Abstract
Non‐contact low‐frequency ultrasound (NCLF‐US) devices have been increasingly used for the treatment of chronic non‐healing wounds. The appropriate dose for NCLF‐US is still in debate. The aims of this pilot study were to evaluate the relationship between dose and duration of treatment for subjects with non‐healing diabetic foot ulcers (DFUs) and to explore the correlation between wound healing and change of cytokine/proteinase/growth factor profile. This was a prospective randomised clinical study designed to evaluate subjects with non‐healing DFUs for 5 weeks receiving standard of care and/or NCLF‐US treatment. Subjects were randomly assigned to one of the three groups: application of NCLF‐US thrice per week (Group 1), NCLF‐US once per week (Group 2) and the control (Group 3) that received no NCLF‐US. All subjects received standard wound care plus offloading for a total of 4 weeks. Percent area reduction (PAR) of each wound compared with baseline was evaluated weekly. Profiles of cytokines/proteinase/growth factors in wound fluid and biopsied tissue were quantified to explore the correlation between wound healing and cytokines/growth factor expression. Twelve DFU patients, 2 (16·7%) type 1 and 10 (83·3%) type 2 diabetics, with an average age of 58 ± 10 years and a total of 12 foot ulcers were enrolled. Average ulcer duration was 36·44 ± 24·78 weeks and the average ABI was 0·91 ± 0·06. Group 1 showed significant wound area reduction at weeks 3, 4 and 5 compared with baseline, with the greatest PAR, 86% (P < 0·05); Groups 2 and 3 showed 25% PAR and 39% PAR, respectively, but there were no statistically significant differences between Groups 2 and 3 over time. Biochemical and histological analyses indicated a trend towards reduction of pro‐inflammatory cytokines (IL‐6, IL‐8, IL‐1β, TNF‐α and GM‐CSF), matrix metalloproteinase‐9 (MMP‐9), vascular endothelial growth factor (VEGF) and macrophages in response to NCLF‐US consistent with wound reduction, when compared with control group subjects. This proof‐of‐concept pilot study demonstrates that NCLF‐US is effective in treating neuropathic diabetic foot ulcers through, at least in part, inhibiting pro‐inflammatory cytokines in chronic wound and improving tissue regeneration. Therapeutic application of NFLU, thrice (3) per week, renders the best wound area reduction.
Keywords: Non‐contact low frequency ultrasound, Chronic wound, Inflammatory cytokines, Matrix metalloproteinase, Vascular endothelial growth factor, Diabetic foot ulcers
Introduction
The impact of ultrasound on wound healing has been investigated in several previous studies 1, 2, 3, 4. Recently, a new method utilising non‐contact low‐frequency ultrasound (NCLF‐US) treatment has been introduced as an effective healing modality for various types of chronic wounds, including venous and ischaemic lower‐extremity ulcers and pressure ulcers 5, 6, 7. Randomised control trials (RCT) of NCLF‐US therapy for the diabetic foot with neuropathic and ischaemic ulcers demonstrated accelerated healing with NCLF‐US treatment compared with standard wound care alone 8. Although positive clinical outcomes have been reported, the mechanisms by which NCLF‐US therapy may accelerate healing of chronic ulcers are not fully clear. Induction of fibroblast proliferation with DNA synthesis, elicitation of VEGF and IL‐8 in osteoblasts, and reduction of bacterial propagation in chronic wounds by the NCLF‐US have been reported in several in vitro studies and animal wound models 9, 10, 11. These studies suggested that the NCLF‐US treatment facilitated immune response via fibroblast activity and release of cytokines 12. However, the scientific evidence of the relationship between biological effects of NCLF‐US and clinical outcomes of ulcer healing in human patients is limited.
The aims of this pilot study were to evaluate the relationship between dose and duration of NCLF‐US treatment for subjects with non‐healing diabetic foot ulcers (DFUs). To our knowledge, this is the first attempt to explore the correlation between wound healing and biological markers of tissue response (cytokines, proteinases and growth factors) in human patients with DFUs in response to NFLU treatment.
Materials and methods
Patients
A total of 12 subjects (age range: 40–72 years old) with chronic non‐healing DFUs were enrolled in the study after obtaining signed informed consent. The consent form and study protocol were approved by the Boston University Medical Campus Institutional Review Board (BUMC IRB). Subjects were chosen based on the inclusion/exclusion criteria (Table 1) at screening. Eligible subjects were randomised into one of the following three groups using a block randomisation scheme:
Group 1: receive standard of care + NCLF‐US therapy thrice per week
Group 2: receive standard of care + NCLF‐US therapy once per week
Group 3: receive standard of care only (without NCLF‐US therapy)
Table 1.
Inclusion and exclusion criteria of patients on screening
| Inclusion criteria |
| Age 18–90 years |
| Diabetes mellitus, type I or II |
| Chronic diabetic foot wound (0·5–15 cm2 area) |
| Wagner grade 1 or 2 |
| TcPO2 > 30 mmHg OR ABI > 0·6 |
| Ulcer size 0·5–15 cm2 |
| Exclusion criteria |
| Treatment with non‐contact ultrasound during the 4 weeks prior to this study |
| Lower extremity malignancy (either limb) |
| Critical limb ischaemia |
| Local infection of limb with target ulcer |
| Systemic infection |
| Pregnancy |
| End‐stage renal disease |
| Severe congestive heart disease |
| Severe liver disease |
| Venous leg ulcer with or without diabetes mellitus |
| Known/suspected lidocaine allergy |
Following screening, all enrolled subjects had a 1‐week washout period and returned to a baseline visit (week 1) for data and sample collection, and treatment based on group assignment. With regard to sample collection, visits not completed within ±2 days were considered a ‘missed’ visit and the subject was scheduled for the subsequent visit.
Standard of care
Debridement, offloading and moist wound care are the fundamental standard of care (SOC) for diabetic foot ulcers at Department of Surgery, Boston University Medical Center. All patients in this study received SOC throughout the treatment period of 4 weeks.
Non‐contact low‐frequency ultrasound therapy
NCLF‐US (MIST Therapy System 5.0®; Celleration Inc., Eden Prairie, MN) is an FDA‐cleared device for the treatment of chronic wounds. This ultrasound therapy was used to treat the ulcers in subjects of Groups 1 and 2. The device operates at a frequency of 40 kHz, and the intensity delivered to the wound surface varies from 0·2 to 0·6 W/cm2. With the US transducer held at a distance of 0·5–1·5 cm from the wound bed, US waves produced in a continuous mode are delivered to the wound surface via a medium of sterile saline mist. Treatment duration varies according to the wound area (i.e. longer durations for larger wound areas), with a single session typically lasting approximately 5 minutes.
Clinical data collection
Clinical data including subject's demographics, medical/surgical history, medication, baseline wound characteristics (i.e. length and width, ulcer type, location, grade, etc.) and response to treatment were collected by trained study staff at weeks 1, 2, 3, 4 and 5 using case report forms. Wounds were measured at the screening visit (week 0), treatment visits (week 1–4) and post‐treatment follow‐up visit (week 5). Collected data were entered into a database using Microsoft® Access 2003.
Wound fluid and tissue specimen collection
Biological samples were collected once per week during study visit at weeks 1–4. Wound fluid was collected using a filter paper (PerioPaper; Oraflow Inc., Smithtown, NY) for 30 seconds as described in prior publications, and ulcer tissue was obtained through tissue biopsies 13, 14. Samples were processed according to established protocols 14, 15, 16 and stored at −80°C until time of analysis.
Determination of biological markers of healing in wound fluids
Wound fluid specimens from the filter strips were retrieved by high‐speed centrifugation in 100 μl phosphate‐buffered saline and used for analyses. Once reconstituted, repeated freeze‐thaw cycles were avoided and assays were performed consecutively. In order to determine the tissue response to treatment, several panels of biological markers have been selected. These included cytokines, which regulate the inflammatory process (IL‐6, IL‐1β, TNF‐α, IL‐8 and GM‐CSF), tissue degrading matrix metalloproteinases‐9 and VEGF. These markers were measured by multiplex xMAP immunoassay (Luminex, Austin, TX) using commercially available panels from Invitrogen (Carlsbad, CA) or Millipore (Chicago, IL) according to the manufacturers' protocols. Data were evaluated against standard curves generated for each analyse and reported as picogram per millilitre.
Immunohistochemistry
Inflammatory response at the tissue level was studied in tissue biopsies obtained during surgical procedures. Tissues were fixed in 4% formaldehyde as previously explained 15, 16 and kept frozen at −80°C until analysis. Five micrometre thick sections were obtained using a cryostat; every fifth section was analysed first for the tissue morphology using the standard haematoxylin‐eosin stain and every sixth section was used for the analysis of CD68+ macrophage numbers as determinants of inflammatory infiltrate. The protocol followed the recommendations of the vendor (R&D Systems, Minneapolis, MN). FITC‐labelled CD68‐positive cells were counted using a fluorescence microscope. The data were reported as macrophage numbers per square millimetre of wound area analysed.
Statistical analysis
The data were summarised using descriptive statistics. Continuous variables were summarised with mean and standard deviation. Categorical variables were summarised with frequency and percentage. Means for continuous variables were compared using analysis of variance (ANOVA) tests with Bonferroni correction for post hoc analyses involving multiple comparisons. A Chi‐square test or a Fisher's exact test was performed to compare proportions for categorical variables. Correlation coefficients between two continuous variables were calculated with correlation analysis. Repeated measures analysis using a proc mixed model on longitudinal data was conducted using SAS 9.2 (SAS Institute, Cary, NC). Actual reading value of cytokines, proteinase and growth factors was log‐transformed prior to analysis due to skewed distribution. Original data are expressed as percent of baseline (week 1) on comparison.
Results
Baseline characteristics of enrolled patients
Table 2 shows baseline characteristics of subjects in each group at baseline. Among 12 subjects with a total of 12 foot ulcers, 2 (16·7%) were type 1 and 10 (83·3%) were type 2 diabetics; the average age was 58 ± 10 years. The average ulcer duration was 36·4 ± 24·8 weeks, and the average ABI was 0·91 ± 0·06. There were no significant differences (P > 0·05) in demographics, ulcer features and comorbidities among three treatment groups prior to enrollment, indicating that randomisation was successful in this study.
Table 2.
Baseline characteristics of Cohort
| Group 1 |NCLF‐US 3×/week | Group 2 |NCLF‐US 1×/week | Group 3 |NCLF‐US 0×/week | P‐value | ||
|---|---|---|---|---|---|
| Age | Mean (SD) | 52·6 (3·8) | 64·8 (8·7) | 50·8 (8·5) | >0·05 |
| Gender | M | 100% (4/4) | 25% (1/4) | 75% (3/4) | >0·05 |
| F | 0% (0/4) | 75% (3/4) | 25% (1/4) | >0·05 | |
| Race | B | 25% (1/4) | 50% (2/4) | 75% (3/4) | >0·05 |
| C | 75% (3/4) | 25% (1/4) | 25% (1/4) | >0·05 | |
| H | 0% (0/4) | 25% (1/4) | 0% (0/4) | >0·05 | |
| Diabetes type | Type 1 | 0% (0/4) | 25% (1/4) | 25% (1/4) | >0·05 |
| Type 2 | 100% (4/4) | 75% (3/4) | 75% (3/4) | >0·05 | |
| Diabetic year | Mean (SD) | 16·25 (10·75) | 17·75 (12·26) | 19·87 (1·84) | >0·05 |
| Retinopathy | 25% (1/4) | 75% (3/4) | 0% (0/4) | >0·05 | |
| Nephropathy | 25% (1/4) | 0% (0/4) | 25% (1/4) | >0·05 | |
| Neuropathy | 100% (4/4) | 100% (4/4) | 100% (4/4) | >0·05 | |
| History of major amputation | 25% (1/4) | 25% (1/4) | 25% (1/4) | >0·05 | |
| History of minor amputation | 0% (0/4) | 50% (2/4) | 50% (2/4) | >0·05 | |
| ABI | Mean (SD) | 0·98 (0·38) | 0·89 (0·19) | 0·85 (0·23) | >0·05 |
| Palpable DP on screening | 100% (4/4) | 100% (4/4) | 75% (3/4) | >0·05 | |
| Palpable PT on screening | 75% (3/4) | 50% (2/4) | 25% (1/4) | >0·05 | |
| Uler location | Plantar | 100% (4/4) | 50% (2/4) | 100% (4/4) | >0·05 |
| Dorsal | 0% (0/4) | 25% (1/4) | 0% (0/4) | >0·05 | |
| Medial | 0% (0/4) | 25% (1/4) | 0% (0/4) | >0·05 | |
| Week 0 wound area (cm2, SD) | 1·9 (1·4) | 2·5 (2·1) | 2·1 (0·9) | >0·05 | |
| Wagner grade | 1 | 50% (2/4) | 75% (3/4) | 25% (1/4) | >0·05 |
| 2 | 50% (2/4) | 25% (1/4) | 75% (3/4) | >0·05 | |
| UT grade | 1A | 50% (2/4) | 75% (3/4) | 25% (1/4) | >0·05 |
| 1B | 0% (0/4) | 0% (0/4) | 0% (0/4) | >0·05 | |
| 2A | 50% (2/4) | 25% (1/4) | 25% (1/4) | >0·05 | |
| 2B | 0% (0/4) | 0% (0/4) | 50% (2/4) | >0·05 | |
| Wound infection | 0% (0/4) | 0% (0/4) | 0% (0/4) | >0·05 | |
| Target ulcer duration (weeks) | Mean (SD) | 16·0 (11·5) | 29·3 (19·7) | 64·0 (65·1) | >0·05 |
| Ankle pressure | Mean (SD) | 121·7 (50·6) | 127·3 (35·5) | 75·3 (5·0) | >0·05 |
B, black; C, caucasian; DP, dorsalis pedis; H, hispanic; PT, posterior tibial; UT, University of Taxes.
Clinical wound reduction by treatment
Percent area reduction (PAR) was calculated as the percentage area reduction at treatment visits compared with the baseline area. Compared with baseline wound area, Group 1 (NCLF‐US thrice per week) had an 86% PAR between weeks 3 and 5; Group 2 (NCLF‐US once a week) had 25% PAR and Group 3 (control) about 39% PAR (Figure 1). Analysis of these longitudinal data using repeated measure proc mixed models showed a significant reduction in PAR for Group 1 at weeks 3, 4 and 5 compared with Groups 2 and 3 (P < 0·05). There were no statistically significant differences in PAR between Groups 2 and 3 (Figure 1), indicating application of NCLF‐US once per week was not sufficient to achieve significant wound reduction. Taken together, the clinical wound data demonstrated that NFLU therapy application, thrice per week, renders the best wound reduction benefits (47% wound reduction benefit in this study).
Figure 1.
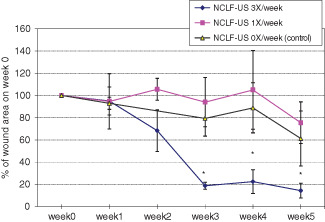
Change of wound size with the non‐contact low‐frequency ultrasound (NCLF‐US) therapy. Twelve eligible subjects with chronic diabetic foot ulcers were randomised into one of the three groups. NCLF‐US therapy (with Celleration MIST Therapy System 5.0®) was applied for the treatment of chronic wounds. Wound sizes were calculated by length × width and expressed as percent change from baseline wound size at week 1.
NCLF‐US on inflammation, tissue healing and regeneration
Substantial trends of profile change in various molecules indicate that inflammation is correlated with clinical outcomes at the tissue level (Figure 2). All pro‐inflammatory cytokines tested (IL‐6, IL‐8, IL‐1β, TNF‐α and GM‐CSF) were reduced in NCLF‐US thrice per week (Group 1) in parallel comparison with control patients (Group 3) as well as in historical comparison with its own baseline values (Figure 2), suggesting that overall inflammatory burden can be reduced by the NCLF‐US therapy. To determine the tissue turnover profile of the levels of MMPs and their tissue inhibitors in the wound fluid, we measured MMP‐1, MMP‐2, MMP‐9 and tissue inhibitors of metalloproteinases‐3 (TIMP‐3). Although MMP‐1, MMP‐2 and TIMP‐3 are not detectable, we observed a 60% reduction in MMP‐9 levels in patients treated with NCLF‐US thrice per week (Figure 3A). MMP‐9 levels were significantly and positively related to reduction in wound area (Figure 3B, P < 0·05).
Figure 2.
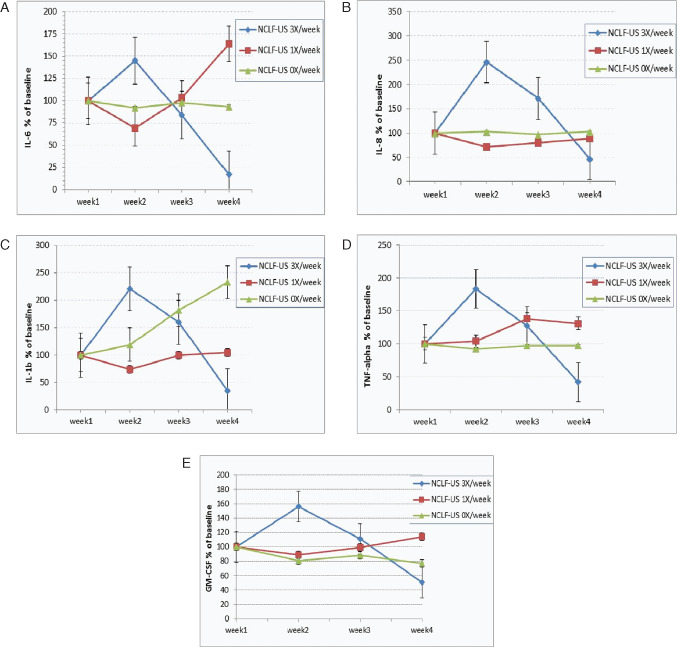
Change of pro‐inflammatory cytokines in wound fluid in response to non‐contact low‐frequency ultrasound (NCLF‐US) therapy. Wound fluids were collected during the study visits. Various molecular markers, including IL‐6, IL‐8, IL‐1b, TNF‐α and GM‐CSF (A–E) were quantified using Luminex 100 multiplex assays. Actual reading values were log‐transformed prior to analysis due to skewed distribution. Results were also expressed as percent change compared with baseline levels of these markers to avoid the discrepancy among baseline values.
Figure 3.
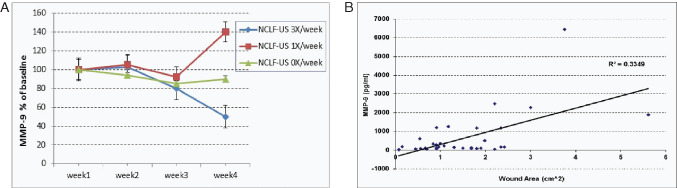
Change of matrix metalloproteinases (MMP)‐9 in wound fluid in response to non‐contact low‐frequency ultrasound (NCLF‐US) therapy. Wound fluids were collected during the study visits. MMPs and TIMPs were quantified using Luminex 100 multiplex assays. Actual reading values were log‐transformed prior to analysis due to skewed distribution. Results were also expressed as percent change compared with baseline (week1) levels of these molecular markers (A). Correlation analysis on MMP and wound size was also performed (B).
We observed remarkable increases in VEGF levels in response to thrice per week application of NCLF‐US (Figure 4A) on week 2 and a trend of decrease from peak, which was consistent with wound area. On week 4, the data showed a trend of VEGF reduction by 50–60% in response to NCLF‐US thrice per week (Group 1) compared with the control group (Group 3) and baseline. However, VEGF was not shown to be significantly and directly correlated with wound reduction in this study. Additionally, VEGF was significantly correlated with MMP‐9 levels (Figure 4B, P < 0·05). The findings of this study suggest that the tissue repair and turnover in connective tissue metabolism were significantly associated with angiogenesis and wound healing, and NCLF‐US, at a dose of thrice per week, significantly improved the overall rate of tissue regeneration.
Figure 4.
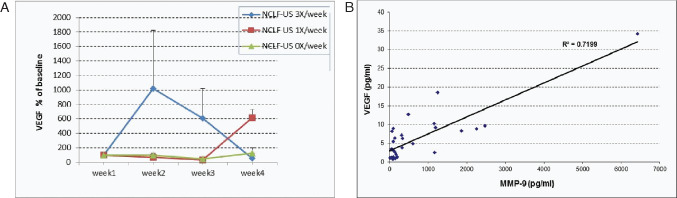
Change of vascular endothelial growth factor (VEGF) in wound fluid in response to non‐contact low‐frequency ultrasound (NCLF‐US) therapy. Wound fluids were collected during the study visits. VEGF were quantified using Luminex 100 multiplex assays. Actual VEGF reading values were log‐transformed prior to analysis because of skewed distribution. Results were also expressed as percent change compared with baseline (week1) levels of these molecular markers (A). Correlation analysis on VEGF and MMP was also performed (B).
Macrophage infiltration during wound healing in diabetic foot ulcers
To study the cellular infiltration and inflammatory response at the tissue level, we obtained wound tissues through biopsies during the treatment period. While the initial intention was to assess specifically the inflammatory cytokines as a measure of inflammation, the tissue shapes and sizes differed considerably due to shrinkage of tissues during wound healing. Therefore, we decided to measure and quantify directly the cellular inflammatory response by macrophage infiltration. A specific and well‐established immunomarker of macrophages (CD68) was chosen and, in order to enhance the visibility of cells positive for CD68, a fluorescent dye (FITC) was used to label the CD68. Using fluorescence microscopy, CD68+ macrophage populations were detected and counted at tissue level in biopsies.
The results (Figure 5) show that the macrophage counts were significantly reduced from baseline by NCLF‐US treatment in Group 1 compared with Groups 2 and 3 (control). These data support the cytokine data showing an overall reduction in inflammatory load in response to NCLF‐US treatment. Within the limits of the study, the reduction in macrophage numbers suggests that the tissue‐healing process by macrophage‐induced homeostasis has been restored after the NCLF‐US treatment.
Figure 5.
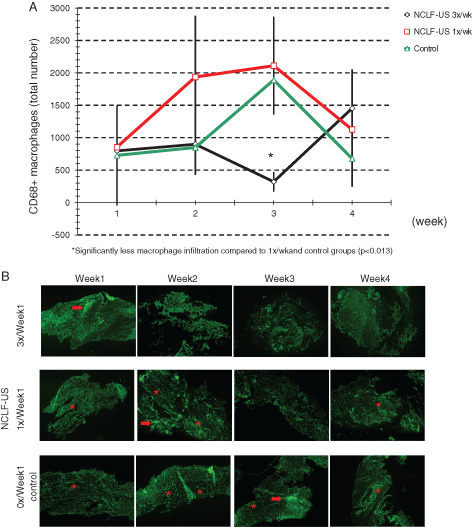
Macrophage infiltration during wound healing in diabetic foot ulcers. Ulcer tissues were obtained during each study visit. Macrophage numbers were quantified as determinants of inflammatory infiltrate (A). CD68 was used as the specific marker of macrophage numbers and fluorescent dye (FITC)‐labelled CD68‐positive cells were counted using a fluorescence microscope (B). Arrows indicate areas of intense CD68+ cell infiltration. Clusters or individual cells are shown with *.
Discussion
The growing incidence of non‐healing ulcers worldwide demands clinicians and researchers to test and introduce novel and effective therapeutic strategies in clinical practice. In recent years, the potential therapeutic applications of ultrasound to wound care have been elucidated by an increasing body of preclinical and clinical studies 1, 2, 3, 4, 12, 17, 18, suggesting that its mechanism of action may be through a combined effect on the inflammatory phase, angiogenesis and the bacterial load 11, 18, 19. Ultrasound may assist in obtaining a more viable granulation tissue and allow a more rapid closure of the wound 20, 21. Our previous meta‐analysis published in 2010 summarised the effects of an NCLF‐US therapy on healing of chronic wounds 21. NCLF‐US was associated with 85·2% area reduction (95% CI 64·7–97·6%) over a mean of 7 weeks. NCLF‐US for treatment of chronic wounds was associated with consistent and substantial wound size reductions, as well as a favourable rate of healing. Cullum et al. 7 reported through analysis of Cochrane Wounds Group Specialised Register for venous leg ulcers that six clinical trials evaluated high‐frequency ultrasound and five of them showed wound healing at 7–8 weeks, whereas low‐frequency ultrasound did not show benefits in two trials. In this study, Group 1 (NCLF‐US thrice per week) had about 86% wound area reduction, whereas Groups 2 (NCLF‐US once a week) and 3 (control) had about 25% and 39% wound area reduction, respectively. PAR at 4 weeks is a validated surrogate marker of healing 22. In a study of the neuropathic DFUs, Sheehan et al. reported that 50% PAR at 4 weeks is a good predictor of healing at 12 weeks 23. Margolis and colleagues have reported that an 80% area reduction at 4 weeks is a predictor of complete DFU wound closure 24. Our results showed that application of NCLF thrice per week for 4 weeks accelerates wound healing as measured by PAR. We conclude that a therapeutic application of NCLF‐US thrice per week renders the best wound reduction benefit and can be considered as a standard dose of NCLF‐US therapy in DFUs patients. This conclusion is supported by the work conducted by Ennis et al. in 2005 8 where, after 12 weeks of care, the proportion of wounds healed (defined as complete epithelialisation without drainage) in the active NCLF‐US therapy group (thrice per week) was significantly higher than that in the sham control group (40·7% versus 14·3%, P = 0·0366, Fisher's exact test).
Wound healing is accomplished by an orderly sequence of three phases: inflammation, proliferation and remodelling 25. However, a prolonged inflammatory phase modulated by excessive pro‐inflammatory cytokines and proteases is one of the biological characteristics of a chronic ‘stalled’ wound 19, 26, 27. Trengove et al. conducted a comparative study of acute and chronic wound fluid and demonstrated high concentration of TNF‐α, IL‐6 and IL‐6 in non‐healing chronic lower extremity ulcers. As the ulcers began to heal, the level of pro‐inflammatory cytokines decreased dramatically as expected, indicating a reduced inflammatory state 28, 29. Chronic DFUs have also been shown to contain abnormally high levels of proteases that prevent normal wound healing, and elevated MMP‐9 was a predictor of a poor healing outcome 25, 30, 31, 32. Our data demonstrate that pro‐inflammatory cytokines (IL‐6, IL‐8, IL‐1b and TNF‐α), along with MMP‐9, are decreased over 4 weeks of NCLF‐US therapy. This indicates that, at least in part, a possible mechanism of NCLF‐US on wound healing may be through reversing the prolonged inflammatory phase of diabetic wounds.
Diabetic ulcers are believed to be caused by neuropathy and subsequent chronic trauma, impaired microvascular circulation and /or peripheral arterial disease. Levels of MMP‐2 and MMP‐9 are elevated in the plasma of diabetic patients, and disruption of balance between MMPs and TIMPs, indicating abnormalities in extracellular matrix metabolism 33, 34. Our current data indicate that MMP‐9 may be a candidate biomarker associated with wound healing status with a direct relationship and positive correlation with wound area (i.e. higher MMP‐9 levels associated with larger wound area and lower MMP‐9 levels with smaller wound area). Our findings also demonstrate a correlation between MMP‐9 and VEGF, thereby indicating that VEGF would also be significantly correlated with wound size although our current data do not show. Moreover, the correlation between MMP‐9 and VEGF suggests that the tissue repair and turnover in connective tissue metabolism are significantly associated with angiogenesis and tissue healing. MMP and VEGF data are truly wide range, so there is no sufficient statistical power to make a conclusive statement. We just reported a trend towards reduction in these molecular markers. Based on trend of reduction, NCLF‐US appears to significantly improve the overall rate of tissue regeneration. Further research is needed to demonstrate effects of NCLF‐US treatment, especially in the area of combined effects of ultrasound therapy with skin grafts, skin substitutes or platelets gels with particular focus on the effects of NCLF‐US on angiogenesis and bio‐burden control.
Conclusion
This proof‐of‐concept study demonstrates that NCLF‐US is effective in treating neuropathic diabetic foot ulcers through, at least in part, inhibiting pro‐inflammatory cytokines in chronic wound and significantly improving tissue regeneration. Therapeutic application of NFLU, thrice per week, renders the best wound area reduction. Because of limited sample size for such a proof‐of‐concept pilot study, we are precautious to generalise conclusion. Larger clinical studies should be considered to verify changing profiles of biomarkers and determine their diagnostic value to predict clinical outcome.
References
- 1. Gehling ML, Samies JH. The effect of noncontact, low‐intensity, low‐frequency therapeutic ultrasound on lower‐extremity chronic wound pain: a retrospective chart review. Ostomy Wound Manage 2007;53:44–50. [PubMed] [Google Scholar]
- 2. Kavros SJ, Liedl DA, Boon AJ, Miller JL, Hobbs JA, Andrews KL. Expedited wound healing with noncontact, low‐frequency ultrasound therapy in chronic wounds: a retrospective analysis. Adv Skin Wound Care 2008;21:416–23. [DOI] [PubMed] [Google Scholar]
- 3. Kavros SJ, Miller JL, Hanna SW. Treatment of ischemic wounds with noncontact, low‐frequency ultrasound: the Mayo clinic experience, 2004‐2006. Adv Skin Wound Care 2007;20:221–26. [DOI] [PubMed] [Google Scholar]
- 4. Kavros SJ, Schenck EC. Use of noncontact low‐frequency ultrasound in the treatment of chronic foot and leg ulcerations: a 51‐patient analysis. J Am Podiatr Med Assoc 2007;97:95–101. [DOI] [PubMed] [Google Scholar]
- 5. Al‐Kurdi D, Bell‐Syer SE, Flemming K. Therapeutic ultrasound for venous leg ulcers. Cochrane Database Syst Rev 2008;CD001180. [DOI] [PubMed] [Google Scholar]
- 6. Baba‐Akbari Sari A, Flemming K, Cullum NA, Wollina U. Therapeutic ultrasound for pressure ulcers. Cochrane Database Syst Rev 2006;CD001275. [DOI] [PubMed] [Google Scholar]
- 7. Cullum NA, Al‐Kurdi D, Bell‐Syer SE. Therapeutic ultrasound for venous leg ulcers. Cochrane Database Syst Rev. 2010;CD001180. [DOI] [PubMed] [Google Scholar]
- 8. Ennis WJ, Foremann P, Mozen N, Massey J, Conner‐Kerr T, Meneses P. Ultrasound therapy for recalcitrant diabetic foot ulcers: results of a randomized, double‐blind, controlled, multicenter study. Ostomy Wound Manage 2005;51:24–39. [PubMed] [Google Scholar]
- 9. Doan N, Reher P, Meghji S, Harris M. In vitro effects of therapeutic ultrasound on cell proliferation, protein synthesis, and cytokine production by human fibroblasts, osteoblasts, and monocytes. J Oral Maxillofac Surg 1999;57:409–19; discussion 420. [DOI] [PubMed] [Google Scholar]
- 10. Lai J, Pittelkow MR. Physiological effects of ultrasound mist on fibroblasts. Int J Dermatol 2007;46:587–93. [DOI] [PubMed] [Google Scholar]
- 11. Reher P, Doan N, Bradnock B, Meghji S, Harris M. Effect of ultrasound on the production of IL‐8, basic FGF and VEGF. Cytokine 1999;11:416–23. [DOI] [PubMed] [Google Scholar]
- 12. Thawer HA, Houghton PE. Effects of ultrasound delivered through a mist of saline to wounds in mice with diabetes mellitus. J Wound Care 2004;13:171–76. [DOI] [PubMed] [Google Scholar]
- 13. Guentsch A, Kramesberger M, Sroka A, Pfister W, Potempa J, Eick S. Comparison of gingival crevicular fluid sampling methods in patients with severe chronic periodontitis. J Periodontol 2011;82:1051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lamster IB, Hartley LJ, Vogel RI. Development of a biochemical profile for gingival crevicular fluid. Methodological considerations and evaluation of collagen‐degrading and ground substance‐degrading enzyme activity during experimental gingivitis. J Periodontol 1985;56(11 Suppl):13–21. [DOI] [PubMed] [Google Scholar]
- 15. Kantarci A, Black SA, Xydas CE, Murawel P, Uchida Y, Yucekal‐Tuncer B, Atilla G, Emingil G, Uzel MI, Lee A, Firatli E, Sheff M, Hasturk H, Van Dyke TE, Trackman PC. Epithelial and connective tissue cell CTGF/CCN2 expression in gingival fibrosis. J Pathol 2006;210:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kantarci A, Nseir Z, Kim YS, Sume SS, Trackman PC. Loss of basement membrane integrity in human gingival overgrowth. J Dent Res 2011;90:887–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ennis WJ, Valdes W, Gainer M, Meneses P. Evaluation of clinical effectiveness of MIST ultrasound therapy for the healing of chronic wounds. Adv Skin Wound Care 2006;19:437–46. [DOI] [PubMed] [Google Scholar]
- 18. Serena T, Lee SK, Lam K, Attar P, Meneses P, Ennis W. The impact of noncontact, nonthermal, low‐frequency ultrasound on bacterial counts in experimental and chronic wounds. Ostomy Wound Manage 2009;55:22–30. [PubMed] [Google Scholar]
- 19. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic‐Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 2008;16:585–601. [DOI] [PubMed] [Google Scholar]
- 20. Driver VR, Yao M. Discussion. Current status of the use of modalities in wound care: electrical stimulation and ultrasound therapy. Plast Reconstr Surg 2010;127 Suppl 1:103S–4S. [DOI] [PubMed] [Google Scholar]
- 21. Driver VR, Yao M, Miller CJ. Noncontact low‐frequency ultrasound therapy in the treatment of chronic wounds: a meta‐analysis. Wound Repair Regen 2010;19:475–80. [DOI] [PubMed] [Google Scholar]
- 22. Cardinal M, Eisenbud DE, Phillips T, Harding K. Early healing rates and wound area measurements are reliable predictors of later complete wound closure. Wound Repair Regen 2008;16:19–22. [DOI] [PubMed] [Google Scholar]
- 23. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4‐week period is a robust predictor of complete healing in a 12‐week prospective trial. Diabetes Care 2003;26:1879–82. [DOI] [PubMed] [Google Scholar]
- 24. Margolis DJ, Gelfand JM, Hoffstad O, Berlin JA. Surrogate end points for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 2003;26:1696–1700. [DOI] [PubMed] [Google Scholar]
- 25. Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen 2011;19:134–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mann A, Breuhahn K, Schirmacher P, Blessing M. Keratinocyte‐derived granulocyte‐macrophage colony stimulating factor accelerates wound healing: Stimulation of keratinocyte proliferation, granulation tissue formation, and vascularization. J Invest Dermatol 2001;117: 1382–90. [DOI] [PubMed] [Google Scholar]
- 27. Yonem A, Cakir B, Guler S, Azal OO, Corakci A. Effects of granulocyte‐colony stimulating factor in the treatment of diabetic foot infection. Diabetes Obes Metab 2001;3:332–37. [DOI] [PubMed] [Google Scholar]
- 28. Trengove NJ, Bielefeldt‐Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non‐healing and healing chronic leg ulcers. Wound Repair Regen 2000;8:13–25. [DOI] [PubMed] [Google Scholar]
- 29. Trengove NJ, Langton SR, Stacey MC. Biochemical analysis of wound fluid from nonhealing and healing chronic leg ulcers. Wound Repair Regen 1996;4:234–39. [DOI] [PubMed] [Google Scholar]
- 30. Liu Y, Min D, Bolton T, Nube V, Twigg SM, Yue DK, McLennan SV. Increased matrix metalloproteinase‐9 predicts poor wound healing in diabetic foot ulcers: Response to Muller et al. Diabetes Care. Nov 2009;32(11):e137. [DOI] [PubMed] [Google Scholar]
- 31. Parks WC. Matrix metalloproteinases in repair. Wound Repair Regen 1999;7:423–32. [DOI] [PubMed] [Google Scholar]
- 32. Liu Y, Min D, Bolton T, Nube V, Twigg SM, Yue DK, McLennan SV. Increased matrix metalloproteinase‐9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care 2009;32:117–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brem H, Sheehan P, Rosenberg HJ, Schneider JS, Boulton AJ. Evidence‐based protocol for diabetic foot ulcers. Plast Reconstr Surg 2006;117(7 Suppl):193S–209S; discussion 210S–11S. [DOI] [PubMed] [Google Scholar]
- 34. Derosa G, D'Angelo A, Tinelli C, Devangelio E, Consoli A, Miccoli R, Penno G, Del Prato S, Paniga S, Cicero AF. Evaluation of metalloproteinase 2 and 9 levels and their inhibitors in diabetic and healthy subjects. Diabetes Metab 2007;33:129–34. [DOI] [PubMed] [Google Scholar]


