Abstract
The aim of this study was to determine specific pattern of port site microbial colonisation, sensitivity and resistance to different antibiotics of bacteria isolated from port site infection (PSI) in low risk patients after elective laparoscopic cholecystectomy in surgical wards at tertiary care hospital of Kashmir. This is a prospective study. The study included 675 consecutive patients of postoperative PSI after elective laparoscopic cholecystectomy for symptomatic cholelithiasis over a period of 12 months. Culture swabs were taken from port sites with signs of PSI and transported to the microbiology laboratory. The positive swab cultures were subjected to antibiotic susceptibility test. The data obtained was analysed by using appropriate statistical analytical tests. The incidence of PSI after elective laparoscopic cholecystectomy is 6·7%. The commonest organism responsible for PSI is pseudomonas, 19 (42·2%) cases. Most of the strains of organisms isolated were resistant to commonly used antibiotics in the hospital,pseudomonas was found 100% resistant to the combination of ampicillin + sulbactum and ceftriaxone and it was sensitive to imipenem, amikacin and vancomycin in 89·47,57 and 52·63% of cases respectively. Our study will be helpful in choosing effective empirical prophylactic antibiotic therapy in cases of elective laparoscopiccholecystectomy and will have a great impact on morbidity and mortality in them because of PSI.
Keywords: Antibiotic resistance, Antibiotic sensitivity, Culture medium, Port site infection
INTRODUCTION
Port site infection (PSI) may be of intrinsic or extrinsic sources. When systemic host resistance is lowered, such as immunosuppresion from medication, disruption of intact cutaneous or mucous membrane as a result of surgical procedures or trauma, patients' bacterial flora may become opportunistic and cause infection. Laparoscopic cholecystectomy has become the preferred method for performing gallbladder surgery in the present era because it is associated with shorter hospital stay and convalescence, less pain and scarring, and lower rates of postoperative surgical site infection (SSI) than open cholecystectomies 1, 2, 3, 4.
The fact that laparoscopic cholecystectomies are associated with fewer SSIs intuitively makes sense as laparoscopy access ports are short in length and only a fraction of the length of the incision used in open laparotomy. Elective laparoscopic cholecystectomy has a low risk for infection 1, 2, 3, 4, 5, but many surgeons still use prophylactic antibiotics as is used for elective open cholecystectomy 6, 7, 8. The rate of PSI and the use of prophylactic antibiotics in elective laparoscopic cholecystectomies may vary from centre to centre. Therefore, every centre should have own evidence basis for the use of prophylactic antibiotics in elective laparoscopic cholecystectomies through the research at their centre.
This study was designed to determine the port (incision) site infection rate and choose effective empirical prophylactic antibiotic therapy in cases of elective laparoscopic cholecystectomy.
PATIENTS AND METHODS
The study included 675 consecutive patients of postoperative PSI after elective laparoscopic cholecystectomy for symptomatic cholelithiasis in surgical units of this hospital over a period of 12 months from 1 April 2010 to 31March 2011 in the Department of Surgery, Government Medical College, in collaboration with the Department of Microbiology. In order to minimise the bias in our observations, the patients with immunosuppresion and patients with known malignancies, as the chances of PSI are more because of their immunocompromised state, and those with antibiotic prophylaxis with acute cholecystitis and PSI after 30 days were excluded from the study. The patients in their extremes of age were also excluded from the study. Only patients with euglycaemia were included in the study. All the patients were admitted 3 hours before the operation with 6 hours of fasting and had scrub bath without shaving the hair of the operative area. After surgery all the port sites were dressed with barrier dressings to prevent introduction of bacteria from the environment. Culture swabs were taken from port sites of all patients with signs of PSI under all aseptic precautions and transported to the microbiology laboratory and then systemic antibiotics were given. In the laboratory the swabs were processed as per the standard microbiological procedure and protocols. First the swabs were incubated at 35°C and observed for evidence of any growth in first 6–18 hours, swab subcultures were subjected to MacConky agar and then blood agar were made from all culture swabs and further incubated for 48 hours. Samples which did not show any growth at 48 hours of incubation were declared culture negative. The positive blood cultures were subjected to antibiotic susceptibility test. The data obtained were analysed by using appropriate statistical analytical tests (percentages and incidence).
RESULTS
In our study the incidence of PSI after elective laparoscopic cholecystectomy was 6·7% (Table 1). The PSI (1, 2) occurred in 45 (6·7%) patients (Table 1). The commonest organism responsible for PSI was Pseudomonas in 19 (42·2%) cases followed by Staphylococcus aureus in 18 (40%) cases and out of which methicillin‐resistant S. aureus (MRSA) in 13 (72·2% ) cases (Table 2). Most of the strains of organisms isolated were resistant to commonly used antibiotics in the hospital, pseudomonas was found 100% resistant to the combination of ampicillin + sulbactum and ceftriaxone and was sensitive to imipenem, amikacin and vancomycin in 89·47, 57 and 52·63% cases, respectively. MRSA was also found resistant to commonly used antibiotics such as ceftriaxone, ampicillin + sulbactum and ceftazidime + calvulanic acid. Linzolid and vancomycin were effective in 84 and 100% cases, respectively (Table 3).
Table 1.
Incidence of port site infection
| Port site infection (culture positive) | Total (%) |
|---|---|
| No | 630 (93·3) |
| Yes | 45 (6·7) |
Figure 1.
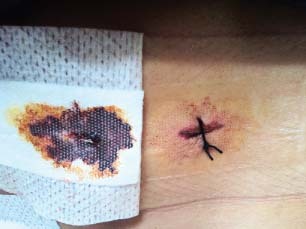
Epigastric port site infection with soakage.
Figure 2.
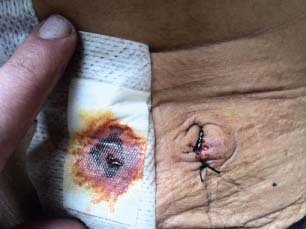
Umbilical port site infection with soakage.
Table 2.
Bacteriological profile of port site
| Microbe | No. of patients | Percent |
|---|---|---|
| Staphylococcus aureus | 5 | 11·1 |
| Pseudomonas aeruginosa | 19 | 42·2 |
| MRSA | 13 | 28·9 |
| Klebsiella species | 2 | 4·4 |
| Escherichia coli | 3 | 6·7 |
| Acinetobacter | 1 | 2·2 |
| Proteus | 2 | 4·4 |
| Total | 45 | 100 |
MRSA, methicillin‐resistant Staphylococcus aureus.
Table 3.
Antibiotic sensitivity of bacteria isolated from infected port site *
| Antibiotic | Staphylococcus aureus (n = 5) | Pseudomonas (n = 19) | MRSA (n = 13) | Escherichia coli (n = 3) | Klebsiella (n = 2) | Proteus (n = 2) | Acinetobacter (n = 1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S (%) | R (%) | S (%) | R (%) | S (%) | R (%) | S (%) | R (%) | S (%) | R (%) | S (%) | R (%) | S (%) | R (%) | |
| Ampicillin + sulbactum | 1 (20) | 4 (80) | 0 | 19 (100) | 0 | 13 (100) | 0 | 3 (100) | 0 | 2 (100) | 0 | 2 (100) | 0 | 1 (100) |
| Amoxiclav | 0 | 5 (100) | 15 (78·9) | 4 (21·1) | 3 (23·7) | 10 (76·92) | 1 (33·33) | 2 (66·66) | 0 | 2 (100) | 1 (50) | 1 (50) | 0 | 1 (100) |
| Ceftriaxone | 2 (40) | 3 (60) | 0 | 19 (100) | 2 (15·38) | 11 (84·61) | 2 (66·66) | 1 (33·33) | 0 | 2 (100) | X | x | X | X |
| Methicillin | 5 (100) | 0 | X | X | 0 | 13 (100) | X | X | X | X | X | x | X | X |
| Ceftazidime + clavulanic acid | 4 (80) | 1 (20) | 3 (15·78) | 16 (84·21) | 0 | 13 (100) | 2 (66·66) | 1 (33·33) | 1 (50) | 1 (50) | 1 (50) | 1 (50) | 0 | 1 (100) |
| Linzolid | 5 (100) | 0 | X | X | 11 (84·61) | 2 (15·38) | X | X | X | X | X | x | X | X |
| Vancomycin | 4 (80) | 1 (20) | 10 (52·63) | 9 (47·36) | 13 (100) | 0 | 1 (33·33) | 2 (66·66) | X | X | X | x | X | X |
| Gentamycin | 3 (60) | 2 (40) | 3 (15·78) | 16 (84·21) | 9 (69·23) | 4 (30·76) | 2 (66·66) | 1 (33·33) | 0 | 2 (100) | 1 (50) | 1 (50) | 1 (100) | 0 |
| Amikacin | 4 (80) | 1 (20) | 11 (57·89) | 8 (42·10) | 9 (69·23) | 4 (30·76) | 2 (66·66) | 1 (33·33) | 1 (50) | 1 (50) | 1 (50) | 1 (50) | X | X |
| Imipenem | 1 (20) | 4 (80) | 17 (89·47) | 2 (10·52) | X | X | 3 (100) | 0 | 2 (100) | 0 | 2 (100) | 0 | X | X |
| Ciprofloxacin | 3 (60) | 2 (40) | 12 (63·15) | 7 (36·84) | 4 (30·76) | 9 (69·23) | 2 (66·66) | 1 (33·33) | 1 (50) | 1 (50) | 1 (50) | 1 (50) | 1 (100) | 0 |
*x means sensitivity not checked.
DISCUSSION
The SSI may be at organ space or incision (port) site. The incision (port) site infection may be superficial involving skin and subcutaneous tissues or deep involving fascia and muscles. It is known fact that the rate of PSI after elective laparoscopic cholecystectomy is lower than the incision site infection after open elective cholecystectomy because laparoscopy access ports are shorter in length than an incision made for open cholecystectomy 1, 2, 3, 4.
The incidence, bacteriological profile and antibiotic sensitivity of the PSI after laparoscopic cholecystectomy in low risk patients have not been extensively studied so far.
The incidence of PSI (1, 2) after elective laparoscopic cholecystectomy observed in our study was 6·7% (Table 1). Shindholimath et al. (7) has also reported an almost similar incidence (6·3%) while Jan et al. (8), Den Hoed et al. (9), Zitser et al. (10) and Colizza et al. (11) reported the incidence of 5·07, 5·3, 2·3 and <2% respectively. The higher incidence of PSI in our study could be because of use of reusable trocars.
The commonest organism responsible for PSI in our study was Pseudomonas aeruginosa in 19 (42·2%) cases followed by S. aureus in 18 (40%) cases, out of which MRSA in 13 (72·2% ) cases (Table 2). These results suggest that most often PSI was of intrinsic source (P. aeruginosa) possibly because of spillage of bile and retrieval of gallbladder through the port site and extrinsic (S. aureus) probably because of reusable trocars. The surgical site infection was found to be most often because of P. aeruginosa when there is break in mucous membrane of a hollow viscus. S. aureus infection suggest intrinsic source from skin or nasal cavity and extrinsic source from reusable trocars which is also supported by the other study 9, 10, 11.
In our study most of the strains of organisms isolated were resistant to commonly used antibiotics in the hospital, pseudomonas was found 100% resistant to the combination of ampicillin + sulbactum and ceftriaxone and was sensitive to imipenem, amikacin and vancomycin in 89·47, 57 and 52·63% cases respectively. MRSA was also found resistant to the commonly used antibiotics such as ceftriaxone, ampicillin + sulbactum and ceftazidime + calvulanic acid. Linzolid and vancomycin were effective in 84 and 100% cases, respectively (Table 3). The antibiotic sensitivity and resistance profile observed in our study were almost similar to the observation of the studies performed by Niemogha et al. (9), Jan et al. (10) and Adegoke et al. (11).
The incidence of PSI is lower in low‐risk patients after elective laparoscopic cholecystectomy 1, 2, 3, 4, 5, 12, 13 but the observed slightly higher incidence in our study could be attributed to the lower ward and theatre hygiene, spillage of bile during the procedure and reuse of trocars. Therefore, this can be minimised by improving the ward and theatre hygiene, preventing the spillage of bile and use of disposable single‐use trocars. Although bile samples were not taken in our study, bile sample should be taken for culture and antibiotic sensitivity if there is any bile spillage during the surgery.
Although studies 12, 13 have shown that the antibiotic prophylaxis is not needed in low‐risk patients after elective laparoscopic cholecystectomy, we recommend that the use of antibiotic prophylaxis should be based on the prevalence of different bacteria and their antibiotic sensitivity and resistance pattern researched in each centre.
CONCLUSION
Hence, we conclude that our study will be helpful in choosing effective empirical prophylactic antibiotic therapy in cases of elective laparoscopic cholecystectomy and this will have a great impact on morbidity and mortality in them because of PSI.
REFERENCES
- 1. Legorreta AP, Silber JH, Costantino GN, Kobylinski RW, Zatz SL. Increased cholecystectomy rate after the introduction of laparoscopic cholecystectomy. JAMA 1993;270:1429–32. [PubMed] [Google Scholar]
- 2. Hendolin HI, Paakonen ME, Alhava EM, Tarvainen R, Kemppinen T, Lahtinen P. Laparoscopic or open cholecystectomy: a prospective randomized trial to compare postoperative pain, pulmonary function, and stress response. Eur J Surg 2000;166:394–9. [DOI] [PubMed] [Google Scholar]
- 3. Zacks SL, Sandler RS, Rutledge R, Brown RS Jr. A population‐based cohort study comparing laparoscopic cholecystectomy and open cholecystectomy. Am J Gastroenterol 2002;97: 334–40. [DOI] [PubMed] [Google Scholar]
- 4. Inoue H, Itoh K‐I, Hori H, Muraoka Y. The cosmetic benefit of three‐port laparoscopic cholecystectomy and umbilical trocar insertion. Dig Endosc 1994;6:49–51. [Google Scholar]
- 5. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections. Am J Infect Control 1988;85:818–27. [DOI] [PubMed] [Google Scholar]
- 6. Ulualp K, Condon RE. Antibiotic prophylaxis for scheduled operative procedures. Infect Dis Clin North Am 1992;6:613–25. [PubMed] [Google Scholar]
- 7. Kellum JM, Duma RJ, Gorbach SL, Sugermen HJ. Single‐dose antibiotic prophylaxis for biliary surgery. Arch Surg 1987;122:918–22. [DOI] [PubMed] [Google Scholar]
- 8. Meijer WS, Schmitz PIM, Jeekel J. Meta‐analysis of randomised, controlled clinical trials of antibiotic prophylaxis in biliary tract surgery. Br J Surg 1990;77:283–90. [DOI] [PubMed] [Google Scholar]
- 9. Niemogha M‐T, Atoyebi Oluwole A, Ogunsola Folashade T, Mofikoya Bolaji O, Egbuna Kathleen N, Emuveyan Ejiro E, Tolu O. Detecting nosocomial intrinsic infections through relating bacterial pathogens of incision. Sierra Leone J Biomed Res 2011;3:13–20. [Google Scholar]
- 10. Jan WA, Khan SM, Jehanzeb M, Muazzam. Surgical site infection and pattern of antibiotic use in a tertiary hospital in Peshawar. J Ayub Med Coll Abbottabad 2010;22:141–5. [PubMed] [Google Scholar]
- 11. Adegoke AA, Tom M, Okoh AI, Jacob S. Studies on multiple antibiotic resistant bacterial isolated from surgical site infection. Sci Res Essays 2010;5:3876–81. [Google Scholar]
- 12. Higgins A, London J, Charland S, Ratzer E, Clark J, Haun W, Maher DP. Prophylactic antibiotics for elective laparoscopic cholecystectomy ‐ are they necessary? Arch Surg 1999;134:611–4. [DOI] [PubMed] [Google Scholar]
- 13. Mahmoud SA, Khafagy WW, Omar W, Atia S, El‐Nashar NM. Antibiotic prophylaxis in elective laparoscopic cholecystectomy: a prospective study. Egypt J Surg 2005;24:145–51. [Google Scholar]


