary lesion (ear, nose and throat, lungs, urinary tract and anal canal) were negative. However, an abdominal wall biopsy demonstrated an infiltrating squamous cell carcinoma similar to that found in the wall of the small intestine.
During the postoperative recovery period, the patient presented dehiscence of the midline scar, with an abdominal evisceration and an enterocutaneous fistula (Figure 1). The patient was referred to our institution in June 2011.
Figure 1.
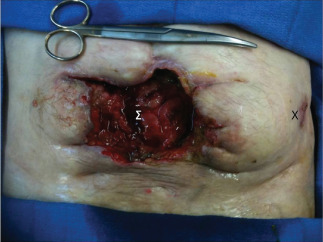
A local view during preoperative vacuum therapy. Σ: enterocutaneous fistula, X: old drain hole in the left flank.
A computed tomography (CT) scan with three‐dimensional reconstruction enabled us to map the entire lesion and showed (i) a 90‐mm long nodular lesion infiltrating the sheath of the rectus abdominis, (ii) a 60‐mm long lesion infiltrating the left oblique muscle and (iii) a 35‐mm long intra peritoneal lesion infiltrating the small intestine, far from the duodenojejunal flexure (Figure 2). We also examined a suspected bilateral inguinal lymph node.
Figure 2.
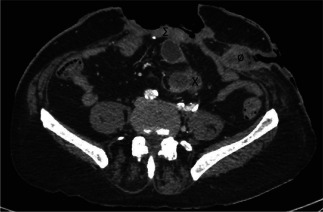
Preoperative CT scan. Σ: A tumour infiltrating the sheath of the rectus abdominis, φ: a tumour infiltrating the left oblique muscle and X: a carcinomatosis lesion infiltrating the small intestine.
Initial treatment consisted of parenteral refeeding for 2 weeks (because of profound hypoalbuminaemia; 17 g/l) and somatostatin analogue treatment. The enterocutaneous fistula was complicated by painful ulceration of the surrounding skin. We therefore decided to initiate vacuum therapy and to change the vacuum drain every 48 h in the operating room (under general anaesthesia).
The patient's records were discussed in an inter‐regional multidisciplinary team meeting. The team decided to suggest the simultaneous resection of all lesions and abdominal wall repair without prior chemotherapy because of the patient's poor overall status and the difficulty of pain management.
The operation was carried out by a gastrointestinal surgeon and several plastic surgeons. The first operating phase consisted in harvesting a musculocutaneous flap bearing the left vastus lateralis as a free flap (Figure 3A). Next, parietectomy was performed (diameter: 25 cm), with removal of the old drain in the left flank and en bloc resection of the intestinal segment involved (including 130 cm of small bowel, starting 160 cm from the angle of Treitz and ending 30 cm before the ileocaecal valve) (Figure 3B). We then performed mesenteric lymphadenectomy and bilateral inguinal lymphadenectomy.
Figure 3.
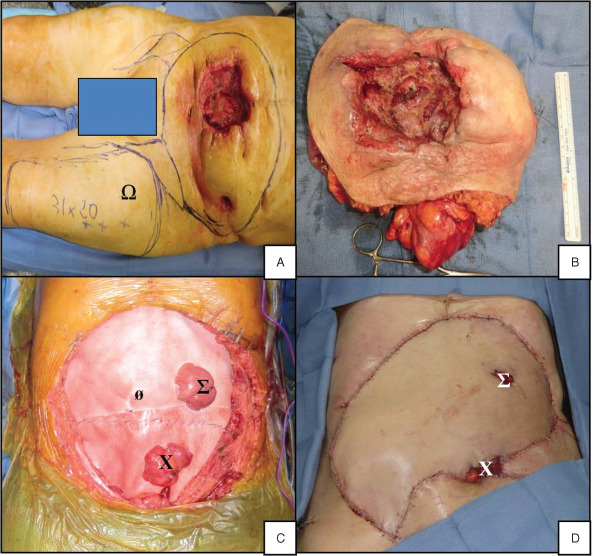
Intraoperative photographs. (A) Plot of the resection area and free flap harvesting zone (Ω). (B) En bloc resection of the abdominal wall and the small intestine. (C) Implementation of bioprostheses (ø) with one ileostomy (Σ) and one colostomy (X) through the prostheses. (D) Local view at the end of surgery.
The second operating phase corresponded to parietal reconstruction. Two bioprostheses (38 × 38 cm2 Permacol®, Covidien, Mansfield, MA) were sutured together and put in place (Figure 3C). One ileostomy and one colostomy were extracted through the bioprostheses. The ileostomy was made through the free flap, whereas the colostomy opened out on the left lower side of the flap and above the inguinal fold. The free flap's pedicle was anastomosed with the right femoral artery and the right saphenous vein (Figure 3D).
Two weeks after surgery, faced with necrotic aspect of the colostomy, we decided to re‐operate: construction of a colocolonic anastomosis and drainage (with two irrigation drains and one vacuum drain) were required. Drain irrigation was initiated on the day after surgery, in order to reduce the likelihood of anastomotic fistula.
Recovery from surgery was marked by the occurrence (on postoperative day 21) of an anastomotic fistula. The latter was well‐tolerated but required continued irrigation and nutritional support (Figure 4A). On postoperative day 24, lower gastrointestinal endoscopy showed almost complete dehiscence of the colocolonic anastomosis and so a 15 cm partially covered metal stent was installed in the colon (Figure 4B). Vacuum therapy was required at the old colostomy site and local treatment of the flap was needed to maintain good tissue perfusion and prevent infection.
Figure 4.
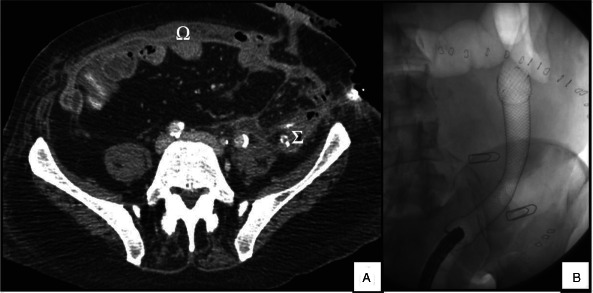
Postoperative CT scan and lower gastrointestinal endoscopy. (A) A CT scan highlighting the bioprostheses in the abdominal wall (Ω) and the colocolonic fistula directed to the abdominal wall (Σ). (B) Lower gastrointestinal endoscopy showing the partially covered colonic stent.
Histological analysis of the surgical specimen showed a well‐differentiated squamous cell carcinoma measuring 15 × 9 × 5 cm3 and infiltrating the skin integument, hypodermis, deep muscle, peritoneum and wall of the small intestine (Figure 5). We also found one metastasis in the left inguinal lymph node and two others in the right inguinal lymph node. The tumour resection was the total (R0).
Figure 5.
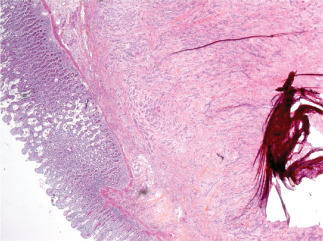
Histological analysis of the surgical specimen.
In the multidisciplinary team meeting, it was decided to initiate Folfox chemotherapy and radiotherapy in the inguinal lymph node area. Given the presence of a colonic fistula and the patient's non‐optimal overall status, it had not been possible to perform adjuvant chemotherapy within the first 3 months following surgery. A CT scan performed 6 months after surgery showed metastases in the lungs and the thoracic lymph nodes. Chemotherapy with cetuximab led to stabilisation of the lung lesions 13 months after the surgery. The abdominal wall has healed completely and the patient can walk unassisted. We were able to remove the colonic stent without prompting recurrence of the colonic fistula.
Discussion
Enterocutaneous fistula is associated with a high morbidity rate and a mortality rate that varies from 5% to 25%, depending on the study in question 2, 3. Preoperative recovery appears to be essential because (i) enterocutaneous fistula causes absorption disorders and local pain (4) and (ii) the tumour observed in the present case leads to hypercatabolism. Optimised preoperative nutrition can improve wound healing and prevent postoperative complications (5).
In most cases, enterocutaneous fistula is caused by a neoplastic process in the digestive tract, (6). To the best of our knowledge, the development of cancer on a chronic enterocutaneous fistula had never been previously reported. We are aware of a case of pilonidal cyst that developed 20 years after the onset of a wound healing disorder (1) and some cases of cancer that emerged as chronic perianal fistulas (7). In the present case, a squamous cell carcinoma had not been removed because of the patient's poor overall condition and the tumour's size. Although this condition is rare, knowledge of this kind helps to avoid useless, exhaustive check‐ups and reduce the degree of supportive care.
Preoperative CT is probably essential in this situation. It maps the lesions, informs the resection strategy and provides measures for the prosthesis used in the subsequent parietal reconstruction. In the present case, single‐step resection was essential for clean surgery and achievement of tumour‐free margins.
We decided that the best way to close the patient's huge abdominal wall defect was to use prosthesis. This approach has been already described in a patient with an abdominal desmoid tumour, although the digestive tract was not resected (8). In fact, our patient's parietal defect was similar to that found in a neonate with omphalocele or gastroschisis, for which bioprostheses are used with success (9). Use of a bioprosthesis in this case was useful because of the high risk of intraoperative contamination of the prosthesis and thus the need to avoid complicated infections (10).
Collaboration between gastrointestinal surgeons and plastic surgeons appears to be essential for the treatment of huge parietal tumours that invade the gastrointestinal tract because coverage with a flap is necessary. Various options have been described in the literature 11, 12. In our case, the large size of the parietal defect meant that only anastomosis between a free flap and a vascular system that was sufficiently large and not too close to the resection area was possible. We decided to use a flap from the vastus lateralis rather than the latissimus dorsi, in anticipation of a long period of postoperative confinement and to facilitate postoperative nursing.
To the best of our knowledge, this is the first report of a patient having been treated initially with a parietal bioprosthesis and then chemotherapy. This case provides useful information on several issues, including (i) the ability of a bioprosthesis to resist systemic chemotherapy and (ii) the management of patients presenting similar pathologies and in whom rapid treatment may improve the short‐ or medium‐term prognosis.
Conclusion
Although the development of squamous cell carcinoma on an enterocutaneous fistula is rare, physicians should be aware of this possibility. This type of pathology requires multidisciplinary assessment and management, together with good technical skills. Collaboration between the gastrointestinal surgeon (who has to make a maximal and complete resection) and the plastic surgeon (who has to reconstruct the abdominal wall) is essential.
Acknowledgement
The authors have no conflict of interest.
References
- 1. Mc Cune WS, Thistlethwaite JR. Fistula cancer. Ann Surg 1959;149:815–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Draus JMJr, Huss SA, Harty NJ, Cheadle WG, Larson GM. Enterocutaneous fistula: are treatments improving? Surgery 2006;140:570–6. [DOI] [PubMed] [Google Scholar]
- 3. Tassiopoulos AK, Baum G, Halverson JD. Small bowel fistulas. Surg Clin North Am 1996;76:1175–81. [DOI] [PubMed] [Google Scholar]
- 4. Lloyd DA, Gabe SM, Windsor AC. Nutrition and management of enterocutaneous fistula. Br J Surg 2006;93:1045–55. [DOI] [PubMed] [Google Scholar]
- 5. Soeters PB, Ebeid AM, Fischer JE. Review of 404 patients with gastrointestinal fistulas. Impact of parenteral nutrition. Ann Surg 1979;190:189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chamberlain RS, Kaufman HL, Danforth DN. Enterocutaneous fistula in cancer patients: etiology, management, outcome, and impact on further treatment. Am Surg 1998;64:1204–11. [PubMed] [Google Scholar]
- 7. Chandramohan K, Mathew AP, Muralee M, Anila KR, Ramachandran K, Ahamed I. Squamous cell carcinoma arising from long‐standing perianal fistula. Int Wound J 2010;7:515–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Overhaus M, Decker P, Fischer HP, Textor HJ, Hirner A. Desmoid tumors of the abdominal wall: a case report. World J Surg Oncol 2003; 1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Tuil C, Saxena AK, Willital GH. Experience with management of anterior abdominal wall defects using bovine pericard. Hernia 2006;10:41–47. [DOI] [PubMed] [Google Scholar]
- 10. Loganathan A, Ainslie WG, Wedgwood KR. Initial evaluation of Permacol bioprosthesis for the repair of complex incisional and parastomal hernias. Surgeon 2010;8:202–5. [DOI] [PubMed] [Google Scholar]
- 11. Sakuraba M, Asano T, Yano T, Yamamoto S, Moriya Y. Reconstruction of an enterocutaneous fistula using a superior gluteal artery perforator flap. J Plast Reconstr Aesthet Surg 2009;62:108–11. [DOI] [PubMed] [Google Scholar]
- 12. Sledzianowski JF, Suc B, Hézard L, Ghannem Y, Grolleau JL, Fourtanier G. [Large abdominal parietectomy for late abdominal wall recurrence of colonic cancer: reconstruction with latissimus dorsi free flap with delayed insertion]. Ann Chir 2001;126:789–93. [DOI] [PubMed] [Google Scholar]


