Dear Editors,
Cervical collars are necessary to immobilise the cervical spine of patients with multiple trauma 1, 2. This devise remains in situ until the radiographic cervical spine clearance is completed 2. A 29‐year‐old male with multiple trauma and subdural haemorrhage admitted to the intensive care unit (ICU) was selected for this case study. The patient had been taken to hospital by emergency medical service (EMS) following an accident. He was fitted with a Philadelphia rigid collar at the site of the accident by EMS technicians. After admission to the hospital and assessment by emergency physicians, neurosurgeons and orthopaedic surgeons, he was transferred to the ICU. Five days later, the spinal clearance process was completed. On day 6 after ICU admission, physicians ordered removal of the cervical collar. After removal of the collar by critical care nurses, two stage IV pressure ulcers were observed. One ulcer developed in his occiput and the other on his chin (Figures 1 and 2). Physicians assessed the ulcers and confirmed that they were caused by the pressure of the cervical collar.
Figure 1.
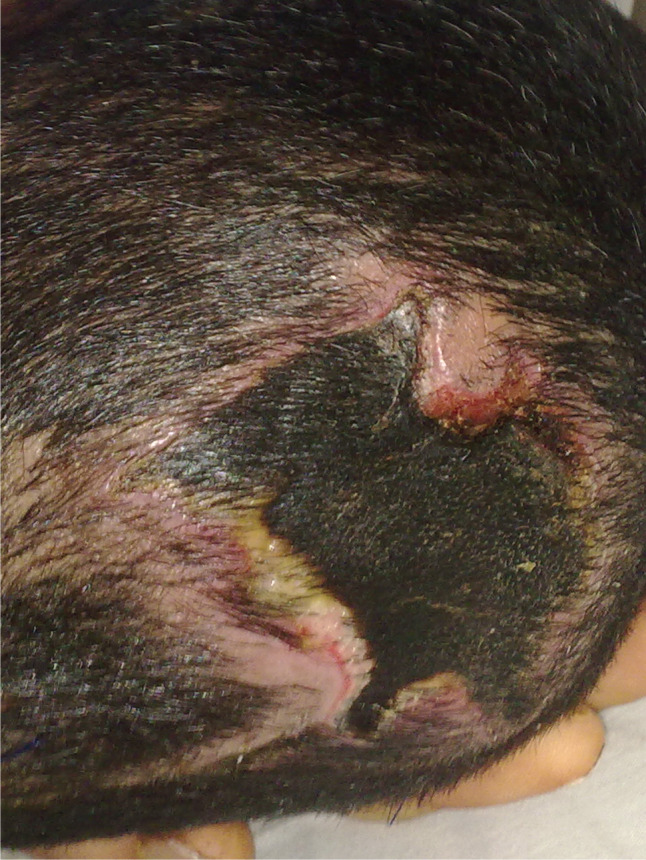
Ulcer in the occiput.
Figure 2.
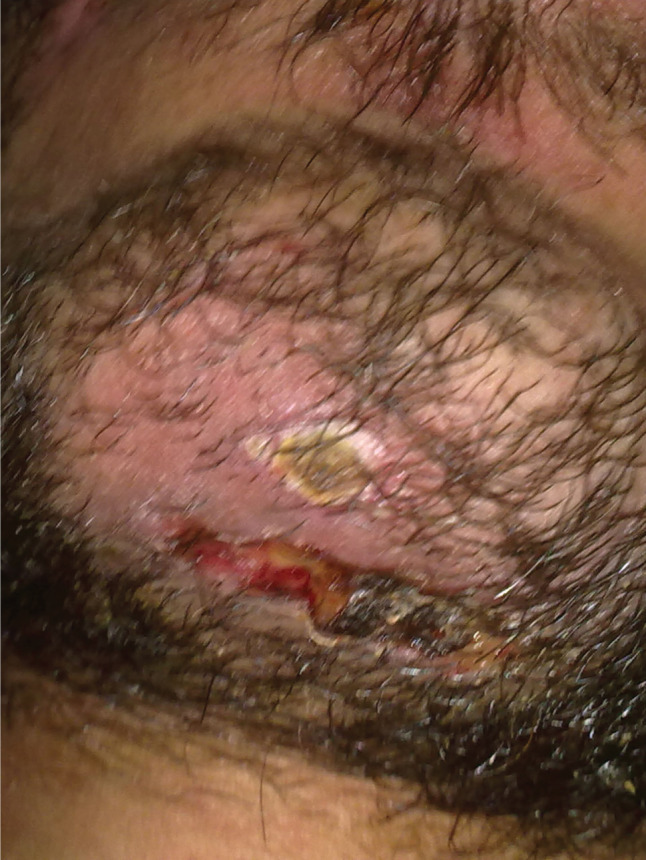
Ulcer on the chin.
Risk of pressure ulcer development increased with the use of medical devices 3, 4, 5, 6. This risk is higher among critically ill patients because this group of patients requires more medical devices for monitoring and therapeutic purposes 4, 7. Coyer et al. reported that the prevalence of medical device‐related pressure ulcer was 3·1% in ICU patients 8. The present case shows that skin may be affected by cervical collar, and as a result pressure ulcer may develop. Ackland et al. determined four risk factors for pressure ulcer development during cervical collar use, which include ICU admission, mechanical ventilation, the necessity for cervical magnetic resonance imaging and the time to cervical spine clearance and collar removal 2. For the prevention of medical device‐related pressure ulcers, the National Pressure Ulcer Advisory Panel (NPUAP) has identified seven recommendations that include: (i) choose the correct size of medical devices to fit the individual; (ii) cushion and protect the skin with dressings in high‐risk areas; (iii) remove or move the device daily to assess skin under device; (iv) avoid placement of device over area of prior or existing pressure ulceration; (v) educate staff on correct use of devices and prevention of skin breakdown; (vi) be aware of oedema under device(s) and potential for skin breakdown; and (vii) confirm that devices are not placed directly under an individual who is bedridden or immobile 9. These recommendations should be considered by health care members, especially nurses who care for critically ill patients.
Abbas Abdoli Tafti, MD1, Sanaz Sajadi, MD2 & Hossein Rafiei, MSc3
1Orthopedic Department
Kashani Hospital, Shahrekord University of Medical Sciences
Shahrekord, Iran
2Emergency Department
Kashani Hospital, Shahrekord University of Medical Sciences
Shahrekord, Iran
3Department of Intensive and Critical Care
School of Nursing and Midwifery, Shahrekord University of Medical Sciences
Shahrekord, Iran
hosseinrafiei21@yahoo.com
References
- 1. Jacobson TM, Tescher AN, Miers AG, Downer L. Improving practice: efforts to reduce occipital pressure ulcers. J Nurs Care Qual 2008;23:283–8. [DOI] [PubMed] [Google Scholar]
- 2. Ackland HM, Cooper DJ, Malham GM, Kossmann T. Factors predicting cervical collar‐related decubitus ulceration in major trauma patients. Spine 2007;32:423–8. [DOI] [PubMed] [Google Scholar]
- 3. Walker J. Pressure ulcers in cervical spine immobilization: a retrospective analysis. J Wound Care 2012;21:323–6. [DOI] [PubMed] [Google Scholar]
- 4. Iranmanesh S, Rafiei H, Esmaeili Abdar M. A case of pressure ulcer development on a patient's ear as a result of pulse oximetry probe. Int Wound J 2012;9:701–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Black JM, Cuddigan JE, Walko MA, Didier LA, Lander MJ, Kelpe MR. Medical device related pressure ulcers in hospitalized patients. Int Wound J 2010;7:358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Webber‐Jones JE, Thomas CA, Bordeaux RE Jr. The management and prevention of rigid cervical collar complications. Orthop Nurs 2002;21:19–25. [DOI] [PubMed] [Google Scholar]
- 7. Black J, Alves P, Brindle CT, Dealey C, Santamaria N, Call E, Clark M. Use of wound dressings to enhance prevention of pressure ulcers caused by medical devices. Int Wound J 2013. DOI: 10.1111/iwj.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coyer FM, Stotts NA, Blackman VS. A prospective window into medical device‐related pressure ulcers in intensive care. Int Wound J 2013. DOI: 10.1111/iwj.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. http://www.npuap.org/resources/educational‐and‐clinical‐resources/best‐practices‐for‐prevention‐of‐medical‐device‐related‐pressure‐ulcers/


