Abstract
Randomised controlled trials in chronic wounds typically exclude patients with comorbidities and confounding factors. Well‐designed observational studies can provide complementary clinical evidence that randomised trials cannot address. This study determined if wound care registry outcomes could be an alternative data source and if the results would be robust and valid. Changes in wound area and depth were hypothesised to be different between run‐in therapies and platelet‐rich plasma (AutoloGel™, Cytomedix, Inc) treatment. From a treatment registry of 285 chronic wounds, 46 had run‐in and post‐treatment data. Seven chronic wound categories were identified. Mean wound age at study start was 52·4 days. General linear model repeated measures showed a credible and robust data set. Statistically significant differences for wound area and depth were observed between run‐in and post‐treatment period at multiple time points. Wound area and depth ≥50% reduction were analysed using Kaplan–Meier methods. During run‐in, 15% of wound area improved compared to 28% post‐treatment and 11% of wound depth improved during run‐in compared to 39% post‐treatment. Significant clinical outcomes indicated many previously non responsive wounds began actively healing in response to platelet‐rich plasma therapy, indicating that registry data can be used as a complementary source of evidence.
Keywords: Clinical study, Platelet‐rich plasma, Practice‐based evidence, Statistical analysis, Wound healing
INTRODUCTION
The healing of chronic wounds is often characterised by periods of relatively steady healing interspersed with plateaus in which the wound does not improve. Converting a plateaued or stalled wound into a healing state is a challenge in wound care practice. The Center for Medicare and Medicaid Services (CMS) utilises the definition of a chronic wound as one that does not heal completely after 30 days of standard medical treatment (1). Clinicians, on the other hand, employ a broader, more practical definition of a wound that cannot heal easily because of overwhelming intrinsic and extrinsic factors 2, 3, 4. A defect in any of the major interrelated phases of healing – clotting, inflammation, proliferation and remodelling – can result in a non healing wound that can be further exacerbated by factors, such as wound age or infection (5). Comorbidities and other factors such as patient age, poor nutrition, concurrent illness, steroids, radiation, immobility, smoking also negatively influence the wound‐healing trajectory and lead to stalled or delayed healing 3, 5.
In the treatment of persistent non healing wounds, a dilemma exists between the competing considerations of cost and time‐to‐heal. While an actively healing wound may be treated with inexpensive agents and dressings, a wound that has stopped healing may require changes in treatment to re‐activate healing 5, 6, 7, 8, 9. Determining how to stimulate wound healing is a high priority for both clinicians and wound researchers. Early intervention with more aggressive treatments may even prevent stalling of the healing process and improve the overall healing trajectory.
Accurate assessment of wound‐healing trajectories is a major challenge for researchers and clinicians applying effective treatment strategies. The Wound Healing Society (WHS) treatment guidelines for diabetic ulcers advises re‐evaluation of the wound and treatment based on failure to reach a 40% reduction of initial wound size by week 4 (6). If there are no clinical signs of infection, pressure ulcers should respond to comprehensive care in as little as 2 weeks. Pressure ulcers that are not responsive prior to surgery and/or to initial comprehensive therapy should be considered candidates for growth factor‐type therapy (8). Initial wound‐healing trajectories have also been correlated to outcomes 10, 11, 12, 13. Diabetic ulcer patients with a 53% reduction in ulcer area at 4 weeks have been shown to exhibit a statistically significant 58% healing rate at week 12, whereas those with less reduction in ulcer area had only a 9% healing rate (13). Early identification of wounds with a low probability of healing allows clinicians to proceed quickly to more effective treatments.
Among several advanced wound‐healing modalities, autologous platelet‐rich plasma (PRP) has been in clinical use for wound healing for more than two decades. PRP consists of cytokines, chemokines and growth factors. The platelet actively mediates wound healing by initiating the clotting cascade and releasing multiple growth factors, such as platelet‐derived growth factor (PDGF), insulin‐like growth factor (IGF), vascular endothelial cell growth factor (VEGF), platelet‐derived endothelial cell growth factor (PD‐ECGF) and transforming growth factor‐β (TGFβ) among others (14). Clinical trials have shown the application of PRP to a wound can activate the healing process 15, 16. Currently, the only way to deliver multiple growth factors simultaneously is to utilise an autologous therapy.
Autologous platelet‐rich plasma (PRP) Gel (AutoloGel™; Cytomedix, Inc, Gaithersburg, MD) is derived from a small sample of the patient's blood and is applied directly to the wound bed. This PRP Gel consists of plasma and platelets, yielding a physiologic concentration of growth factors, cytokines, chemokines and a fibrin scaffold. The platelet and plasma fraction is mixed with ascorbic acid and calcified thrombin to activate platelets and form a fibrin gel. Unlike other PRP products, this autologous PRP Gel is applied topically to a wound at near physiological levels 16, 17. The PRP Gel technique received FDA 510(k) device permission in 2007, and is used for chronic and acute wounds, such as venous leg, pressure and diabetic ulcers and for the management of mechanically or surgically debrided wounds (18). The mechanism of action for PRP is presumed to be the molecular and cellular induction of normal wound‐healing responses. Accordingly, this therapy should have clinical activity regardless of wound aetiology.
A prospective, randomised, controlled, blinded, multicenter study of PRP Gel was performed in 72 diabetic foot ulcer (DFU) patients (15). The study demonstrated complete healing in 81·3% of common‐sized ulcer wounds treated with PRP Gel compared with 42·1% in controls for a subgroup per protocol analysis, P = 0·036 (15). Frykberg et al. described an observational clinical study of autologous PRP Gel treatment in 65 complex wounds of different aetiologies, including pressure ulcers, diabetes, venous, arterial, surgical, trauma and sickle cell wounds, and several key clinical parameters (16). Despite the failure to heal in pretreatment, improvements in wound area and volume, undermining or sinus tracts/tunnelling were observed in 97% of the wounds within 2·8 weeks following 3·2 PRP Gel applications. These findings indicate PRP Gel may offer clinicians a biologic healing stimulant for recalcitrant wounds.
Although randomised controlled trials (RCTs) are considered to be the gold standard in generating clinical evidence, the clinical effects of interventions in ‘real‐world’ patients in less controlled conditions are becoming recognised as important for study 19, 20, 21. Because ‘real‐world’ patients are clinically complex, often presenting with multiple comorbidities, underlying aetiologies and compliance issues, RCT‐proven therapies may fail to be effective. This might be attributed to RCT designs that do not reflect the complex issues faced in clinical practice and are limited by the challenges of designing such studies given wound heterogeneity 19, 20, 21, 22, 23. Use of practice‐based evidence (PBE) or observational study data obtained in a real‐world setting may provide data complementary to RCT evidence. Most observational studies, however, are not rigorous in their design, execution and analysis, and therefore their conclusions are not always considered valid for the practice of evidence‐based medicine (EBM). Consequently, the most important designs will continue to be cohort, case control and comparative designs which have a higher level of evidence than a large case series.
To increase the level of evidence and to overcome criticism of observational studies due to lack of rigor, a comparative design utilising specific statistical methods was used to analyse a wound outcomes registry. A wound care registry contained a dataset of chronic wounds with pretreatment run‐in data. The data was analysed using general linear modelling (GLM) techniques on a time scale basis over run‐in and treatment periods. A Kaplan–Meier (KM) analysis was performed in which each wound served as its control during run‐in and treatment phases.
The hypothesis of this study was threefold: (i) wound care registry data could generate clinically meaningful evidence when the outcomes are appropriately analysed as an alternative source of data; (ii) the robustness and validity of the results could be demonstrated by statistical analysis; and (iii) significant differences in wound area and depth between PRP Gel treatment compared to pretreatment run‐in therapies could be detected.
METHODS
Database creation and management
A platelet‐rich plasma gel (PRP Gel, AutoloGel™) treatment registry is maintained by Cytomedix, Inc. The wound registry is a centralised database of data provided voluntarily by clinicians as they treated patient's wounds during an evaluation of the use of the PRP Gel for the treatment of chronic wounds. Data is only tabulated during this evaluation period. Clinicians do not provide data following the evaluation period. All information on patient wound and treatment are voluntarily submitted by clinicians using this PRP Gel. No other PRP gels are included in the registry. No other restrictions are applied on the data submitted, nor is submission required as part of the product usage. Information on registry wounds includes a variety of information that is normally recorded and/or measured during normal standard of care evaluations and procedures and charted per the policy of each institution. Clinicians can submit this information to the registry. Registry data includes wounds that improved, showed no change or worsened. This PRP Gel database was accessed with provider and company permission to acquire the data for this study.
Privacy of all patients within the database was protected by de‐identification of patient data in compliance with the Health Insurance Portability and Accountability Act (HIPAA) regulations for studying aggregate data for research purposes, per the National Institutes of Health Authorization for Research Uses and Disclosures. According to the HIPAA Privacy Rule, Internal Review Board's approval and informed consents are not required as the data was gathered by the clinicians during normal clinical care and all protected health information about the patients were de‐identified prior to analysis (24). The data submitted to the registry and the conduct of the study to evaluate the data also complies with the ethical guidelines of the 1975 Declaration of Helsinki.
Inclusion/exclusion criteria
In this study, the run‐in period is defined as the pretreatment period during which standard or advanced treatment modalities were used.
This study evaluated the changes in wound dimensions between pretreatment therapy impact and post‐PRP Gel treatment impact. The registry data was from multiple outpatient and long term acute care (LTAC) centres. The study population consisted of a general chronic wound population. Unlike a randomised controlled trial, the data in the registry is voluntarily submitted and reflects clinical practice treatment and outcome data in these real‐world patients.
Because comparing the run‐in pretreatment outcomes to the PRP Gel post‐treatment outcomes was essential to the study hypothesis, all registry patients were reviewed. Patients included those who received at least one PRP Gel treatment and had at least three wound measurements, including the transition baseline measurement, prior to PRP Gel treatment, a baseline measurement at the time of PRP treatment, and at least one post‐PRP treatment measurement. Out of the 285 wounds (n = 200 patients) in the database, 49 (n = 35 patients) had run‐in data available. Three wounds were not included because the data from the run‐in period was over 100 days with no baseline measurements recorded immediately prior to PRP treatment preventing assessment of the baseline status. Detailed information on the preparation and use of the PRP treatment analysed in this study has been described previously (16).
Wound measurements
Clinicians using PRP Gel were previously trained to use a comprehensive wound measurement technique to ensure uniformity of wound assessment. Disposable paper rulers with centimeter markings, and cotton‐tipped applicators were used to probe and measure length, width and depth of the visible wound as well as undermining, sinus tracts and tunnelling. Measurements were taken and recorded just prior to each PRP Gel application and after the last PRP Gel treatment. Length and width of the wound opening was measured using the standard ‘clock face’ method (25). Length is 12:00 to 6:00 with 12:00 towards the head, width at 3:00 to 9:00. Depth measurement was taken from the deepest point of the wound bed to the level of usual skin surface and at a 90° angle to skin surface.
To ensure more accurate area measurement for all wound types, area was calculated in the registry as an ellipse using the longest and shortest linear measurements of the wound perpendicular to each other as the major and minor axes (length × width × 0·7854). The common calculation of area (length × width) is more appropriate for square and rectangular shapes rather than elliptical shapes seen in a clinical population. Use of an ellipse for calculating wound measurement has been used in a database of more than 120 000 wounds and in RCTs 26, 27. Depth was analysed as a separate variable to better assess the changes observed instead of using a volume calculation.
Assessment times
Data on wound variables were available at seven general assessment times: start of run‐in period, farthest from baseline (T_3); second run‐in time point, second farthest from baseline (T_2); run‐in time point, closest to baseline (T_1); baseline, run‐in treatment discontinued, PRP Gel initiated (T0); first post‐PRP Gel treatment point (T1); second post‐PRP Gel treatment point (T2); third post‐PRP Gel treatment point (T3). Data for area (i.e. length and width) and depth were recorded at each assessment time (measured in days before baseline for the run‐in period, and after baseline during PRP Gel treatment). Because the registry is a compilation of clinical practice, assessment times were not the same for different wounds (i.e. for one wound, T_3 might be 21 days, while for another it might be 7 days). Because 28% (n = 13) and 35% (n = 16) of the data were missing at T_2 and T2, respectively, these assessment times were not used in the analysis. In addition, for some wounds, T_3 was equal to T_1, and T1 equal to T3, respectively, when only one measurement before or after baseline was available.
Wound type
The aetiology of each wound type was documented in the database with seven types identified: arterial, diabetic foot, pressure or venous ulcer; dehisced, surgical wound and wound of other aetiology. Surgical and dehisced wounds were combined to form one category because of the sample size and the related nature of these wounds. Because of the small numbers of arterial ulcers and wounds of other aetiology, these two types of wounds were combined in the statistical analysis to form a mixed group. Categorised and aggregate results were used to display the outcome variations in these chronic wounds.
Data transformation
Because the range of wound depth and area was large, the depth or area was set at 100% for start of run‐in period (T_3) data, and all measurements at other assessment times were calculated as percentages in relation to the initial depth or area to this time point.
Statistical analysis
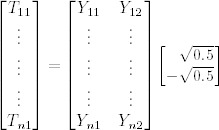
The primary variables used in the analysis were wound depth and area. To test whether the percentage of area or depth significantly decreased from the run‐in period to the end of the treatment period, a GLM repeated measures approach was taken (formal H0 hypothesis: the percentage of depth and area in relation to initial depth and area are equal at each time point). GLM repeated measures is a statistical approach designed to compare time points that contain data for multiple variables in a longitudinal manner (28). The general equation is: T = YM, where T is time at multiple points, Y represents the variables at each time and M is the orthonormal contrast matrix. The equation as a whole when tested is (28):
 |
Models can be simple within‐group factors or complex involving covariates and other between‐group factors. Models were developed using T_3 data for the initial time point and four time points (pretreatment run‐in, baseline, post‐PRP treatment 1 and post‐PRP treatment 3) with and without wound type as additional factors. Initial models were multivariate (depth and area). Robustness of models was tested using non transformed and log‐transformed variables. The effect of outliers was examined by using a cut off of standardised residuals >3·3. The significance of estimated marginal mean differences between the time points was adjusted for multiple comparisons using Sidak.
Another way to capture the events that happen within a longitudinal time frame is to use KM analysis in which events are treated according to survival algorithms. By using patients as their own controls, the run‐in period can be compared to the treatment period provided wounds that meet the event criteria are not represented in both groups (i.e. no double counting). To determine clinical outcomes of PRP Gel treatment, a KM analysis was also carried out in which an event was defined as a wound reduced by 50% or more in area or depth by the time the treatment ended using 100% as the initial value. First, data were categorised for two groups: run‐in pretreatment and post‐PRP treatment. Second, while a wound could decrease by 50% in area or depth between any two assessment points, the reduction in depth or area had to be improving (i.e. show a 50% decrease in area or depth) for the event to be valid. Third, if a wound was reduced by 50% during the run‐in period, then the wound was not analysed in the treatment period to avoid skewing the data in either direction. Statistical analysis was performed using PASW 19 (SPSS, Inc, Chicago, IL).
RESULTS
General
The entire wound registry consisted of 285 wounds of various aetiologies. Within the registry, there was a group of patients with run‐in historical data on 46 wounds. Mean age of all patients was 59·6, whereas run‐in data patients were of an average age of 58·3 years (range 25–89 years). Mean wound age of the entire registry was 48·2 weeks with a median of 22 weeks and the run‐in dataset mean was 52·4 weeks and median of 34 weeks showing the run‐in group had a similar but longer wound duration. The predominant wound for the registry and the run‐in data set was a pressure ulcer (n = 142 and 20, respectively) followed by surgical wounds (n = 38 and 8, respectively). Other wounds included in the registry and this study were dehisced wounds (n = 24 and 6), diabetic ulcers (n = 41 and 4), venous ulcers (n = 32 and 3), sickle cell (n = 1 and 1) and other (n = 5 and 2 respectively). Mean baseline area for all registry wounds was 26·0 cm2 and mean baseline depth was 1·40 cm. The mean area for the run‐in wounds was 32·3 cm2 at baseline [standard deviation (SD): 79·69; range: 0·4–530·2], while mean depth at baseline was 1·63 cm (SD: 1·48; range: 0·1–5·0). The mean percent reduction in area (33%) and depth (44%) between baseline and the final treatment assessment point for the 46 wounds was similar compared to the entire 285 wound registry (area 38·8%, depth 37·7% and volume 54·3%), suggesting that the run‐in wound dataset had a similar wound trajectory to the entire dataset. All of these data characteristics indicated the two groups were similar in wound characteristics and trajectory.
Of the 46 wounds with run‐in data (N = 34 patients), the mean age of the wound at first assessment was 52·4 days (SD: 71·62). Mean time from first wound assessment during run‐in (T_3) to baseline (T0) was 22·2 days (SD: 22·84, range: 2–87) and mean time from baseline at the third assessment (T3) during treatment was 13·4 days (SD: 9·88, range: 3–36). The first run‐in assessment of mean area was 33·9 cm2. At the final post‐treatment assessment, however, the average area was 22·6 cm2 resulting in a 33% decrease in size from first run‐in assessment. For depth, the first run‐in assessment was 1·8 cm. At the final post‐treatment assessment though, the mean depth was 1·0 cm, a 44% decrease. Compared to first assessment, dehisced/surgical wounds, as well as diabetic foot ulcers showed the largest decrease in percentage of area (46 and 53%; Figure 1), while diabetic foot and venous ulcers showed the greatest decrease in percentage of depth (54 and 58%; Figure 2). While a few wounds substantially increased in depth during the run‐in period (120% for other types of wounds; Figure 2), both diabetic foot ulcers and other types of wounds had very large increases in area during the run‐in period (>200%; Figure 1). Moreover, while most wounds had larger areas at baseline compared to the first assessment period during run‐in, dehisced or surgical wounds were approximately 80% of original areas at baseline. Given the vast increased pretreatment wound size, using error bars and showing the entire graph is impractical. It should be noted that for eight wounds, the start of the run‐in period (T_3) was equal to the run‐in time point closest to baseline (T_1) and for 12 wounds, the first treatment period (T1) was equal to the third treatment period (T3), that is only one measurement from baseline was available.
Figure 1.
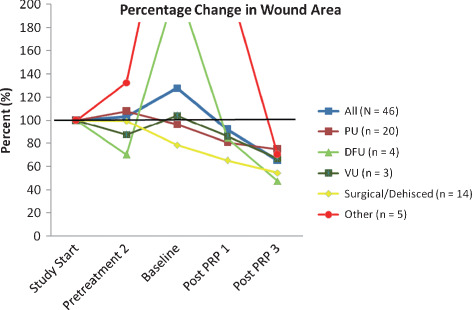
Percentage changes in area by wound type in regard to assessment period, using first assessment values as 100%.
Figure 2.
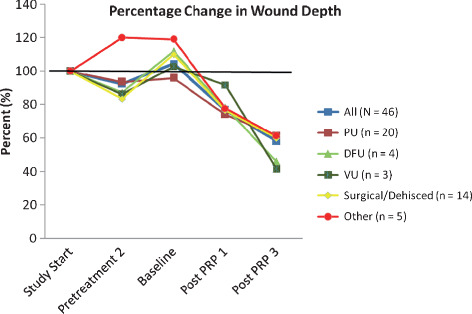
Percentage changes in depth by wound type in regard to assessment period, using first assessment values as 100%.
GLM repeated measures
Although heteroscedasticity was observed at some time points in the variables and two outliers had a few residuals that exceeded 3·3, the overall results of the models did not justify the use of outlier deletion or log‐transformation given the small size of the sample. The multivariate analysis demonstrated significant interaction between area and depth (percentage‐transformed variables), as well as significant differences between pairs at different time points. Multivariate tests showed significant effects between‐subjects and within‐subjects (between‐subjects: Wilks' lambda = 0·155, P = 1·6 × 10−18; within‐subjects: Wilks' lambda = 0·452, P = 9·3 × 10−6). Adding a wound type factor did not improve the model significantly. Sphericity is a measure of the variance–covariance matrix in which the covariance between any two variables is equal to the average of their variances minus a constant. If it is significantly violated, it has to be corrected so that the overall F test of the model is valid. Table 1 shows that there is significant violation of sphericity. The most commonly used method to correct for sphericity violation is epsilon adjustment, of which Greenhouse‐Geisser (GG) is considered conservative in a small sample size. The epsilon adjustments using Huynh‐Feldt and lower‐bound methods are also included as they are normally reported in this kind of analysis. Table 2 shows the univariate effects. Note that while power is above 0·8 for the GG correction for depth, it is below this level for the GG for area (0·620). This means that in respect of area it is still possible that a type II error may be possible in which more significant effects may be apparent. However, given the simplicity of the model, this is an acceptable limitation. From the tests of within‐subjects contrast, it was noted that for depth, a quadratic effect (P = 0·005) was also apparent, suggesting that a nonlinear component might be present (data not shown). This is also a limitation of the model, in that linear effects are assumed. No significant nonlinear contrasts were apparent for area.
Table 1.
Mauchly's test of sphericity (tests the null hypothesis that the error covariance matrix of the orthonormalised transformed dependent variables is proportional to an identity matrix)
| Within‐subjects effect † | Measure | Mauchly's W | Approx. chi‐square | df | P | Epsilon * | ||
|---|---|---|---|---|---|---|---|---|
| Greenhouse‐Geisser | Huynh‐Feldt | Lower‐bound | ||||||
| Assessment | Depth | 0·516 | 28·931 | 5 | 2·4 × 10−5 | 0·722 | 0·759 | 0·333 |
| Area | 0·109 | 96·980 | 5 | 2·4 × 10−19 | 0·450 | 0·459 | 0·333 | |
df, degrees of freedom.
*May be used to adjust the degrees of freedom for the averaged tests of significance. Corrected tests are displayed in the tests of within‐subjects effects table.
†Design: intercept. Within‐subjects design: assessment. While the GG values substantially deviate from 1·0, the correction is sufficiently good enough that the model is valid.
Table 2.
Univariate tests
| Source | Measure | Type III sum of squares | df | Mean square | F | P | Partial eta squared | Observed power * | |
|---|---|---|---|---|---|---|---|---|---|
| Assessment | Depth | Sphericity assumed | 55 411·86 | 3 | 18 470·62 | 13·480 | 9·5 × 10−8 | 0·231 | 1·000 |
| Greenhouse‐Geisser | 55 411·86 | 2·165 | 25 599·76 | 13·480 | 3·7 ×10−6 | 0·231 | 0·998 | ||
| Huynh‐Feldt | 55 411·86 | 2·278 | 24 327·28 | 13·480 | 2·2 × 10−6 | 0·231 | 0·999 | ||
| Lower‐bound | 55 411·86 | 1·000 | 55 411·86 | 13·480 | 0·001 | 0·231 | 0·949 | ||
| Area | Sphericity assumed | 93 243·32 | 3 | 31 081·11 | 4·379 | 0·006 | 0·089 | 0·864 | |
| Greenhouse‐Geisser | 93 243·32 | 1·349 | 69 102·02 | 4·379 | 0·030 | 0·089 | 0·620 | ||
| Huynh‐Feldt | 93 243·32 | 1·376 | 67 755·97 | 4·379 | 0·029 | 0·089 | 0·626 | ||
| Lower‐bound | 93 243·32 | 1·000 | 93 243·32 | 4·379 | 0·042 | 0·089 | 0·535 | ||
| Error (Assessment) | Depth | Sphericity assumed | 184 974·43 | 135 | 1370·18 | ||||
| Greenhouse‐Geisser | 184 974·43 | 97·405 | 1899·03 | ||||||
| Huynh‐Feldt | 184 974·43 | 102·499 | 1804·64 | ||||||
| Lower‐bound | 184 974·43 | 45·000 | 4110·54 | ||||||
| Area | Sphericity assumed | 958 199·32 | 135 | 7097·77 | |||||
| Greenhouse‐Geisser | 958 199·32 | 60·721 | 15 780·34 | ||||||
| Huynh‐Feldt | 958 199·32 | 61·927 | 15 472·95 | ||||||
| Lower‐bound | 958 199·32 | 45·000 | 21 293·32 |
df, degrees of freedom.
*Power is below 0.8 for the GG area indicating a type II error may be possible.
Method used to correct for sphericity violation and its P value are bolded.
Paired mean comparisons were made for the different time points. Time points were defined as run‐in pretreatment 2; baseline; post‐PRP treatment 1 and post‐PRP treatment 3. The initial run‐in assessment is excluded as it is set at 100%. For area data, there was a significant difference (P = 0·002) between the run‐in and post‐PRP treatment 3 period. There were multiple statistically significant differences for depth between run‐in pretreatment and post‐PRP treatment 3, baseline and post‐PRP treatment 1, baseline and post‐PRP treatment 3 and post‐PRP treatment 1 and treatment 3 (Table 3).
Table 3.
Pairwise comparisons based on marginal means; P values adjusted for multiple comparisons (Sidak; significant values in bold)
| Measure | (I) Assessment | (J) Assessment | Mean difference (I−J) | Standard error | P | 95% CI for difference |
|---|---|---|---|---|---|---|
| Depth | Pretreat2 | Baseline | −12·2 | 10·32 | 0·809 | −40·63 to 16·14 |
| Post‐PRP1 | 15·5 | 8·10 | 0·318 | −6·77 to 37·79 | ||
| Post‐PRP3 | 34·1 | 7·72 | 0·00037 | 12·86 to 55·35 | ||
| Baseline | Post‐PRP1 | 27·8 | 6·45 | 0·001 | 10·02 to 45·49 | |
| Post‐PRP3 | 46·4 | 7·81 | 2·3 ×10−6 | 24·87 to 67·83 | ||
| Post‐PRP1 | Post‐PRP3 | 18·6 | 4·84 | 0·002 | 5·28 to 31·91 | |
| Area | Pretreat2 | Baseline | −24·480 | 22·41 | 0·861 | −86·15 to 37·19 |
| Post‐PRP1 | 11·3 | 10·80 | 0·885 | −18·46 to 40·96 | ||
| Post‐PRP3 | 38·2 | 10·00 | 0·002 | 10·64 to 65·69 | ||
| Baseline | Post‐PRP1 | 35·7 | 16·45 | 0·193 | −9·52 to 80·98 | |
| Post‐PRP3 | 62·6 | 26·52 | 0·128 | −10·33 to 135·63 | ||
| Post‐PRP1 | Post‐PRP3 | 26·9 | 12·61 | 0·209 | −7·78 to 61·61 |
CI, confidence interval.
Time points defined as follows: run‐in pretreatment 2; baseline; post‐PRP treatment 1; and post‐PRP treatment 3.
KM analysis
Seven wounds were reduced in area by 50% during the run‐in period, compared to 11 wounds during the treatment period. Mean time for the run‐in period was more than 2·5 times longer than that of the treatment period: (66·0 vs. 25·0 days, respectively; standard error: 7·15 vs. 2·29, respectively). The majority of the data are essentially right truncated rather than right censored because run‐in times are far longer on average than PRP Gel treatment times. The data showed that one minus cumulative survival probability was significantly better for the PRP Gel treatment group compared to the run‐in group (logrank Mantel‐Cox test: P = 0·028; Figure 3).
Figure 3.
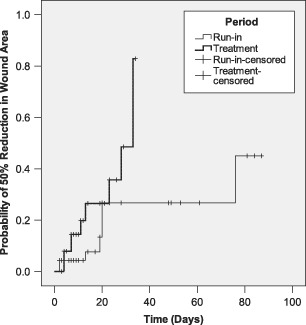
Kaplan–Meier analysis for run‐in and treatment times for time to 50% reduction in area.
Five wounds were reduced in depth by 50% during the run‐in period compared to 16 wounds during the treatment period. Mean time for the run‐in period was also more than three times longer than that of the treatment period: (72·9 vs. 22·3 days, respectively; standard error: 6·18 vs. 2·47, respectively). The majority of this data are also essentially right truncated because the run‐in times are far longer on average than PRP treatment times. One minus cumulative survival probability was significantly better for the PRP Gel treatment group compared to the run‐in group (log rank Mantel‐Cox test: P = 0·00034; Figure 4).
Figure 4.
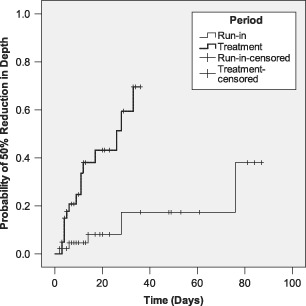
Kaplan–Meier analysis for run‐in and treatment times for time to 50% reduction in depth.
DISCUSSION
An RCT in diabetic foot ulcers previously demonstrated the efficacy of this PRP gel (15). The data presented in this study complements the RCT results and effectiveness across a large variety of wound etiologies. When the clinical impact of a treatment can be demonstrated in controlled situations (i.e. RCT) as well as less controlled settings, clinicians can be more certain that the intervention has utility in a wide variety of clinical settings. Even though this study population was small, the outcome results and trajectories were similar to those achieved in much larger study sizes (15, 16, 34).
Observational studies describe outcomes in which selection criteria are less restrictive than RCTs. When used in conjunction with RCT results, PBE data can add to an enhanced evidence pool for clinical decision making. Wound care registries can be extremely useful in analysing clinical practice outcomes and the use of pretreatment run‐in data for self control provided a unique, but valid approach to obtaining real‐world PBE. To overcome the limitations encountered in many observational studies, comparative run‐in data and statistical methods were used in this study to compare the impact between the run‐in pretreatment and post‐PRP treatment on the same wounds. Using run‐in data not only raises the level of comparative clinical evidence in this study, but also acts further as a complementary set of evidence to prior RCT results (15). Furthermore, using the run‐in pretreatment data and post‐PRP treatment data provides insight into the clinical practice and realities encountered by chronic wound care clinicians. At this time, appropriately designed observational studies providing valid evidence for clinical effectiveness in patients with comorbidities and refractory wounds are rare.
Although values of the marginal means and probability values changed with different general linear repeated measures models and with the inclusion of all data or removal of two outliers, the overall outcome pattern did not. Consequently, given the small sample size, the multivariate model using all data and untransformed variables was preferred and judged sufficiently robust that its major conclusions are reliable. Although less common in wound research, KM analysis was used to analyse two groups in which patients constitute their own controls so that pretreatments and treatment can be compared (29). Thus, the statistical techniques used in this study are valid for comparatively analysing a wound care registry.
It is well accepted that reducing wound size has clinical significance in terms of improvement in healing and patient Quality of Life (QoL) measures (20). For prospective studies, a predefined reduction of the wound size by >50% has served as a surrogate endpoint for healing (20). In this study, when using a surrogate marker of a reduction of ≥50% of wound area during the pretreatment period, only 15% of wounds improved whereas post‐PRP treatment, 28% were actively healing. The difference was greater when the surrogate marker was applied to depth. During the pretreatment period, only 11% of wounds improved compared to the post‐PRP treatment of 39% actively healing by ≥50%. These results could indicate that the short‐term use of PRP Gel appears to convert many non healing wounds into actively healing ones. Analysing time and 50% reduction in size, the KM analysis also showed the pretreatment run‐in period was much longer than post‐PRP treatment period to achieve the same amount of improvement. Because the analysis was limited to available data, the time to complete healing is not known.
The results of this study demonstrated that more non healing wounds had reduced area and depth between the run‐in pretreatment period and the post‐PRP Gel treatment period. The KM analysis indicated that PRP Gel treatment statistically reduced the wound area or depth by ≥50% more than the pretreatment run‐in period (area P = 0·028, depth P = 0·00034). The results appeared to be more pronounced for depth measurements than area calculations. One possible reason for this observation is that many chronic wounds, such as DFUs and pressure ulcers, can have deep tissue involvement resulting in deep wounds. By contrast, venous leg ulcerations are more superficial, but must have a granulated wound bed prior to healing by epithelialisation. Because the body must replace absent tissue before more superficial layers can heal, the data could be interpreted as an observation of the wound healing from the bottom up (i.e. depth) more than healing from the sides (e.g. length, width and contraction). Healing by granulation tissue deposition to reduce the wound depth could prevent further deep tissue involvement and associated comorbidities such as infection and amputation 30, 31. All of the wound types showed improved depth and area post‐PRP treatment, but diabetic foot and venous ulcers showed the greatest depth reduction, whereas dehisced and surgical wounds and diabetic foot ulcers had the greatest decrease in area within 4 weeks. Because surgical and dehisced wounds were previously treated to heal by primary intention, it is possible the base of the wound bed was shallow enough that once granulated, the area quickly decreased as contraction and epithelialisation began to occur.
The chronic wounds in this study were recalcitrant to standard and advanced wound care (i.e. negative pressure wound therapy) and remained unhealed during the run‐in period. The pretreatment run‐in period was 2·5 times longer for area and more than three times longer for depth compared to the treatment period. Wounds incur a treatment cost no matter if they are healing or not, yet even basic inexpensive care becomes expensive when it is ongoing and ineffective 2, 3. A cost effectiveness model analysed cost and QoL benefits for PRP Gel treatment and alternative wound care treatments for DFUs. It documented that PRP Gel was less expensive with increased QoL benefits over a 5‐year period than the alternative treatments including standard of care, tissue engineered skin substitutes, non contact ultrasound, single growth factor, and negative pressure wound therapy (32). In this wound care registry study, the results support the idea of cost savings based on mean treatment times. The mean time for pretreatment run‐in was 9·5 and 10·5 weeks for area and depth compared to 3·5 and 3 weeks for post‐PRP treatment. Such a reduction in treatment time, cost and QoL should impact important clinical and financial decisions.
Unlike a case series, this analysis utilised prospectively collected data from patients as part of their standard wound care treatment. Because each patient served as their own control and the pretreatment run‐in periods were included, the study has a higher level of evidence (33). In addition, wound data and outcomes came from real‐world patients with a variety of wound aetiologies, comorbidities and other confounding factors like PBE studies. Study limitations included missing data points for some wounds and not all time points were able to be analysed resulting in missing potentially useful ancillary data. Use of grouped assessment time points is a study limitation because it adds variability to the dataset; however, using such a grouping of general days allows the actual clinical practice to be analysed. Often pretreatment run‐in data was not available in chronic non healing wounds which resulted in a small sample size and even smaller wound subcategory sizes. While the small sample size is a limitation, particularly in regard to individual wound types, other larger clinical results suggest similar outcomes. Also, the GLM model on depth uses the assumption that healing is linear, but the data suggest a nonlinear effect may be present. The GLM model on area does not seem to have this nonlinear effect and the assumption is correct. Lastly, the observation that not all wounds were ‘kick started’ is valid, but the reason why the majority of wounds began healing actively and others did not, is not known at this time.
This study demonstrates the utility of wound care registry data when appropriately analysed. Both analytical methods, GLM and KM, demonstrated that the results obtained using registry data were in broad agreement with the results obtained from the RCT and other studies (15, 16, 34). By including run‐in pretreatment and post‐PRP treatment data, the statistical analysis showed important clinical outcomes resulting from various treatments. Including run‐in treatment and outcome data for each wound raises the level of evidence in this study. A predefined surrogate marker for healing, reduction of the wound size by >50%, was used to assess clinical improvement (20). Greater than 50% reduction in area and depth following PRP Gel treatment in a relatively short time appears to suggest many non responsive wounds developed a strong healing trajectory. The significant clinical outcomes in response to PRP Gel therapy complement clinical results obtained from prior clinical studies. We propose that this analytical approach can be used with data on other wound care registries in other products or interventions.
ACKNOWLEDGEMENTS
The authors would like to thank the following individuals and institutions for their contributions to the study: Laurie M. Rappl (Cytomedix, Inc); Bob Desotelle (Asheville Specialty Hospital); Boston Medical Center and Boston University School of Medicine; Carol Anderson (Advanced Therapy Surfaces, Maplewood, MN); Caroline E. Fife (IntelliCure, Inc, The Woodlands, TX); Cynthia A. Dowd (Wound Care and Outpatient Department, Mary Immaculate Wound Center, Newport News, VA); Dawn Meader, (Solara Hospital Harlingen, Brownsville, TX) and Baylor College of Medicine.
Financial support for manuscript preparation was provided by Cytomedix, Inc. MJC, WWL and TES are consultants for Cytomedix. CPF is employed by Cytomedix, Inc. Payment is not dependent upon publication of this manuscript or wound outcomes of this manuscript.
REFERENCES
- 1. Lau J, Tatsioni A, Balk E, Chew P, Kupelnick B, Wang C, O’Donnell T. Usual care in the management of chronic wounds: A review of the recent literature. Agency for Healthcare Research and Quality Technology Assessment Program. Rockville, MD: Department of Health and Human Services, 2005. March 8. [PubMed]
- 2. Falanga V. The chronic wound: failure to heal. In: Falanga V, editor. Cutaneous wound healing. Florence: Martin Dunitz Ltd., 2001:155–64. [Google Scholar]
- 3. Fowler E. Chronic wounds: an overview. In: Krasner D, editor. Chronic wound care, a clinical source book for healthcare professionals. King of Prussia: Health Management Publications Inc, 1990:12–8. [Google Scholar]
- 4. Robson MC, Barbul A. Guidelines for the best care of chronic wounds. Wound Repair Regen 2006;14:647–8. [DOI] [PubMed] [Google Scholar]
- 5. Lawrence WT. Clinical management of nonhealing wounds. In: Cohen IK, Diegelmann RF, Lindblad WJ, editors. Wound healing: biochemical and clinical aspects. Philadelphia: Saunders, 1992:541–61. [Google Scholar]
- 6. Steed DL, Attinger C, Colaizzi T, Crossland M, Franz M, Harkless L, Johnson A, Moosa H, Robson M, Serena T, Sheehan P, Veves A, Wiersma‐Bryant L. Guidelines for the treatment of diabetic ulcers. Wound Repair Regen 2006;14:680–92. [DOI] [PubMed] [Google Scholar]
- 7. Robson MC, Cooper DM, Aslam R, Gould LJ, Harding KG, Margolis DJ, Ochs DE, Serena TE, Snyder RJ, Steed DL, Thomas DR, Wiersma‐Bryant L. Guidelines for the treatment of venous ulcers. Wound Repair Regen 2006;14:649–62. [DOI] [PubMed] [Google Scholar]
- 8. Whitney J, Phillips L, Aslam R, Barbul A, Gottrup F, Gould L, Robson MC, Rodeheaver G, Thomas D, Stotts N. Guidelines for the treatment of pressure ulcers. Wound Repair Regen 2006;14:663–79. [DOI] [PubMed] [Google Scholar]
- 9. Carter MJ, Fife CE. Factors affecting the healing of chronic wounds: an iconoclastic view. In: Percival SL, editor. Microbiology of chronic wounds. London: CRC Press,2010:345–72. [Google Scholar]
- 10. Tallman P, Muscare E, Carson P, Eaglstein WH, Falanga V. Initial rate of healing predicts complete healing of venous ulcers. Arch Dermatol 1997;133:1231–4. [PubMed] [Google Scholar]
- 11. Kantor J, Margolis DJ. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. Br J Dermatol 2000;142:960–4. [DOI] [PubMed] [Google Scholar]
- 12. Weingarten MS, Neidrauer M, Mateo A, Mao X, McDaniel JE, Jenkins L, Bouraee S, Zubkov L, Pourrezaei K, Papazoglou ES. Prediction of wound healing in human diabetic foot ulcers by diffuse near‐infrared spectroscopy: a pilot study. Wound Repair Regen 2010;18:180–5. [DOI] [PubMed] [Google Scholar]
- 13. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4‐week period is a robust predictor of complete healing in a 12‐week prospective trial. Diabetes Care 2003;26:1879–82. [DOI] [PubMed] [Google Scholar]
- 14. Jacobson M, Fufa D, Abreu EL, Kevy S, Murray MM. Platelets, but not erythrocytes, significantly affect cytokine release and scaffold contraction in a provisional scaffold model. Wound Repair Regen 2008;16:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Driver VR, Hanft J, Fylling CP, Beriou JM, AutoloGelTM Diabetic Foot Ulcer Group. A prospective, randomized, controlled trial of autologous platelet‐rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage 2006;52:68–87. [PubMed] [Google Scholar]
- 16. Frykberg RG, Driver VR, Carman D, Lucero B, Borris‐Hale C, Fylling CP, Rappl LM, Clausen PA. Chronic wounds treated with a physiologically relevant concentration of platelet‐rich plasma gel: a prospective case series. Ostomy Wound Manage 2010;56:36–44. [PubMed] [Google Scholar]
- 17. Reese RJ. Autologous platelet rich plasma (PRP): what do we know? Important concepts relevant to hair restoration surgery. Hair Transplant Forum International 2010;20:14–17. [Google Scholar]
- 18. FDA 510(k) clearance, BK060007 . AutoloGel System. Rockville (MD): Cytomedix, Inc, 2007. [Google Scholar]
- 19. Horn SD, Gassaway J. Practice‐based evidence study design for comparative effectiveness research. Med Care 2007;45:S50–7. [DOI] [PubMed] [Google Scholar]
- 20. A EWMA patient outcome group document. Outcomes in controlled and comparative studies on non‐healing wounds; recommendations to improve the quality of evidence in wound management. J Wound Care 2010;19:239–68. [DOI] [PubMed] [Google Scholar]
- 21. Bagshaw SM, Bellomo R. The need to reform our assessment of evidence from clinical trials: a commentary. Philos Ethics Humanit Med 2008;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moffatt CJ, Doherty DC, Smithdale R, Franks PJ. Clinical predictors of leg ulcer healing. Br J Derm 2010;162:51–8. [DOI] [PubMed] [Google Scholar]
- 23. Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 2000;342:1887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. U.S. Department of Health and Human Service . National Institutes of Health. HIPAA Privacy Rule. URL : http://privacyruleandresearch.nih.gov/pr_08.asp8b [accessed on 8 December 2010].
- 25. Sussman C. Wound measurements. In: Sussman C, Bates‐Jensen B, editors. Wound care: a collaborative practice manual for physical therapists and nurses. Gaithersburg: Aspen, 2001:120–41. [Google Scholar]
- 26. Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic ulcers receiving standard treatment. A meta‐analysis. Diabetes Care 1999;22:692–95. [DOI] [PubMed] [Google Scholar]
- 27. Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum‐assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care 2008;31:631–36. [DOI] [PubMed] [Google Scholar]
- 28. Hill T, Lewicki P. Statistics: general linear models. Methods and Applications. Tulsa: StatSoft,2007:245–6. [Google Scholar]
- 29. Lockhart AC, Braun RD, Yu D, Ross JR, Dewhirst MW, Humphrey JS, Thompson S, Williams KM, Kiltzman B, Yuan F, Grichnik JM, Prola AD, Conway DA, Hurwitz HI. Reduction of wound angiogenesis in patients treated with BMS‐275291, a broad spectrum matrix metalloproteinase inhibitor. Clin Cancer Res 2003;9:586–93. [PubMed] [Google Scholar]
- 30. Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998;21:855–9. [DOI] [PubMed] [Google Scholar]
- 31. Lavery L, McGuire J, Baranoski S, Ayello EA. Diabetic foot ulcers. In: Baranoski S, Ayello EA, editors. Wound care essentials: practice principles. Ambler, PA: Lippincott Williams & Wilkins, 2006:338–62. [Google Scholar]
- 32. Dougherty EJ. An evidence‐based model comparing the cost‐effectiveness of platelet‐rich plasma gel to alternative therapies for patients with nonhealing diabetic foot ulcers. Adv Skin Wound Care 2008;21:568–75. [DOI] [PubMed] [Google Scholar]
- 33. Manchikanti L, Singh V, Derby R, Schultz DM, Benyamin RM, Prager JP, Hirsch JA. Reassessment of evidence synthesis of occupational medicine practice guidelines for interventional pain management. Pain Physician 2008;11: 393–482. [PubMed] [Google Scholar]
- 34. de Leon JM, Driver VR, Fylling CP, Carter MJ, Anderson C, Wilson J, Dougherty RM, Fuston D, Trigilia D, Valenski V, Rappl LM. The clinical relevance of treating chronic wounds with an enhanced near‐physiological concentration of platelet‐rich plasma gel. Adv Skin Wound Care 2011;24:357–68. [DOI] [PubMed] [Google Scholar]


