Abstract
Marjolin's ulcer refers to malignant degeneration in a chronic wound. Although originally described in an area of burns scar, many other chronic wounds such as osteomyelitis sinus tracts, venous stasis ulcers and chronic pressure sores have the potential to undergo malignant transformation. We present an interesting case of malignant degeneration in a male paraplegic patient with chronic sacral and ischial pressure sores. By discussing our radical surgical solution to this problem, we aim to highlight the importance of prompt diagnosis.
Keywords: Fillet flap, Marjolin, Paraplegia, Ulcer
INTRODUCTION
Marjolin's ulcer classically refers to squamous cell carcinoma (SCC) arising in an area of burns scar, although, nowadays, the term is used to describe malignant degeneration in any chronic wound. Wounds such as chronic osteomyelitis sinus tracts, venous stasis ulcers and pressure sores all have the potential to undergo malignant change.
Pressure sores are a common problem plaguing the elderly and immobile. Sustained pressure, shear or friction leads to tissue microvascular occlusion, ischaemia and necrosis. Once formed, they are resistant to intervention and often reflect a steady decline in a patient's condition. It is the aim of this report to bring attention to malignant degeneration in the chronic pressure sore, particularly in the younger patient suffering spinal cord injury. We highlight how important it is for these patients and those responsible for their care to be aware of the signs suggesting malignant change and the importance of biopsy not only at these times but also at regular intervals during the life cycle of any chronic wound.
CASE REPORT
A 41‐year‐old paraplegic male was admitted with increasing pain and infective complications of extensive chronic sacral and ischial pressure sores. His paraplegia was a result of a motorcycle accident at the age of 15. Examination showed multiple areas of pressure necrosis over the sacrum, buttocks and perineum, surrounding erythema and an offensive smelling, purulent discharge. Groin examination showed palpable lymphadenopathy. The patient was pyrexial although all other observations were normal. A septic screen confirmed grossly elevated inflammatory markers and he was commenced on empirical antibiotics. Magnetic resonance imaging (MRI) suggested osteomyelitis of the left and right ischial tuberosity, sacrum and left femoral head. The patient was taken to theatre for debridement and groin node biopsy. Tissue samples confirmed widespread moderately differentiated SCC from the multiple areas of ulceration. Lymph node biopsy showed no evidence of metastases. Staging computed tomography showed no evidence of pelvic or distal disease. Furthermore, MRI showed peri‐rectal inflammation raising the concern of tumour involvement.
Following multiple discussions with the patient, the decision was made to attempt curative resection and reconstruction under the combined care of orthopaedics, colorectal and plastic surgery. The patient was extremely malnourished with a pre‐operative weight of just 36 kg (normal weight 45 kg). The patient was taken to theatre for abdomino‐perineal resection and colostomy, radical excision of sacral and ischial pressure sore SCC, debridement of osteomyelitic left hemi‐pelvis, disarticulation and excision of left femoral head, femur, tibia and fibula, and complete left lower limb myocutaneous fillet flap reconstruction, pedicled on the femoral vessels. The operation was technically successful. The flap remained in good health for the remainder of the patient's admission and he was discharged to a rehabilitation centre after approximately 4 months in hospital.
Histology confirmed a multi‐focal, moderately differentiated SCC with a highly infiltrative growth pattern and evidence of perineural and perivascular invasion. There was no involvement of the anal canal or rectum. Two lymph nodes isolated from mesorectal tissue showed reactive changes only. Deep and peripheral margins were clear. The decalcified specimen confirmed no bony involvement.
DISCUSSION
The development of SCC in pressure sores is uncommon with one study quoting an incidence of 0·5% (1). Although falling under the umbrella term of Marjolin's ulcer, some consider pressure sore carcinoma as a separate, more aggressive entity (2). The time taken for malignant transformation can be in the region of 30 years although reports exist in the literature of accelerated transformation (3). Our patient had suffered from pressure sores for approximately 10 years before the diagnosis of carcinoma. The appearance of a mass, onset of pain and change in the character of discharge can signal malignant degeneration (4). Increasing pain and acute infection resulted in the admission of our patient and also prompted tissue biopsy during initial debridement. This is the major learning point of this report.
Several theories exist to explain the formation of Marjolin's ulcers. Chronic irritation and repeated attempts at healing provide a prolonged stimulus for cellular proliferation and may increase the rate of spontaneous mutations. Toxins released by necrotic tissue may have a directly mutagenic effect on cells (5). Mutations in genes responsible for cell division and apoptosis result in increased rates of cancer and not surprisingly, these mutations have been showed in patients with Marjolin's ulcers 6, 7. The cocarcinogen theory states that the physical or chemical insult causing the original wound is not in itself carcinogenic but it may cause pre‐existing neoplastic cells to become activated or more susceptible to other well‐known carcinogenic factors (8). Areas of chronic scar tissue may become deplete of immunological cells normally resident in the skin. As a result, tumours can evade immuno‐detection, increasing their aggressiveness and ability to metastasise (9). It is likely that the pathogenesis is multi‐factorial having both environmental and genetic contributions.
Management of Marjolin's ulcers consists mainly of radical excision. Sentinel lymph node (SLN) biopsy can have a useful role in clinically node negative patients (10). Our patient had bilateral palpable groin nodes, which were sampled before definitive reconstruction and therefore SLN biopsy was not performed. Adjuvant radiotherapy and chemotherapy can be useful in those with inoperable metastatic disease.
Large sacral and ischial pressure sores often have underlying osteomyelitis and require the excision of large volumes of soft tissue and bone. This situation is exacerbated when malignant transformation has occurred. Residual cavities are often too great to be reconstructed with conventional soft tissue flaps. The ‘three muscle flap’ using the thigh musculature has been widely described (11). The total thigh flap with lower leg amputation has also been described (12). Preservation of the lower leg can be useful for future reconstruction although tissue demands often prevent this. Disused atrophy in paraplegia can limit tissue availability and in this case necessitated the use of the entire limb.
The first fillet flap was performed in the 1950s and its application to pressure sores was described in 1961 (13). The entire leg can be used when necessary and these flaps have been described as both pedicled and free 14, 15. Absolute contra‐indications include tumour extension into the limb and the absence of suitable vessels. Relative contra‐indications include infection and lymphoedema. Although our patient had previous surgery around the posterior aspect of the proximal thigh, the femoral vessels were intact. Ulceration was diffuse and extended over a significant portion of the buttocks (Figure 1). With exception of the discarded foot, we used a pedicled musculocutaneous flap of the entire leg. The lower leg was entirely de‐epithelialised and used to fill the pelvic cavity preventing herniation of abdominal viscera and obviating the need for additional synthetic support. The postoperative result is shown in Figure 2.
Figure 1.
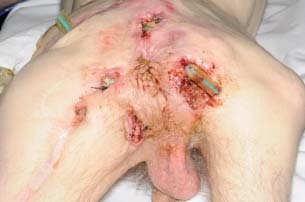
Widespread ischial and sacral pressure sores with malignant degeneration.
Figure 2.
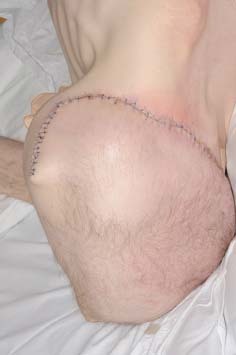
Postoperative result following radical excision and complete left lower limb pedicled, myocutaneous fillet flap reconstruction.
Other reported alternatives in extensive cases include hemi‐corporectomy. First performed in the 1960s, these procedures are associated with huge postoperative physiological changes and have a high mortality. In selected cases, it can offer the only prospect of long‐term disease control (16).
The prognosis of Marjolin's ulcer and specifically malignancy arising in a chronic pressure sore is poor. This is a result of the insidious nature of malignant transformation, a lack of awareness and the resultant late diagnosis. Rates of metastases in pressure sore carcinoma have been reported as high as 61% (17). This is significantly greater than figures reported for Marjolin's ulcers originating in burns scar and ostemyelitis 18, 19. In one small series of patients with pressure sore carcinoma, 80% of patients died of recurrence within 18 months of resection and flap reconstruction (20). In another more recent series of patients with spinal cord injury, these aggressive tumours were uniformly fatal (21).
CONCLUSION
Malignant degeneration in a pressure sore is a particularly aggressive form of Marjolin's ulcer. Although surgery offers hope of curative resection, for many these operations are palliative. The alleviation of debilitation, depression and the social vulgarity associated with an extensive, offensive smelling wound can radically improve the patient's life. As the management of pressure sores predominantly occurs in the community, it is essential that the patient, their relatives, tissue viability nurses and primary care physicians are aware of the signs and symptoms consistent with carcinogenesis.
REFERENCES
- 1. Mustoe T, Upton J, Marcellino V, Tun CJ, Rossier AB, Hachend HJ. Carcinoma in Chronic pressure sores: a fulminant disease process. Plast Reconstr Surg 1986;77:116–21. [DOI] [PubMed] [Google Scholar]
- 2. Stancard CE, Cruse CW, Wells KE, Karl R. Chronic pressure ulcer carcinomas. Ann Plast Surg 1993;30:274–7. [DOI] [PubMed] [Google Scholar]
- 3. Thio D, Clarkson JH, Misra A, Srivastava S. Malignant change after 18 months in a lower limb ulcer: acute Marjolin's revisited. Br J Plast Surg 2003;56:825–8. [DOI] [PubMed] [Google Scholar]
- 4. Esther RJ, Lamps L, Schwartz HS. Marjolin ulcers: secondary carcinomas in chronic wounds. J South Orthop Assoc 1999;8:181–7. [PubMed] [Google Scholar]
- 5. Treves N, Pack GT. The development of cancer in burn scar: an analysis and report of thirty‐four cases. Surg Gynecol Obstet 1930;58:749–51. [Google Scholar]
- 6. Harland DL, Robinson WA, Franklin WA. Deletion of the p53 gene in a patient with aggressive burn scar carcinoma. J Trauma 1997;42:104–7. [DOI] [PubMed] [Google Scholar]
- 7. Lee SH, Shin MS, Kim HS. Somatic mutations of Fas (Apo‐1/CD95) gene in cutaneous cell carcinomas arising from a burn scar. J Invest Dermatol 1999;114:122–6. [DOI] [PubMed] [Google Scholar]
- 8. Konigova R, Rychterova V. Marjolin's ulcer. Acta Chir Plast 2000;42:91–4. [PubMed] [Google Scholar]
- 9. Bostwick J 3rd, Pendergrast WJ Jr, Vasconez LO. Marjolin's ulcer: an immunologically privileged tumor? Plast Reconstr Surg 1976;57:66–9. [PubMed] [Google Scholar]
- 10. Eastman AL, Erdman WA, Lindberg GM, Hunt JL, Purdus GF, Fleming JB. Sentinel lymph node biopsy identifies occult nodal metastases in patients with Marjolin's ulcer. J Burn Care Rehabil 2004;25:241–5. [DOI] [PubMed] [Google Scholar]
- 11. Acartürk TO. Treatment of large ischial ulcers communicating with the hip joint with proximal femoral resection and reconstruction with a combined vastus lateralis, vastus intermedius and rectus femoris musculocutaneous flap. J Plast, Reconstr Aesthet Surg 2009;62:1497–502. [DOI] [PubMed] [Google Scholar]
- 12. Berger SR, Rubayi S, Griffin AC. Closure of multiple pressure sores with split total thigh flap. Ann Plast Surg 1994;33:548–51. [DOI] [PubMed] [Google Scholar]
- 13. Berkas EM, Chesler MD, Sako Y. Multiple decubitus ulcer treatment by hip disarticulation and soft tissue flaps from the lower limbs. Plast Reconst Surg 1961;27:618–9. [Google Scholar]
- 14. Butler C. Reconstruction of an extensive hemipelvectomy defect using a pedicled upper and lower leg in‐continuity fillet flap. Plast Reconst Surg 2002;109:1060–5. [DOI] [PubMed] [Google Scholar]
- 15. Yamamoto Y, Minakawa H, Takeda N. Pelvic reconstruction with a free fillet lower leg flap. Plast Reconst Surg 1997;99:1439–41. [DOI] [PubMed] [Google Scholar]
- 16. Peterson R, Sardi A. Hemi‐corporectomy for chronic pressure ulcer carcinoma: 7 years of follow‐up. Am Surg 2004;70:507–11. [PubMed] [Google Scholar]
- 17. Tutela RR Jr, Granick M, Benevenia J. Marjolin's ulcer arising in a pressure ulcer. Adv Skin Wound Care 2004;17:462–7. [DOI] [PubMed] [Google Scholar]
- 18. Sabin SR, Goldstein G, Rosenthal HG, Haynes KK. Aggressive squamous cell carcinoma originating as a Marjolin's ulcer. Dermatol Surg 2004;30:229–30. [DOI] [PubMed] [Google Scholar]
- 19. Lifeso RM, Rooney RJ, El‐Shaker M. Post traumatic squamous cell carcinoma. J Bone Joint Surg 1990;72:12–8. [PubMed] [Google Scholar]
- 20. Grottig JC, Bunkis J, Vasconez LO. Pressure sore carcinoma. Ann Plast Surg 1987;18:527–32. [DOI] [PubMed] [Google Scholar]
- 21. Eltorai IM, Montroy RE, Kobayashi M, Jakowatz J, Guttierez P. Marjolin's ulcer in patients with spinal cord injury. J Spinal Cord Med 2002;25:191–6. [DOI] [PubMed] [Google Scholar]


