Abstract
The objective of this study is to develop a chitosan gel formulation containing liposomes loaded with epidermal growth factor (EGF) and to evaluate their effects on the healing of second‐degree burn wounds in rats by immunohistochemical, histochemical and histological methods. EGF‐containing multilamellar liposomes which were carried in chitosan gel, EGF gel and EGF‐loaded liposome formulations were prepared. The in vivo experiments were performed on female Sprague Dawley rats. Second‐degree standard burn wounds were formed on rats and liposomes containing 10 µg/ml EGF in 2% chitosan gel, EGF‐chitosan gel and EGF‐loaded liposome formulations were applied daily to the burn wounds and biopsies were taken at the 3rd, 7th and 14th day of the treatment. When the results were evaluated immunohistochemically, there were significant increases in cell proliferation observed in the EGF‐containing liposome in chitosan gel (ELJ) formulation applied group (P < 0·001). The histochemical results showed that the epithelisation rate in the ELJ group was the highest compared with the other group results (P < 0·001). The histological results indicated and supported these findings and faster epithelisation was observed in the ELJ group compared with the other groups.
Keywords: Burn wound, Chitosan gel, EGF, Healing, Liposomes
INTRODUCTION
Every year in the world many patients suffer from burn wounds that do not completely heal or result in scarring. Researchers have had a difficult time developing treatments for burn injuries because they had little idea of what drug delivery was involved in healing and at what stages during the healing process it becomes important. A burn is a type of injury to the skin caused by heat, electricity, chemicals or radiation. Second‐degree burns involve the superficial (papillary) dermis and may also involve the deep (reticular) dermis layer. These kinds of burns produce blisters, severe pain and redness. The main causes of second‐degree burns are especially scalds from hot water and liquids.
Healing is the process whereby the cells in the body regenerate and repair to reduce the size of a damaged or necrotic area. The wound repair process involves a set of events that include inflammation surrounding the region of injury, wound cell migration and mitosis, angiogenesis and the development of granulation tissue, repair of the connective tissue, regeneration of the extracellular matrix and remodelling that leads to a healed wound (1). Growth factors regulate these processes.
Epidermal growth factor (EGF) and other growth factors such as platelet‐derived growth factor, fibroblast growth factor and transforming growth factor‐β have been shown to accelerate cellular proliferation and synthesis of the extracellular matrix in wound healing process. The use of topical application of growth factors therapy has been fundamental in that regard and has helped to minimise the incidence of having burn wound scar and healing in long‐term time periods.
Recent data suggest that the actions of wound cells may be regulated by local production of peptide growth factors which influence wound cells 1, 2 and also recent data have shown that EGF, TGFα or related peptides may play vital roles in wound repair after burn injury (3).
EGF is a single‐chain polypeptide containing 53 acid residues and 3 disulphide bridges. It has a molecular weight of 6216 Da. It is also present in many mammalian species in a variety of tissues and body fluids. EGF has an important effect on wound healing, which has been shown to stimulate keratinocyte division in vitro and epidermal regeneration in vivo (4). Repeated doses of EGF increased tensile strength of the incisions (5). It was also reported that EGF formulated in multilamellar liposomes was retained for prolonged periods in rat incisions and significantly increased their tensile strength (1). EGF accelerates wound healing (6) and dermal regeneration of partial thickness burns or split‐thickness incisions in vivo (7). It has been shown that repeated injections of EGF or sustained release of EGF from inert polymers stimulated DNA synthesis and elevated the level of DNA and also stimulated collagenase production by fibroblasts.
Liposomes are vesicular structures consisting of hydrated bilayers (8). They are non toxic, biodegradable, biocompatible, able to protect encapsulated matter from hostile environments and capable of acting as sustained release systems.
EGF like all other proteins and peptides has low stability. Thus, EGF encapsulated in liposomes would have had more stability and could be safely used in the treatment of burns.
Chitosan has many useful and advantageous biological properties in its application as a wound dressing, namely biocompatibility, biodegradability, haemostatic activity, anti‐infective activity and ability to accelerate wound healing. The application of chitosan hydrogels may effectively interact with and protect the wound, ensuring a good, moist healing environment (9).
Chitosan is a polysaccharide comprising copolymers of glucosamine and N‐acetylglucosamine and can be derived by partial deacetylation of chitin from crustacean shells (10).
Chitosan enhances the functions of inflammatory cells, such as polymorphonuclear leucocytes, macrophages and fibroblasts (11). Chitosan lacks irritant or allergic effects and is biocompatible with both healthy and infected human skin (12) so that chitosan‐based healing agents are very useful for the treatment of burn wounds. The other advantage of using chitosan hydrogel is the bactericidal effect of chitosan (13).
In this study, we aimed to develop EGF containing multilamellar liposomes which were carried in chitosan gel. To investigate the burn wound healing effects of this formulation, second‐degree uniform deep burn wounds were formed on the backs of the rats. The animals were divided into groups and treated with EGF‐liposome (ELP), chitosan gel with EGF (EJ) and EGF‐liposome‐chitosan gel (ELJ) formulations for 14 days. The healing was evaluated immunohistochemically and histochemically. The results we have obtained with this study were found to be consistent with our previous studies 14, 15. We have obtained an enhanced efficacy on burned skin with the developed liposome formulations containing EGF in chitosan gel.
MATERIALS AND METHODS
Materials
Human epidermal growth factor was purchased from Sigma, St. Louis, MO. Chitosan H was kindly provided from Dainichiseika Color & Chemicals Mfg., Co., Ltd., Tokyo, Japan. Dipalmitoylphosphatidyl choline (DPPC) was purchased from Acros Organics, Belgium, USA and cholesterol was from Sigma. Bromodeoxyuridine (BrdU) and Anti‐bromodeoxyuridine (Anti‐BrdU) were also provided from Sigma. All other chemicals were of analytical grade.
METHODS
In vitro studies
Preparation of the chitosan gel
Glacial acetic acid (0·5%) was added to half of the required water. The weighed amount of chitosan was added and stirred slowly. After swelling, the remaining amount of water was added and mixed. Gel was kept at room temperature overnight before the application to remove the air bubbles.
Preparation of EGF‐liposome
Multilamellar vesicle (MLV) liposomes were prepared using the film hydration method. Cholesterol and DPPC were added in 1:1 molar ratios. After dissolving with chloroform– methanol mixture, the solvents were evaporated under rotavapor and dry film was then obtained. The film was hydrated by phosphate buffer containing EGF and liposomes which were formed after vortex mixing and ultrasonication for 30 minutes. The dispersion was centrifuged 27,000g for 60 minutes at 4°C to separate the MLV from the aqueous solution. Liposome formulations were freshly prepared and used just before the experiments to avoid any degradation.
Preparation of EGF‐liposome‐gel formulation
Finally, after liposomes were prepared, the liposomes‐loaded EGF were dispersed into chitosan gel (2%).
Measurement of the particle size of liposomes
The particle size of liposomes was determined using laser diffraction particle sizer (Helos laser diffraction particle sizer, Symphatec GmbH, Bergstadt‐Clausthal‐Zellerfeld, Germany).
Determination of the type of liposomes and encapsulation efficiency
Physical properties of the liposomes were investigated by inverted microscope (Olympus CK2, Tokyo, Japan) and appearances were framed. Liposomes were centrifuged at 4°C and 27,000g. The EGF contents of liposomes were determined using spectrofluorometre after extraction processes.
In vivo studies
Design of animal experiments
All animal experiments were conducted under the protocols approved by the Animal Care and Use Committee of Gulhane Military Medical Academy. For the in vivo experiments, female Sprague Dawley rats weighing 250 ± 10 g were used.
Rats were housed in individual cages with unrestricted food and water access. The animals were divided into six groups. The unburned group consisted of 4 rats and there were 12 animals in each group. The design of the animal groups is shown in Table 1.
Table 1.
Design of experimental animal groups
| Group codes | Treatment |
|---|---|
| S | Unburned |
| Y | Burned but untreated |
| J | Burned and treated by chitosan gel without EGF |
| EJ | Burned and treated by chitosan gel with EGF |
| LP | Burned and treated with liposome formulation without EGF |
| ELP | Burned and treated with EGF‐liposome formulation |
| ELJ | Burned and treated with EGF solution |
| Burned and treated with EGF‐liposome in chitosan gel formulation | |
| ES | Burned and treated by EGF solution |
| SIL | Burned and treated by commercial product (Silverdin®) |
Formation of the burn wounds
The trauma was performed by exposing the shaved backskin of anaesthetised animals to hot water. For this procedure, a cylindrical shaped bar with the radius of 1 cm was placed on the backs of rats and then hot water (94 ± 1°C) was poured into this bar and held for 15 seconds (16). After the formation of standard, second‐degree burn wounds, the formulations were repeatedly applied (one application every day) to the burned areas for 14 days. Full thickness skin biopsies were collected at the 3rd, 7th and 14th day after wound formation, and the degree of healing was evaluated both immunohistochemically and histochemically.
Immunohistochemical studies
BrdU technique was used for the evaluation of the healing. BrdU is a pyrimidine analogue that is incorporated into DNA‐synthesising nuclei. In immunohistochemical studies, the BrdU incorporated into DNA has been detected using antibodies against BrdU 17, 18.
One hour before animals were sacrificed, BrdU (100 mg/kg body weight) dissolved in saline was injected i.p. The animals were then euthanised and full‐thickness skin biopsies were collected.
After all the steps of immunohistochemical staining, the samples were assessed using Ks400 Vision Screening Analysis Program under Zeiss Axioskop light microscope and results were represented as BrdU per 10 high power field.
Histochemical studies
The skin biopsies taken from all groups were embedded in paraffin and the sections were stained using haematoxylin and eosin and trichrome staining techniques and examined by light microscope.
Measurement of epidermal thicknesses
Increment in the epidermal thickness is one of the important indicators of wound healing. Measurements were carried out using KS400 Vision Screening Analysis Program using light microscope. Skin samples were collected and subjected to the KS400 Vision Screening Analysis Program after trichrome staining (14). All measurements were performed and represented as mean values (n = 6).
Measurement of fibroblast nucleus area
The area of the fibroblast nuclei was measured using Ks400 Vision Screening Analysis Program under Zeiss Axioskop light microscope after trichrome staining. An increment in the fibroblast nucleus sizes indicates a faster healing process. Fifteen different areas from each preparation were examined.
Histological studies
To compare the healing effects on ELP, SIL and ES groups and untreated control groups [unburned (S) and untreated (Y) rats] histologically, structural changes in the skin layers were examined using transmission electron microscope (TEM 911 Carl Zeiss). Tissues were fixed in phosphate‐buffered solution containing 2·5% glutaraldehyde for 2 hours, and were then postfixed in 1% osmium tetroxide (OsO4) and dehydrated in a series of graded alcohols. After passing through propylene oxide, the specimens were embedded in Araldyt CY212, 2‐dodecen‐1‐yl succinic anhydride and benzyldimethyl amine. Ultrathin sections were stained with uranyl acetate and lead citrate and examined with the electron microscope.
Statistical analysis
All data are expressed as means ± SD. Statistical analysis of data was performed using one way ANOVA.
RESULTS
Determination of the pH and viscosity of chitosan gel
The pH of the gel was measured as 5·32. After the preparation of the chitosan gel, the required amount of EGF solution was added and the final concentration was 10 µg/mL. The molecular range of chitosan is 650000 and viscosity value of 2% chitosan gel is 7903 mPa at 25°C.
Measurement of the particle size of liposomes
The type of the liposomes was determined as MLV using inverted microscope. The particle size of the liposomes was investigated by laser diffraction particle sizer and the mean particle size of liposome was found as 4·44 ± 0·03 µm (SD, n = 6).
Determination of encapsulation efficiency of liposomes
The EGF contents of liposomes were determined using spectrofluorometre after extraction processes. The encapsulation efficiency of EGF in the liposomes was found to be 58·1%.
Measurement of epidermis thicknesses
Increment in the epidermis thickness is one of the important indicators of wound healing. The 14th day epidermis thickness results were measured according to the groups (Table 2; Figure 1). Measurements were carried out using Ks400 Vision Screening Analysis Program.
Table 2.
The 14th day epidermis thickness results according to the groups
| Groups | Average epidermis thickness (µm) ± SD | SD |
|---|---|---|
| S group | 6·03 | 0·09 |
| Y group | – | – |
| J group | – | – |
| EJ group | 10·2 | 0·0 |
| LP group | 8·01 | 0·06 |
| ELP group | 10·1 | 0·0 |
| SIL group | 9·02 | 0·05 |
| ELJ group | 12·1 | 0·1 |
| ES group | 11·5 | 0·4 |
‘–’ Indicates ulcered tissue.
Figure 1.
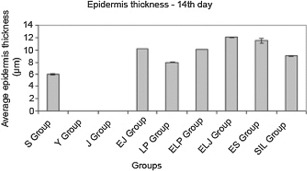
Epidermis thickness results from the groups (n = 6) at the 14th day of the treatment (EJ‐ELP: insignificantly, others: P < 0·001).
Measurement of fibroblast nucleus area
The increment in the fibroblast nucleus areas indicates a faster healing process. Fifteen different areas of each preparation were examined and areas of the fibroblast nucleus were measured shown in Table 3 and Figure 2A–C.
Table 3.
Average fibroblast nucleus areas for experimental groups
| Groups | Average fibroblast nucleus areas (µm2) ± SD | ||
|---|---|---|---|
| 3rdDay | 7thDay | 14th Day | |
| S groups | 10·3 ± 2·6 | 10·3 ± 2·6 | 10·3 ± 2·6 |
| Y groups | 10·3 ± 2·7 | 16·2 ± 3·7 | 13·7 ± 5·3 |
| J groups | 11·0 ± 2·5 | 14·3 ± 4·2 | 14·7 ± 6·3 |
| EJ groups | 12·2 ± 3·3 | 16·3 ± 6·5 | 15·7 ± 8·1 |
| LP groups | 11·3 ± 3·1 | 18·7 ± 7·9 | 16·3 ± 6·6 |
| ELP groups | 11·4 ± 3·0 | 17·7 ± 5·7 | 14·5 ± 6·9 |
| ELJ groups | 11·2 ± 2·9 | 18·0 ± 5·3 | 17·1 ± 6·6 |
| ES groups | 13·0 ± 3·0 | 16·3 ± 6·2 | 17·6 ± 9·1 |
| SIL groups | 12·5 ± 3·3 | 18·8 ± 5·0 | 15·7 ± 5·3 |
Figure 2.
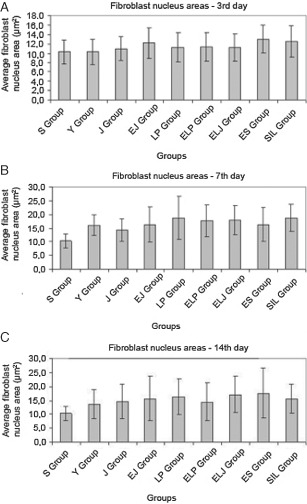
The fibroblast nucleus areas for experimental groups at (A) 3rd, (B) 7th and (C) 14th day (P > 0·05).
Evaluation of the immunohistochemical studies
Full thickness skin biopsies from the 3rd, 7th and 14th day of therapy from all animal groups were examined immunohistochemically using BrdU technique.
A sample image of the proliferated cell stained with BrdU technique is shown in Figure 3.
Figure 3.
Stained proliferated cell (shown with arrows).
The average numbers of proliferated cells (%) labelled with BrdU are shown in Table 4 and Figure 4A–C at the 3rd, 7th and 14th day, respectively. When the results were evaluated immunohistochemically, the rate of healing in the EJ group was found to be increased (P < 0·001).
Table 4.
The percentage of cell number labelled with BrdU for experimental groups
| Groups | The average cell number labelled with BrdU (%) ± SD | ||
|---|---|---|---|
| 3rd Day | 7th Day | 14th Day | |
| S group | 2·11 ± 0·12 | 2·10 ± 0·10 | 2·13 ± 0·15 |
| Y group | – | 1·27 ± 0·15 | 2·33 ± 0·38 |
| J group | 1·20 ± 0·17 | 2·43 ± 0·61 | 3·10 ± 0·68 |
| EJ group | 1·63 ± 0·35 | 4·20 ± 0·18 | 4·95 ± 0·39 |
| LP group | 1·10 ± 0·08 | 3·20 ± 0·18 | 4·20 ± 0·18 |
| ELP group | 1·23 ± 0·21 | 5·27 ± 0·15 | 5·85 ± 0·71 |
| ELJ group | 1·28 ± 0·22 | 4·18 ± 0·22 | 5·27 ± 1·15 |
| ES group | 1·43 ± 0·28 | 4·25 ± 0·13 | 3·90 ± 0·64 |
| SIL group | 1·48 ± 0·29 | 3·27 ± 0·15 | 4·53 ± 0·53 |
‘–’ Indicates no healing.
Figure 4.
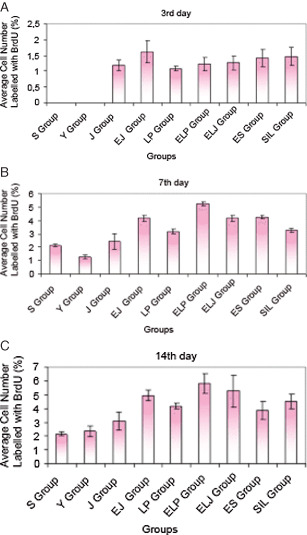
The average cell number labelled with BrdU (A) at the 3rd day of the treatment for the different groups (%) (n = 4) (all groups with each other: P > 0·05); (B) at the 7th day of the treatment for the different groups (%), (n = 4) (S‐Y, EJ‐ELJ, EJ‐ES, ELJ‐ES: unsignificant, others: P < 0·001); (C) at the 14th day of the treatment for the different groups (%), (n = 4) (S‐EJ, S‐LP, S‐ELP, S‐SİL, S‐ELJ, S‐ES, Y‐EJ, Y‐LP, Y‐ELP, Y‐SİL, Y‐ELJ, Y‐ES, J‐EJ, J‐ELP, J‐ELJ, LP‐ELP, ELP‐ES: P < 0·001, others: unsignificant).
Evaluation of the histochemical studies
The full‐thickness skin samples of the untreated group, the gel without EGF applied group, the EGF‐gel formulation applied group and the Silverdin® (commercial silver sulfadiazine cream) applied group were examined at the 14th day of the treatment after trichrome staining and they are shown in Figure 5A–E.
Figure 5.
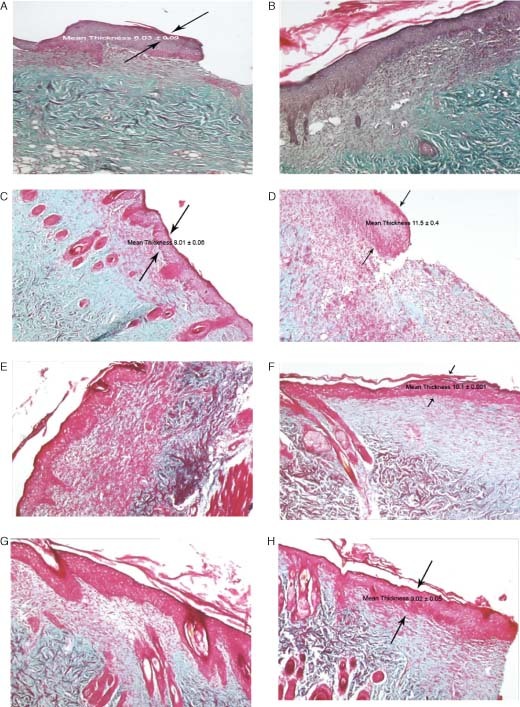
The epidermis thickness of different experimental groups: (A) untreated group (Y); (B) gel formulation without EGF applied group (J); (C) liposome formulation without EGF applied group (LP); (D) EGF‐solution formulation applied group (ES); (E) EGF‐gel formulation applied group (EJ); (F) EGF‐liposome formulation applied group (ELP); (G) EGF‐liposome in chitosan gel formulation (ELJ); (H) Silverdin® (commercial silver sulfadiazine cream) applied group (SIL) (at the 14th day of treatment after trichrome staining; light microscope ×5).
Evaluation of the histological studies
The wound tissues were investigated by ultrathin sectional preparation under TEM at the 14th day of treatment. The results are summarised as follows:
Healthy experimental group (S group). Skin layers were clearly identified, and epidermis and dermis were observed to be normal. Abundant fibrils were also observed (Figure 6A).
Figure 6.
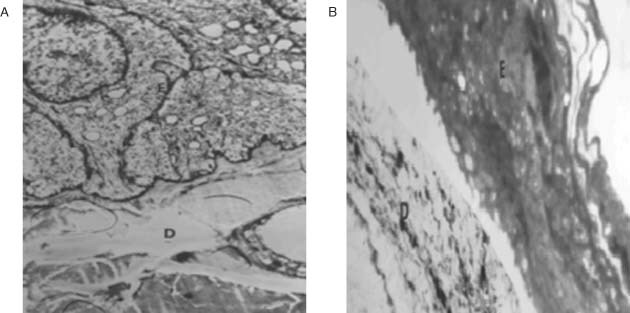
The microscopic image of the epidermis and dermis from the different experimental groups: (A) healthy group, (B) untreated group, at the 14th day of the treatment. E: Epidermis, D: Dermis (×3000).
Burn wound made but no treatment received (Y group). Inflamed cells were observed around the burn wound. Burned cells were observed, but detailed structure could not be identified. Cell response was observed to be inadequate at deeper layers and epithelial tissues and collagen fibres were found to be irregular. Neutrophilic infiltrations were present at dermis surface and the beginning of scar tissue formation was observed as well as of formation of burn wound (Figure 6B).
Burn wound made and EGF solution applied group (ES group). Although epidermis could not be observed under the wound scab, some inflamed cells were found. Infiltrations were observed; irregular myofibroblast distribution, collagen fibres and numerous fibroblasts were noted. The continuation of fibroblastic activity, wound scab and invasive inflamed cell infiltrations underneath were also noticed. A degeneration of cells at epithelial surface was seen, but some myofibroblasts, which were accepted as an indication of wound healing, were observed at dermis. In Figure 7C, the image of the EGF solution group at the 14th day of the treatment is shown.
Figure 7.

The microscopic image of the epidermis and dermis from the different experimental groups: (A) gel formulation without EGF applied group; (B) EGF‐gel formulation applied group; (C) EGF solution applied group; (D) Silverdin® (commercial silver sulfadiazine cream) applied group, at the 14th day of the treatment. E: Epidermis, Mf: Myofibroblast, F: Fibroblast (×3000).
Burn wound made and gel formulation without EGF applied group (J). The continuation of fibroblastic activity was observed. Although no differentiation of epithelial basal cell was observed, some degeneration of mitochondria and loss of crista were noted. There was some wound healing, indicated by active fibroblasts observed at dermis (Figure 7A).
Burn wound made and EGF containing gel applied group (EJ). Emphasised epidermis layers and swollen cells were observed under the wound scar. Some vacuolisation of the dermal cells and healing were observed. An infiltration of inflammed cells was noticed. The structures of surface epithelial cells were normal, but some active fibroblasts and inflammed cells were present at connective tissue layer. The microscopic image of the EGF‐gel formulation applied group at the 14th day of the treatment is shown in Figure 7B.
Burn wound made and commercial Silverdine®cream applied (SIL group). Inflamed cell infiltrations were observed. At intercellular space in the dermis, enlarged epidermal cells and irregular formations were noticed. Random distributions of myofibroblasts were present. There were also some vacuoles and the healing process of the wound was not found to be completed. Epithelisation was found to be higher than observed in the LP and Y groups. But wound scab was still present. The image of SIL group at the 14th day of the treatment is shown in Figure 7D.
Burn wound made and EGF containing liposome formulation applied (ELP group). Epithelial tissue was found to be almost fully recovered, but these new epithelial layers were irregular. Broad collagen fibre bundles were obvious and dermis was normal. Active fibroblast distributions were also noticed at the dermis and connective tissues. Endoplasmic reticulum was observed to be dilated, but active. Therefore, the synthesis of collagens or in other words the formation of wound contraction was also observed. Basal membrane was found to be as healthy as that of the control group (Figure 8A,B).
Figure 8.
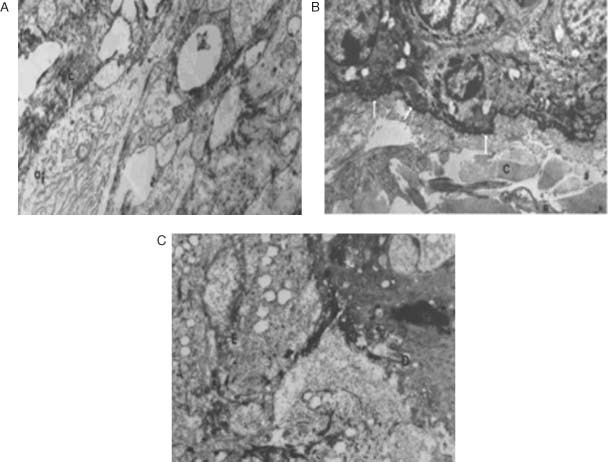
The microscopic image of the epidermis and dermis from the (A) and (B) EGF‐containing liposome formulation applied group (ELP); (C) EGF‐liposome in chitosan gel formulation (ELJ), applied group at the 14th day of the treatment. mf: Myofibroblast, af: active fibroblast, c: collagen, E: epidermis, D: dermis, basal membrane (arrows). (Transmission Electron Microscope ×3000).
Burn wound made and EGF containing liposome in gel formulation applied (ELJ group). This group was found to be the closest group to the healthy experimental group (S). Epithelisation was observed to be completed and regular. Mitotic activities were observed at basal cells of epidermis. Basal membrane and dermis cells were healthy and normal. There was no neutrophilic infiltration observed (Figure 8C).
DISCUSSION
Burn wounds are one of the main problems which have been met during daily life. Burn wounds can cause serious problems according to their depth and surface area. Nowadays, the therapy for the second‐degree burn wounds mainly focused on the therapeutic agents to wounds and burns, be it an established antibiotic to treat infection and the prevention of wound from infections and application of antiseptic and anti‐inflammatory drugs 19, 20, 21. Chitosan is a biodegradable polymer and it accelerates wound healing. It also permits regeneration of tissue elements in skin wounds and has positive application effects on wound healing 14, 22, 23. It has been reported that treatment with EGF–chitosan gel formulation decreased the wound healing period, accelerated epidermal regeneration and tissue formation could be obtained (23). In this study, it was aimed to develop liposome, chitosan gel and liposome in chitosan gel formulations containing EGF and to determine their effects on the healing of second‐degree burn wounds. All results were compared with Silverdin® ointment (SIL) applied group, liposome with and without EGF (ELP, LP) applied group, chitosan gel with and without EGF (EJ, J) applied group, EGF‐containing liposomes in chitosan gel (ELJ) applied group, EGF solution (ES) applied group and untreated control groups [Unburned (S) and untreated (Y) rats].
It was reported in the literature that MLV‐type liposomes can be produced by the film formation method 15, 24, 25. The MLV‐type liposomes of EGF were prepared according to the procedure of film formation method using DPPC, cholesterol, chloroform and methanol. To characterise the liposomes, particle size, shape and amount of encapsulated EGF in the liposome were determined in this study. This technique used for the liposome formation is also reported to be effective 26, 27.
The encapsulation efficiency was found to be 87% for modified MLV type of EGF‐containing liposome with hyaluronic acid in the literature (21). The encapsulation efficiency of vinblastine containing MLV type of liposome as 51% in one of the studies (28) and also 50% encapsulation efficiency was good enough for MLV type of vesicles in another study 8, 29. The encapsulation efficiency of EGF in the liposomes was then calculated and found to be 58·1% in this study which can be accepted as sufficient (15).
In our study, the liposomes were framed under light and inverted microscopes. The particle size of the liposomes, both at 25°C and 4°C, was measured over 6 months by laser diffraction particle sizer. After 6 months, the particle sizes of the liposomes had not changed significantly at 4°C (P > 0·05); however, the particle sizes of the liposomes had increased significantly after the third month at 25°C (P < 0·001).
The results showed that the pH values of gels which were kept at 4°C increased significantly after 180 days (P < 0·001). The pH values of the gels at 40°C increased significantly after 210 days (P < 0·001) and pH values of the gels at 25°C increased significantly after 300 days (P < 0·01) (14).
The in vivo experiments were performed on female Sprague Dawley rats. After shaving the backs of rats under anaesthesia, a cylindrical shaped bar was placed on the back of the rats and then water, heated up to 94 ± 1°C, was poured into this bar and waited for 15 minutes. After the formation of second‐degree burn wounds, the EGF formulations were repeatedly applied on the burned areas with the dose of 0·160 µg/ml for 14 days. Skin biopsies were collected at the 3rd, 7th and 14th day after the wound formation and the degree of healing of the wounds was evaluated histologically, immunohistochemically and histochemically.
EGF (10 µg/ml) containing liposome, gel and liposome in gel formulations were repeatedly applied separately on the burned areas at a dose 0·160 µg/cm2 for 14 days as one application per day after the formation of second‐degree burn wounds. Skin biopsies were collected at 3rd, 7th and 14th day after the wound formations and the degree of healing was evaluated histologically, immunohistochemically and histochemically.
BrDU‐positive cells were detected by immunohistochemical staining method. Cell proliferation is an indication of burn wound healing. BrDU is an analogue of pyrimidine and is a useful alternative to labelling proliferated cells 17, 18. When the results were evaluated immunohistochemically, at the 7th day of the therapy, the maximum cell proliferation was found in ELP group. The healing in EJ, ELJ and ES groups were also found to be rather good and it was seen that the healing ratios were very close to each other (P > 0·05). It was also observed that the cell proliferation in LP, J and other control groups (S and Y groups) were extremely low. There were significant increases of cell proliferation observed in EGF‐containing groups (P < 0·001). At the 14th day of the therapy, it was seen that the healing in ELP and ELJ groups was found to be faster than that of other groups, but there was no significant difference between these two groups (P > 0·05). When ELP group and ELJ group were compared, the healing and the rate of healing were found to be increased.
Similar to the fibroblastic activity accompanied with the diametre of fibroblasts, epidermal thickness, protein deposition and DNA synthesis, the metabolic activity of the skin cells at wounded area can be accepted as an indicator of healing process (30). The reason for this is that as the DNA synthesis is increased and this proliferation reflected on by DNA synthesis, the reepithelisation of a skin wound demands keratinocyte proliferation. This process of DNA synthesis also required angiogenesis and the generation of vascular endothelial cells. The healing process is closely related to the rate of DNA synthesis. Moreover, protein deposition and synthesis play a key role in the wound healing process. The majority of tissue loss after burn injuries are related to protein loss, which makes the protein deposition necessary for repairing the tissue. Both protein synthesis and protein deposition are crucial in constructing a functional dermal base and new blood vessels/supply in supporting reepithelialisation with nutritional support to build new healthy skin 3, 30.
The histochemical results showed that the increase in epidermis thickness in ELJ group was the fastest one. When the fibroblast nuclei areas were evaluated, at the 7th day, the healing rate in ELJ, ELP and EJ groups were close to each other, and the healing rate in ELJ group was found to be the fastest. The healing rates were found to be with the following sequence: ELJ, ELP and EJ groups. At the 14th day, in ELJ and ES group, the healing was found to be the fastest.
Although an effect on the fibroblastic cells was observed, with the use of commercially available Silverdin® ointment, it has not been suggested to use silver sulfadiazine‐containing preparations for the treatment of severe burn wounds, because silver molecules can penetrate through damaged skin cells in high extent and the toxic effect of silver should be considered (31).
The histological results indicated that the healing in ELJ group was better compared with other groups. ELJ group healed rapidly than ELP and EJ groups.
In conclusion, when the results were evaluated altogether, it can be concluded that EGF‐containing formulations are effective in wound healing process. Growth factor formulations, which play an important role in burn wound treatment, are thought to be promising in human health.
EGF, like all other growth factors, has low stability 15, 24, 32, 33. Thus EGF‐containing liposomes in chitosan gel would be more stable and could be retained for long periods in the rat wounds and safely used in the treatment of burns. It was reported that EGF formulated in MLV liposomes was retained for prolonged periods in the rat incisions 1, 15.
Growth factors and other biologic agents will be used more specifically in future.
ACKNOWLEDGEMENT
This study was supported by a research grant from the Gazi University (SBE‐11/2002‐14). The authors are grateful to Prof. Dr Mustafa Şengezer, Assist. Prof. Dr Fatih Zor, Assist. Prof. Dr Melih Alömeroğlu for providing the facilities for the in vivo experiments, and to Prof. Dr Deniz Erdoğan and Assist. Prof. Dr Ahmet Nacar for their kind assistance in histological analysis.
REFERENCES
- 1. Schultz G, Rotatori DS, Clark W. EGF and TGF‐α in wound healing and repair. J Cell Biochem 1991;45:346–52. [DOI] [PubMed] [Google Scholar]
- 2. Fu X, Shen Z, Chen Y, Xie J, Guo Z, Zhang M, Sheng Z. Randomised placebo‐controlled trial of use of topical recombinant bovine basic fibroblast growth factor for second‐degree burns. Lancet 1998;352:1661–4. [DOI] [PubMed] [Google Scholar]
- 3. Wenczak BA, Lynch JB, Nanney LB. Epidermal growth factor receptor distribution in burn wounds. J Clin Invest 1992;90:2392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Çelebi N, Erden N, Gönül B, Koz M. Effects of epidermal growth factor dosage forms on dermal wound strength in mice. J Pharm Pharmacol 1993;46:386–7. [DOI] [PubMed] [Google Scholar]
- 5. Brown GL, Curtsinger LJ, White M, Mitchell RO, Pietsch J, Nordquist R, Fraunhofer A, Schultz GS. Acceleration of tensile strength of incisions treated with EGF and TGF‐β. Ann Surg 1988;208:788–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kiyohara Y, Nishiguchi K, Komada F, Iwakawa S, Hirai M, Okumura K. Cytoprotective effects of epidermal growth factor (EGF) ointment containing nafamostat, a protease inhibitor, on tissue damage at burn sites in rats. Biol Pharm Bull 1993;16:1146–9. [DOI] [PubMed] [Google Scholar]
- 7. Schultz GS, White M, Mitchell R, Brown G, Lynch J, Twardzik DR, Todaro GJ. Epithelial wound healing enhanced by transforming growth factor‐α and vaccinia growth factor. Science 1987;235:350–2. [DOI] [PubMed] [Google Scholar]
- 8. Crommelin DJA, Schreler H. Liposomes. In: Kreuter J, editor. Colloidal drug delivery systems. New York: Marcel Dekker Inc, 1994:73–157 [Google Scholar]
- 9. Ishihara M, Ono K, Sato M, Nakanishi K, Saito Y, Yura H, Matsui T, Hattori H, Fujita M, Kikuchi M, Kurita A. Acceleration of wound contraction and healing with a photocrosslinkable chitosan hydrogel. Wound Rep Reg 2001;9:513–21. [DOI] [PubMed] [Google Scholar]
- 10. Illum L. Chitosan and its use as a pharmaceutical excipient. Pharm Res 1998;15:1326–31. [DOI] [PubMed] [Google Scholar]
- 11. Ueno H, Mori T, Fujinaga T. Topical formulations and wound healing applications of chitosan. Adv Drug Del Rev 2001;52:105–15. [DOI] [PubMed] [Google Scholar]
- 12. Dodane V, Vilivalam VD. Pharmaceutical applications of chitosan. PSTT 1998;1:246–53. [Google Scholar]
- 13. No HK, Park NY, Lee SH, Meyers SP. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol 2002;74:65–72. [DOI] [PubMed] [Google Scholar]
- 14. Alemdaroğlu C, Değim Z, Çelebi N, Zor F, Öztürk S, Erdoğan D. An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns 2006;32:319–27. [DOI] [PubMed] [Google Scholar]
- 15. Alemdaroglu C, Degim Z, Celebi N, Sengezer M, Alomeroglu M, Nacar A. Investigation of epidermal growth factor containing liposome formulation effects on burn wound healing. J Biomed Mater Res Part A 2008;85A: 271–83. [DOI] [PubMed] [Google Scholar]
- 16. Uhl KV, Furnschlief E, Wagner A, Ferko B, Katinger H. Topically applied liposome encapsulated superoxide dismutase reduces postburn wound size and edema formation. Eur J Pharm Sci 2001;14:63–7. [DOI] [PubMed] [Google Scholar]
- 17. Sugihara H, Hattori T, Fukuda M. Immunohistochemical detection of bromodeoxyuridine in formalin fixed tissues. Histochemistry 1986;85: 193–5. [DOI] [PubMed] [Google Scholar]
- 18. Montero‐Montoya R, Serrano L, Ostrosky‐Wegman P. In vitro induction of micronuclei in lymphocytes: the use of bromodeoxyuridine as a cell proliferation marker. Mutat Res 1997;391:135–41. [DOI] [PubMed] [Google Scholar]
- 19. Mohammed Haneefa KP, Shahima Hanan K, Saraswathi R, Guru Prasad M, Chandini N. Formulation and evaluation of herbal gel of Pothos scandens Linn. Asian Pac J Trop Med 2010: 988–92.
- 20. Goertz O, Abels C, Knie U, May T, Hirsch T, Daigeler A, Steinau H‐U, Langer S. Clinical safety and efficacy of a novel thermoreversible polyhexanide‐preserved wound covering gel. Eur Surg Res 2010;44:96–101. [DOI] [PubMed] [Google Scholar]
- 21. Ryssel H, Germann G, Riedel K, Reichenberger M, Hellmich S, Kloeters O. Suprathel‐acetic acid matrix versus acticoat and aquacel as an antiseptic dressing: an in vitro study. Ann Plast Surg 2010;65:391–5 [DOI] [PubMed] [Google Scholar]
- 22. Conti B, Giunchedi P, Genta I, Conte U. The preparation and in vivo evaluation of the wound‐healing properties of chitosan microspheres. STP Pharm Sci 2000;10:101–4. [Google Scholar]
- 23. Değim Z, Çelebi N, Sayan H, Babül A, Erdoğan D, Take G. An investigation on skin wound healing in mice with a taurine‐chitosan gel formulation. Amino Acids 2002;22:187–98. [DOI] [PubMed] [Google Scholar]
- 24. Yerushalmi N, Arad A, Margalit R. Molecular and cellular studies of hyaluronic acid‐modified liposomes as bioadhesive carriers for topical drug delivery in wound healing. Arch Biochem Biophys 1994;313:267–73. [DOI] [PubMed] [Google Scholar]
- 25. Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol 1965;13:238–52. [DOI] [PubMed] [Google Scholar]
- 26. Al‐Angary AA, Bayomi MA, Khidr SH, Al‐Meshal MA, Al‐Dardiri M. Characterization, stability and in vivo targeting of liposomal formulations containing cyclosporin. Int J Pharm 1995;114:221–5. [Google Scholar]
- 27. Lichtenstein A, Margalit R. Liposome‐encapsulated silver sulfadiazine (SSD) for the topical treatment of infected burns: thermodynamics of drug encapsulation and kinetics of drug release. J Inorg Biochem 1995;60:187–98. [DOI] [PubMed] [Google Scholar]
- 28. Yerushalmi N, Margalit R. Bioadhesive, collagen‐modified liposomes: molecular and cellular level studies on the kinetics of drug release and on binding to cell monolayers. Biochim Biophys Acta 1994;1189:13–20. [DOI] [PubMed] [Google Scholar]
- 29. Lasic DD. Novel applications of liposomes. TIB TECH 1998;16:307–21. [DOI] [PubMed] [Google Scholar]
- 30. Zhang XJ, Chinkes DL, Ramanujam VMS, Wolfe RR. Local injection of insulin‐zinc stimulates DNA synthesis in skin donor site wound. Wound Rep Reg 2007;15:258–65. [DOI] [PubMed] [Google Scholar]
- 31. Degreef H, Michiels JL. 1% Silver sulfadiazine cream as a treatment for infected leg ulcers. Curr Therapeutic Res 1984;36:1190–4. [Google Scholar]
- 32. Allen TM. Liposomes. Opportunities in drug delivery. Drugs 1997;54(Suppl. 4):8–14. [DOI] [PubMed] [Google Scholar]
- 33. Celebi N, Turkyilmaz A, Gonul B, Özoğul C. Effects of epidermal growth factor microemulsion formulation on the healing of stress‐induced gastric ulcers in rats. J Control Release 2002;83: 97–210. [DOI] [PubMed] [Google Scholar]


