Abstract
The main objective of this case‐cohort‐type observational study conducted at different Surgical Departments of the Charité‐Universitätsmedizin in Berlin was to evaluate the sequential use concept first described by Systagenix Wound Management in 2007. Fifty‐two patients with different wound healing by secondary intention were treated for 7 weeks at the Charité‐Universitätsmedizin in Berlin. A multidisciplinary team worked together to reach consensus in wound assessment; in classification of infection status according to the criteria described by European Wound Management Association (EWMA); in treatment protocol and on dressings to be used to ‘cover’ wounds. Before dressing application, all wounds were cleaned from debris. Following the sequential use concept, wounds classified as stages 2 and 3 were dressed with SILVERCEL® and TIELLE® or TIELLE PLUS® to ‘clean’ the wounds. After 2–3 weeks, treatment was changed to PROMOGRAN PRISMA® and TIELLE® to ‘close and cover’ wounds, thus providing optimal wound healing. Wounds classified as non infected were dressed with PROMOGRAN PRISMA® and TIELLE® during the complete treatment period. Patients were asked to evaluate the treatment using a simplified questionnaire developed at the Charité‐Universitätsmedizin in Berlin. Wounds comprised 37 surgical procedures, 8 chronic mixed ulcer, 4 pressure sores, 1 diabetic foot ulcer, 1 venous leg ulcer, and 1 mixed arterial/venous ulcer. At baseline, 12 wounds were classified as stage 3, 38 wounds as stage 2 and 2 wounds as stage 1. After 7 weeks of treatment, all patients showed a positive clinical response to the sequential use treatment. Results of wound size showed a high significant progression of wound healing expressed with a profound reduction of wound area (P in all measurements <0·001, chi‐square test) and improved granulation. This study summarises the clinical experiences derived from the evaluation of the sequential use concept in the daily clinical practice of wound treatment. On the basis of the wound healing results, patients' evaluation of treatment and the clinicians' and staff experiences, this concept was implemented at different Surgical Departments of the Charité‐Universitätsmedizin in Berlin.
Keywords: Daily clinical practice, Sequential use concept, Surgery, Wound exudate management Wound healing
INTRODUCTION
Chronic wounds and wound healing by secondary intention cause discomfort to patients, rise health care costs and increase workload to staff involved in the treatment of these wounds. Ideally, wound treatment should follow an interdisciplinary approach involving clinicians, surgeons, chiropodists and nurses. Daily clinical practice in Germany has shown that the workload of staff involved in wound treatment has increased. There is a real need for the implementation of a systematic clinical decision‐making processes to ensure the application of best practice, the consideration of advances in knowledge of wound biology and of concepts for best wound treatment. Recent advances in our understanding of chronic wound biology have led to the development of diverse novel treatments that offer renewed hope to patients with ulcers and other chronic wounds (1). Defining the role of these new treatments, in the context of the increasing number of patients with chronic wounds, represents the next challenge (2). The most recent example of a concept wound healing process that was translated into a more precise and systematic approach to define appropriate treatment and dressing selection is the wound bed preparation (WBP) concept (3). The wound management principles underlying the WBP concept are debridement, wound bioburden management and exudate management. These issues have to be incorporated in a precise and systematic approach, thus leading to the appropriate dressing selection (4). There is no ideal dressing for all wound types and clinical experience has shown that dressings are often chosen on the basis of local practices and empirical experiences. The type of wound, its appearance, the amount of exudate and the absence or presence of pain should assist in the selection of an appropriate dressing and the adoption of novel dressings should be based on scientific evidence. The aim of this case‐cohort‐type observational study was to implement a precise and systematic treatment concept to improve the healing of chronic wounds and for wound healing by secondary intention, establish an easy to follow treatment routine in daily clinical practice and to reduce the number of products used to dress wounds. A multidisciplinary team worked together to conduct a detailed wound assessment, to reach consensus in treatment regime to follow and to decide on the appropriate dressings that were to be used to cover wounds according to the healing status of the wound.
MATERIALS AND METHODS
This case‐cohort‐type observational study was approved by the Ethical Committee of the Charité‐Universitätsmedizin in Berlin. The study describes the experiences of implementing the sequential use concept (5) firstly described by Systagenix Wound Management in 2007 at three different Departments of Surgery of the largest University Hospital in Germany, the Charité‐Universitätsmedizin in Berlin. This simple concept considers and defines the optimum combination of products throughout the wound healing process towards the desired outcome of complete wound closure. Ultimately, the concept strives for simplicity in terms of product selection and ease of use for the treatment of chronic wounds and for wound healing by secondary intention. In principle, the concept recommends first to clean the wound by applying specific products that are proven to control bacteria, manage exudate, control odour and to debride slough, and then to close the wounds using products known to facilitate healing, control inflammation, prevent re‐infection and manage exudate. The final piece of the sequential use concept is covering wounds with an appropriate secondary dressing to create an optimal moist wound healing environment. Best practice experience of clinicians and the staff involved in this study are considered and patients' overall impression on dressing performance is considered to choose the appropriate wound treatment regime.
Patient population
In total, 52 patients were enrolled in this study. Patient recruitment occurred between May and October 2008. Twenty‐two patients were treated at the Charité‐Universitätsmedizin in Berlin Campus Mitte, Department of General, Visceral, Vascular and Thoracic Surgery; 18 patients were treated at the Charité‐Universitätsmedizin in Berlin, Campus Benjamin Franklin, Department of Plastic and Reconstructive Surgery; and 12 patients were treated at the Charité‐Universitätsmedizin in Berlin Campus Virchow, Department of General, Visceral and Transplantation Surgery.
Treated patients met the following inclusion criteria: diagnosed with venous and mixed aetiology leg ulcers, diabetic foot ulcers, pressure ulcers and surgical wound healing by secondary intention; with a wound size ≥1 cm (2); wounds to be free of necrosis; patients readiness and capability to adhere to medical instructions and to comply with the scheduled visits at one of the three medical centres of the Charité‐Universitätsmedizin in Berlin. All patients gave prior, written informed consent to participate and willingness to be followed up for 6 weeks.
Patients were excluded from the study if they met any of the following criteria: they were aged under 18 years, to have known incompatibility to silver or silver‐containing products and known hypersensitivity to any of the dressing components. Also excluded were patients known for current abuse of drugs or current use of drugs without medical prescription, known current excessive alcohol consumption, pregnancy or lactation, had progressive or advanced malignant disease, were undergoing chemotherapy and patients showing non concordance with the wound care regime.
Duration of wound treatment was 7 weeks with a follow‐up period of 6 weeks.
Criteria for defining infection status of wounds
The infection status of treated wounds was defined following the criteria described by the European Wound Management Association (EWMA) (6)
-
1
Stage 1: A few subtle signs of infection (some malodour, pain or exudate), but wound healing is progressing normally.
-
2
Stage 2: Increasing signs of infection (increasing malodour, pain or exudate) and healing is no longer progressing normally.
-
3
Stage 3: Overt signs of local infection (discharge of pus, malodour, pain, erythema and local warmth), with evidence of surrounding tissue involvement. The wound appears unhealthy or is deteriorating.
-
4
Stage 4: Overt signs of local infection and systemic infection (pyrexia and raised white blood cell count), with possible evidence of surrounding tissue involvement. This can lead to sepsis and organ failure, which can be life‐threatening.
Only patients with stages 1, 2 or 3 wounds were included in the study. Swabs were taken from the wound bed for microbiology before treatment to determine bioburden level.
Intervention
All three centres at the Charité‐Universitätsmedizin in Berlin followed the same treatment concept and dressing protocol. Wounds were treated for 7 weeks with a follow‐up period of 6 weeks.
For those wounds classified as stages 2 and 3, treatment was initiated with SILVERCEL® to ‘clean’ the wounds from bacteria, manage wound exudate, control wound odour and debride the wound bed from slough. SILVERCEL® is a conformable silver antimicrobial dressing known to combine the potent broad‐spectrum antimicrobial action of silver with the enhanced exudate management properties of hydroalginate technology 7, 8, 9, 10. To ‘cover’ wounds, maintain a moist wound environment and avoid leakage in highly exuding wounds and TIELLE® or TIELLE PLUS® was used as secondary dressings (11). The products used were achieved from Systagenix Wound Management, Norderstedt, Germany, formerly Johnson & Johnson Wound Management.
SILVERCEL® was applied in accordance with the manufacturer's instructions and changed two or three times a week, depending on the exudate level and the wound's healing status. It was applied for a period of 2–3 weeks, until the wounds were no longer in stage 2 or 3. Treatment was then changed to PROMOGRAN PRISMA®, a biodegradable ORC/Collagen dressing with silver, known to control inflammation, prevent re‐infection and manage wound exudate to promote the formation of granulation tissue, epithelialisation and provide optimal wound healing 12, 13. PROMOGRAN PRISMA® was used in combination with TIELLE® dressing as a secondary dressing for 3–5 weeks. Wounds classified as non infected wounds were treated with PROMOGRAN PRISMA® in combination with TIELLE® dressing during the complete wound treatment period of 7 weeks. Dressing changes occurred two to three times a week, depending on the exudate level and the wound's healing status. Any adverse effects were documented.
Clinical evaluation
All patients received a complete clinical examination prior to their enrollment in the study. Clinical assessment of the wounds and assessment of healing was evaluated by an interdisciplinary approach involving nurses, podologists, surgeons and clinicians. Clinical data were collected on a weekly basis during the 7‐week treatment period and during the follow‐up period of 6 weeks. After initial wound assessment, wound debridement was conducted as required to remove debris and bacteria. For those cases, where extensive debridement was necessary, patients underwent general anaesthesia. Prior to dressing application, wounds were disinfected and cleaned with polyhexanide 0·04% solution.
Wound assessment included definition of type of wound, infection status, presence of wound odour, amount of exudation, inflammatory status, quality of surrounding tissue and patients' general condition. A clinical wound score was used to assess the progress of healing and the quality of granulation tissue (14). The score includes a maximum of seven points, with each parameter of wound appearance having a maximum number of points. A higher score is consistent with a better healing potential. This score assessed granulation, colour and consistency of granulation tissue (Table 1). A score of six points or more designates new tissue of good quality and therefore the ideal basis for further complete epithelialisation, for example, healing.
Table 1.
| Wound quality | Finding | Score points |
|---|---|---|
| Granulation | Absent | 0 |
| One fourth of ulcer area | 1 | |
| Half of ulcer area | 2 | |
| Three fourth of ulcer area | 3 | |
| Complete | 4 | |
| Colour | Pale | 0 |
| Pink | 1 | |
| Bright red | 2 | |
| Consistency | Spongy | 0 |
| Solid | 1 | |
| Maximum total score | 7 |
*A higher score is consistent with a better healing potential.
The surrounding skin was evaluated for maceration and signs of inflammation and wounds were documented using digital photography. Following initial wound assessment, wounds were assessed at each dressing change. Dressing changes were performed by wound specialists. Progress of wound healing was determined by measurement of wound area from tracings of the wound using transparencies and a marker pen. Wound area was calculated using the formula π/4 × maximum length × maximum width.
Patients' overall impression of dressing treatment
Patient involvement is a key aspect in choosing an appropriate dressing for wound management. Therefore, a simplified questionnaire was developed at the Charité‐Universitätsmedizin in Berlin for patients to comment on the treatment. At baseline, after 3 and 7 weeks of treatment, patients were invited to rate dressing comfort and their impression on treatment using a 6‐point scale from 1 (excellent), 2 (good), 3 (satisfying), 4 (sufficient), 5 (problematic) to 6 (insufficient).
STATISTICAL ANALYSIS
Patients' code numbers including clinical data collected were used for statistical analysis with the statistical programme SPSS 16.0. Results are presented as mean ± SD for normal distribution and as median and range for abnormal distributed values. Chi‐square test was used for statistical evaluation of wound size reduction.
RESULTS
Patient characteristics
Fifty‐two patients (29 male and 23 female) were enrolled in this study. The median age of male patients was 59·8 years (range from 21 to 82 years) with a median body mass index (BMI) of 24·8 (range from 19·3 to 40·3). The median age of female patients was 54·0 years (range from 23 to 83 years) with a median BMI of 30·1 (range from 18·7 to 41·4). Wounds comprised 37 surgical procedures, 8 chronic mixed ulcer, 4 pressure sores, 1 diabetic foot ulcer, 1 venous leg ulcer and 1 mixed arterial/venous ulcer. Multiple comorbidities were found in 17 patients, whereas 11 patients were detected for COLD/smoker, 5 patients suffered diabetes mellitus, 2 patients were diagnosed with peripheral artery occlusive disease, 1 patient suffered from heart failure and 2 patients were diagnosed with kidney and liver malfunction. Treated wounds were localised on abdomen (n = 26), left foot (n = 10), right foot (n = 5), flank (n = 1) and other localisations (n = 10).
Wound infection and dressing treatment regime
At baseline, 12 wounds were classified as stage 3 (showing signs of local infection and high exudate levels), 38 wounds were classified as stage 2 (increasing signs of infection, were colonised by bacteria, had low or moderate exudate levels) and 2 wounds were classified as stage 1 (subtle signs of infection, no exudate). All wounds were cleaned from debris before dressing application, 14 wounds received NU‐GEL Hydrogel to debride and 38 wounds underwent surgical sharp debridement.
Treatment regime was dictated by the results of initial wound assessment and the classification of infection status; 44 wounds were classified as stages 3 and 2, these were treated with SILVERCEL® and TIELLE® for the first 4–5 weeks and then with PROMOGRAN PRISMA® and TIELLE® for the following 2–3 weeks. The remaining eight wounds classified as stage 1 were treated with PROMOGRAN PRISMA® and TIELLE® for the duration of the study (7 weeks). Dressing changes were mainly performed by wound care specialists.
Wound healing
All patients showed a positive clinical response to the sequential use concept (5) of wound treatment. Weekly results of median and range values of wound area during the treatment time of 7 weeks are summarised in Figure 1. A clear trend towards rapid healing and significant reduction of wound size was observed shortly after initiating treatment and during the treatment time of 7 weeks. Statistical analysis of wound size results showed a highly significant progression of wound healing expressed with a profound reduction of wound surface (P in all measurements <0·001, chi‐square test).
Figure 1.
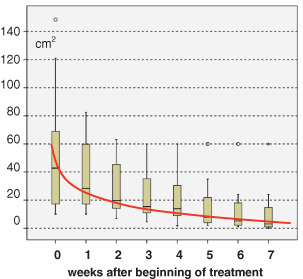
Significant reduction (P < 0·001, chi‐square test) in wound area (median and range values) during treatment time (7 weeks).
Wound score
The wound score (14) as assessed in this case‐cohort‐type observational study can range between 0 (poor healing potential) and 7 (maximum healing potential). Results of wound scoring indirectly describe the effectiveness of the sequential use treatment concept.
Weekly results of median and range wound score values are summarised in Figure 2. With progress of healing and reduction of wound area, a clear trend towards improvement of quality of new tissue can be observed with 50% of patients showing a wound score of 6, 5 weeks after beginning of wound treatment.
Figure 2.
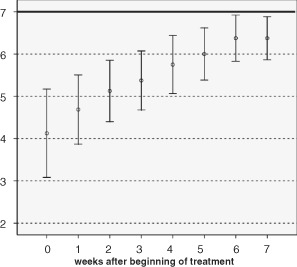
Clear trend towards improvement of healing and improved quality of tissue; median and range wound score values presented during treatment time of 7 weeks.
Patients' evaluation
Patients' overall impression of treatment received was assessed mainly by evaluating the dressings used to treat wounds before, during and after treatment following the sequential use concept. Considering that most patients were not able to answer a more complicated document such as the SF‐36 questionnaire generally used to document quality of life aspects. The scoring system used for this evaluation was feasible and easy to handle in daily clinical practice. Results of the evaluation are summarised in Figure 3. At the start of the study, treatment was evaluated by patients between ‘satisfying’ (school grade 3) and ‘sufficient’ (school grade 4), after 3 weeks between ‘satisfying’ and ‘good’ (mean 2·75; SD 0·2), and with ‘satisfying’ and ‘good’ (mean 2·5; SD 0·1) after 7 weeks of treatment (nearly at wound closure).
Figure 3.
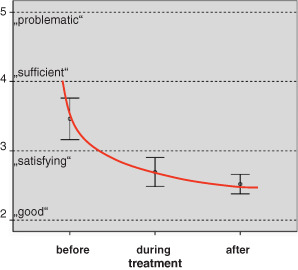
Patients' overall impression of treatment.
DISCUSSION
Chronic wounds and wound healing by secondary intention represent a major burden to patients, health care professionals and the health care system. Special wound treatment is required for these wounds and an accurate diagnosis of wounds is essential for selection of appropriate dressings to be used. Modern wound dressings can influence and accelerate healing and contribute to the improvement of patients' quality of life. However, there is no one dressing that possesses all the ideal properties of a modern wound dressing 14, 15. There is an increasing number of dressings available in the market and choosing the appropriate dressing should be based on scientific evidence. Selection of appropriate dressings should also be based on the type of wound, healing status, bioburden present and amount of exudation. This study evaluated the applicability of an uncomplex sequential use concept for treatment of most wound healing by secondary intention. Evaluation of applicability of the concept at three different Departments of Surgery of the Charité‐Universitätsmedizin in Berlin implied that current knowledge of wound healing and existing experience in wound treatment derived from daily clinical practice of clinicians and clinical staff involved in this project was considered. At the beginning of this study, more than 150 dressings for moist wound treatment (various sizes of products included) were listed since 2006 in all departments of the Charité‐Universitätsmedizin in Berlin. As a result, dressings were chosen on the basis of local practice and personal empirical experience. The authors decided to apply the sequential use concept in daily clinical practice to evaluate the results derived from the use of three products for the treatment of most chronic wounds and wound healing by secondary intention. Extensive wound debridement before first dressing application, control of systemic and local infection and a general treatment of the comorbidities were basic components of the approach. A weekly interdisciplinary meeting including a profound discussion on wound status and healing progress was implemented in the treatment routine to improve standards and patients' quality of life. The authors decided to follow the approach that the first wound assessment was performed by the treating consultant, preferably a wound specialist, and continued treatment by a wound specialist. At the Charité‐Universitätsmedizin hospital, wound specialists are certified and responsible for the treatment (and stoma supply) of all chronic wounds or difficult to heal wounds. Outcomes of the treatment were evaluated weekly or immediately if complications were noticed.
The standardised documentation used in this study for collecting data on healing progress was shown to be easy to follow and a practical instrument for an objective evaluation of the healing process as well as for the evaluation of the patients' quality of life. The authors learned from daily clinical practice that the application of modern dressings, as the ones we used in this study, should preferably be performed by experienced professionals. In general and compared with past experiences, wounds had to be re‐dressed at longer intervals, because of the high absorption capacity of dressings used allowing them to be left in place longer. However, the time needed for dressing change was relatively long with approximately 17 minutes (mean time) for each change. This could be mainly attributed to the standard procedure of dressing changes that includes dressing removal, wound inspection and description of the wound, cleaning debris, disinfection, dressing preparation, sodium chloride application if necessary and application of the secondary dressing.
In our evaluation, we observed that nearly 80% of the surgical wounds treated with the sequential use concept healed within 7 weeks of treatment. Results of wound size reduction corresponded with results derived from use of the wound score (14) to evaluate progress of healing. In the first step of the treatment and after debridement, wound bed of wounds showing local signs of infection were cleaned using a silver dressing. Our results do not support the conclusions of a Cochrane Review on silver dressings and insufficient evidence for their use in infected or contaminated chronic wounds (16). However, it has to be considered that nearly half of the patients treated had severe comorbidities and are therefore not comparable to older multimorbid patients with chronic wounds (e.g. diabetic foot ulcers) with wound duration of more than 6 months.
Although the protocol followed was standardised, this study has some limitations. The majority of wounds treated were surgical wounds and were patients who had a long hospital stay as a result of patients' underlying disease and the complications occurring. The hospital stay is subject to many factors in Germany with a health care system that discourages outpatient treatment and early discharge after surgical debridement in different ways: financial disincentives (decreasing reimbursement by 800 to 900 €/day if patients are discharged before the day advised), fear of legal practice suits if complications occur after discharge and readmission rate as quality of care marker.
In this study, aspects of patients' quality of life were considered by evaluating patients' overall impression of the treatment received in using a simplified questionnaire developed at the Charité‐Universitätsmedizin in Berlin, meaning that the evaluation was not as broad as using formal questionnaires like the SF‐36 format. However, the authors decided to stick to feasible methods and in developing simple questions to address social, financial and physical parameters of patients' treated, to reflecte the reality of daily clinical practice at the Charité‐Universitätsmedizin in Berlin.
CONCLUSION
This study summarises the clinical experiences at three different Surgical Departments at the Charité‐Universitätsmedizin in Berlin in evaluating the sequential use concept (5). The results show that this is an easy to use concept that is applicable for the treatment of most wound healing by secondary intention and also simplifies the decision process to choose the right dressing for covering the wound at different stages of healing. Clinicians and staff involved in this study decided to implement the sequential use concept in daily clinical practice based on the clinical results for wound healing and on the reduced number of dressings held by the hospitals.
ACKNOWLEDGEMENTS
We thank Christine Smoliner for data collection and organisation of study meetings. Additional support was provided by Birgit Klatt, Guenter Gerig (both Campus Mitte) and Uwe Balzke (Campus Virchow Klinikum) – wound care specialists of the Charité‐Universitätsmedizin in Berlin. We also thank PD Dr Infanger (Campus Mitte and Benjamin Franklin) for his support. Our best thanks are given to Dr C. Welling for her continuous and constructive encouragement. This study was funded by Systagenix Wound Management, Norderstedt, Germany, formerly Johnson & Johnson Wound Management.
REFERENCES
- 1. Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Rep Reg 1996;4:411–20. [DOI] [PubMed] [Google Scholar]
- 2. Thomas S. Wound management and dressings. London: The Pharmaceutical Press, 1990. [Google Scholar]
- 3. Schultz GS, Sibbald GR, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Rep Reg 2003;11:1–28. [DOI] [PubMed] [Google Scholar]
- 4. Gray D, Cooper P, White R, Kingsley A. Applied wound management: a clinical decision making framework. Supplement Wounds UK 2007.
- 5. Cullen B, Ivins N. Promogran™ & Promogran Prisma™ made easy. Wounds Int 2010;1. URL http://www.woundsinternational.com [Google Scholar]
- 6. Vowden P, Cooper RA. An integrated approach to managing wound infection. In: European Wound Management Association (EWMA). Position document: management of wound infection. London: MEP Ltd, 2006. [Google Scholar]
- 7. Addison D, Rennison T, Del Bono M, Stephens S, Boothman S. Evaluation of the antimicrobial properties silver release profile & absorbency characteristics of an antimicrobial silver hydroalginate wound dressing. Poster presentation EWMA 2005.
- 8. Addison D, Rennison T, Del Bono M, Steadman M, Stephens S. An antimicrobial silver alginate dressing for the management of chronic wounds. Poster Presentation, SAWC, San Antonio, 2006.
- 9. Teot L, Maggio G, Barrett S. The management of wounds using SILVERCEL hydroalginate. Wounds UK 2005;1:70–7. [Google Scholar]
- 10. Meaume S, Vallet D. Evaluation of a silver‐releasing hydroalginate dressing in chronic wounds with signs of local infection. J Wound Care 2005;14:411–9. [DOI] [PubMed] [Google Scholar]
- 11. Diehm C, Lawall H. Evaluation of Tielle hydropolymer dressings in the management of chronic exuding wounds in primary care. Int Wound J 2005;2:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cullen B, Smith R, McCulloch E, Silcock D, Morrison L. Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Rep Reg 2002;10:16–25. [DOI] [PubMed] [Google Scholar]
- 13. Wollina U, Schmidt WD, Krönert C, Nelskamp C, Scheibe A, Fassler D. Some effects of a topical collagen‐based matrix on the microcirculation and wound healing in patients with chronic venous leg ulcers. Preliminary observations. Low Extrem Wounds 2005;4:214–24. [DOI] [PubMed] [Google Scholar]
- 14. Schmidt W‐D, Liebold K, Faßler D, Wollina U. Contact‐free remission spectroscopy of leg ulcers: principle, technique, and calculation of spectroscopic wound scores. J Invest Dermatol 2001;116:531–5. [DOI] [PubMed] [Google Scholar]
- 15. Vermeulen H, van Hattem JM, Storm‐Versloot MN, Ubbink DT. Topical silver for treating infected wounds. Cochrane Database Syst Rev 2007, Issue 1. Art. No.: CD005486, doi: 10.1002/14651858.CD 005486.pub2. [DOI] [PubMed] [Google Scholar]
- 16. Lawrence JC. What materials for dressings? Injury 1982;13:500–512. [DOI] [PubMed] [Google Scholar]


