Abstract
Wound dehiscence is a surgical complication caused by the application of opposing and distracting forces tending to pull apart the suture line. In recent years, a novel negative pressure surgical management system has been developed to prevent surgical wound complications. This system creates a closed environment that removes exudates and other potentially infectious material, protects the surgical site from external contamination, provides support in holding the edges of the incision together and promotes wound healing. In this study, we describe our first experience with Prevena™, a closed incision negative pressure management system used on suture line following wide pathological scars excision for the prevention of postoperative wound dehiscence. Eight patients with wide and mature pathological skin scars were treated with Prevena™. The device was positioned directly after surgical correction for 8 days with a continuous application of −125 mmHg negative pressure. All treated patients had no postoperative surgical wound dehiscence. In one case, a limit of the device was represented by its poor adherence on hairy surface, hampering the maintenance of an appropriate local negative pressure. In another case, suture line was longer than Prevena™ foam and it was covered partially. Prevena™ system appears to be safe, easy to use and may represent a support technique to wide pathological skin scars surgical correction.
Keywords: Negative pressure wound therapy, Prevena, Scar, Wound healing
Introduction
Reconstructive approach after scar excision includes grafting with split‐ or full‐thickness skin, local or distant flaps or, simply, direct suture of the edges, depending on the surface of the excised area 1, 2, 3. However, surgical excision and direct suture is the usual treatment of pathological scars 3. This surgical procedure can be complicated by wound dehiscence caused by the application of opposing and distracting forces (muscle pull, elastic forces of adjacent skin or external and internal pressure) tending to pull apart the suture line 4. Tension‐free skin closure represents the best option to avoid complications 5, 6, 7 but it is not always possible as it depends on anatomic site or width of the corrected scar.
Different surgical approaches have been tested to reduce wound dehiscence, such as reverse tissue expansion by liposuction 8, keeping a base of scar tissue and suture of the wound edges over the base (Millard technique) 9 and tissue expansion as well as serial excision 10, providing good results.
However, no study has focused on the use of negative pressure surgical management (NPSM) to prevent wound dehiscence after scar excision and direct closure.
On the basis of the worldwide positive experience with negative pressure wound therapy (NPWT) for the management of soft tissue defects, some authors have already underlined the beneficial effects of NPWT for the management of postoperative incision wounds 11, 12, 13, 14, 15.
The benefits of the negative pressure include maintenance of a closed wound environment, reduction of oedema, increased perfusion, removal of exudates and splinting at the incision line 14, 16.
In this study, we describe our first experience with Prevena™ (Kinetic Concepts Inc, San Antonio, TX), a NPSM system, on the suture line after wide pathological scars excision. Our aim was testing safety, intraoperative application and postoperative management of the device.
Patients and methods
Between January 2010 and December 2011, eight patients (six females and two males) affected by large pathological scars were treated with Prevena™. Mean patient age was 33 years (range 20–60 years). All patients signed a written informed consent before surgery.
Treated areas were mature, not active, usually sequelae of hypertrophic scars, with an average size of 12·7 (length) × 3·1 cm (width) and ranges from 8 × 2 cm to 19 × 3 cm. They were located on body areas determining skin stretch during the flexion/extension movements: on the thigh in three patients, on the forearm in two patients, on the leg, the knee and the lower abdomen, respectively, in three different patients (Table 1).
Table 1.
Patients characteristics
| Age (years) | Sex | Scar dimension (cm) | Scar site | Surgery | Complications | |
|---|---|---|---|---|---|---|
| 1 | 20 | F | 10 × 5 | 1/3 proximal leg | SE + SR + DC | None |
| 2 | 25 | F | 13 × 3 | Anterior 1/3 middle forearm | SR + DC | None |
| 3 | 28 | F | 10 × 2 | Posterior 1/3 middle forearm | SR + DC | None |
| 4 | 40 | M | 18 × 2 | Lateral surface thigh | SR + DC | None |
| 5 | 25 | M | 19 × 3 | Knee | SR + DC | None |
| 6 | 38 | F | 12 × 2 | Lower abdomen | SR + DC | Poor adherence of system (hairy area) |
| 7 | 60 | F | 8 × 2 | Posterior 1/3 middle thigh | SR + DC | None |
SE, skin expansion; SR, scar revision; DS, direct suture.
Prevena™ is a battery‐powered device that consists of a suction unit with a 45‐ml canister for fluid collection, a tube and a sponge dressing with non adherent layer and a semipermeable adhesive drape. The system also has a bactericidal silver (0·0019% ionic silver) interface between sponge and skin (to prevent possible bacterial growth in the dressing), a small carrying case to minimise effects on mobilisation and a pressure indicator manufactured into the dressing that gives a visual clue to (in)sufficient negative pressure.
All surgical procedures were performed by the same surgeon.
After scar excision, direct skin closure was obtained by means of a two‐layer multifilament absorbable suture and a non absorbable monofilament intradermic suture in all eight patients (Figure 1A–C).
Figure 1.
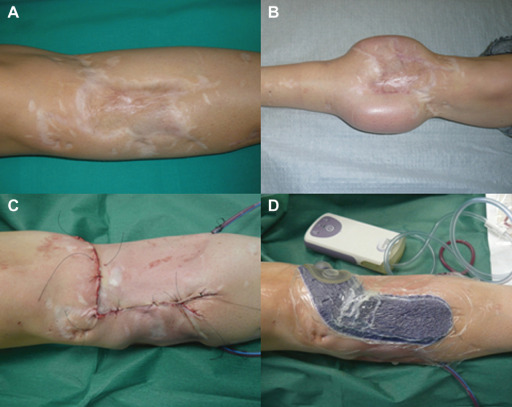
Case 1: (A) Preoperative scar, (B) soft tissue expansion, (C) linear suture after scar excision and (D) Prevena™ application.
In two cases, scar excision followed the expansion of local adjacent skin (Figure 1B). Final suture technique after skin expander removal was the same.
Wound closure was followed by the activation of the device. Then Prevena™ foam was placed over the suture at the end of the surgical procedure and maintained for 8 days with a pre‐set continuous pressure of −125 mmHg (Figure 1D). According to the manufacturer, in order to get maximum benefit, Prevena™ was applied on a clean and dried incision site to ensure proper fixation of the adhesive drape.
Suction drain was positioned outside the field of adherence of the Prevena™ system in all patients and they were discharged the day after surgery.
Device management was totally home care providing a good compliance of patients.
Results
Seven patients had successfully completed the treatment.
In one patient, where scar was located nearby pubic hairy body area, the Prevena™ system did not show a good skin adherence, despite shaving, because of the failed air‐proof closure necessary to maintain an appropriate local negative pressure. The patient stopped the treatment after 1 day.
In the other seven cases, no complications occurred during 8 days therapy. Removal of the dressing showed a suture with a firm apposition of wound edges without infection signs (Figure 2A). The canister never contained exudates or liquids while the foam showed good absorption property (Figure 3).
Figure 2.
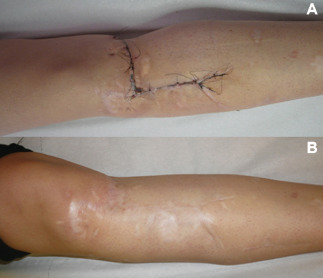
Case 1: (A) Scar at Prevena™ removal – 8th day and (B) scar at 12‐month follow‐up.
Figure 3.
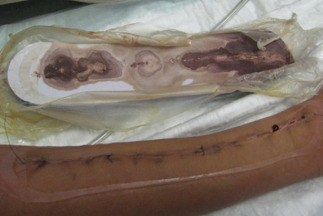
Absorption property of Prevena™ foam.
Moreover, all the treated patients reported a good compliance in daily device management.
Intradermic suture was removed on 12th day and a mean 9‐month follow‐up confirmed the fine aspect of resulting linear scar (Figure 2B).
In one case, suture line was longer than the Prevena™ foam, and therefore it was only partially covered (Figure 4).
Figure 4.
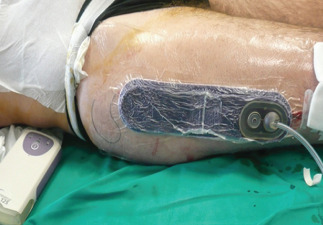
Partial coverage of linear incision by Prevena™ system.
Discussion
Primary closed incisions with high risk of surgical complications include different surgical procedures such as abdominal laparotomy 17, cardiothoracic procedure 18, hip and knee arthroplasty 19 and lower extremities bypass 20. These surgical procedures can be affected by seroma and hematomas formation, because of the pooling of serum and blood in tissue space, resulting to be the primary cause of surgical site complication such as infection, impaired healing and wound dehiscence.
To prevent these complications, the wound care of surgical incision provides different techniques and devices such as hydrocolloid dressing 21, growth factors application 22 and ultrasound therapy 23.
Recently, a new system based on negative pressure has been introduced for the management of surgical incision (NPSM).
Two articles were published showing mechanism of action of NPSM. Wilkes et al. 14 demonstrated that the negative pressure decreases lateral tissue stresses and changes the direction of tissue tension forces like in intact skin. Kilpadi and Cunningham 16 described, in a porcine model, an important reduction of seroma and hematoma formation due to an increased lymph clearance using the Prevena™ System, mechanism confirmed in a human study by Pachowsky et al. 24.
Other studies showed evidence on preventive actions of NPSM on surgical complications of the incision site 11, 13, 25. Atkins et al. 12 reported the results of a retrospective study using NPSM in 57 patients with high risk of sternal wound infection after cardiac surgery. No complications in the NPSM group and one infection in the control group were reported, concluding that the NPSM should be considered in patients with an increased risk for sternal wound infections.
Our clinical report describes the first use of NPSM with the Prevena™ system in wide pathological scar treatment. Selection of wide scars located on body areas determining skin stretch during movements allowed to test the system on high‐risk patients for secondary complications.
Modulation of biomechanical environment around the incision site by the NPSM permitted an uncomplicated wound healing.
No postoperative surgical wound dehiscence confirmed the role of NPWT to maintain the integrity of a closed incision by the reduction of lateral tissue stresses and the increase of appositional strength of the wound edges.
No wound infection supported the hypothesis of preventive action of NPSM on infective postsurgical complications. Supposed mechanisms of protection from external contaminations may be related to the high absorption property of the Prevena™ foam, to the bactericidal characteristics of the silver‐containing wound dressing 26 and to the close environment that avoids secondary colonisation.
After 8 days of treatment, the absence of exudates in all canisters confirmed the supposed endogenous mechanisms of lymph clearance of fluid from the subcutaneous dead space activated by Prevena™. Moreover, the suction drain positioned deeply for 1–2 days permitted early fluid evacuation.
A limit of the device, observed during the treatment of a scar located in the lower abdomen, is represented by poor adherence of the system on hairy body areas, despite shaving, because of the failed air‐proof closure necessary to maintain an appropriate local negative pressure.
For a correct application of the device, to obtain a perfect adhesion of the drape to the skin, scars located close till 2 cm from a hairy body area should be excluded from the treatment with Prevena™ system.
Finally, an additional limit of the device could be related to the standard length of Prevena™ foam, limiting a complete treatment to surgical incisions no longer than 24 cm.
Easy intraoperative application and postoperative management, associated to the good compliance of patients, make Prevena™ a safe home‐care device.
Its usefulness as adjuvant technique in scar treatment is demonstrated by the prevention of postoperative wound complications.
Conclusion
Although the casuistic is small, this article represents the first clinical report about the role of NPSM after surgical scar revision. Selection of high‐risk patients for secondary complications due to high‐tension closure associated to particular anatomical site (upper and lower extremities) could be the starting point for further studies regarding the use of NPSM in postoperative care.
References
- 1. Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scar and keloids. Plast Reconstr Surg 2010;125:557–68. [DOI] [PubMed] [Google Scholar]
- 2. Mustoe TA, Cooter RD, Gold MH, Hobbs FD, Ramlet A-A, Shakespeare PG, Stella M, Tèot L, Wood FM, Ziegler UE. International clinical recommendation on scar management. Plast Reconstr Surg 2002;110:560–71. [DOI] [PubMed] [Google Scholar]
- 3. Wolfram D, Tzankov A, Pulzi P, Piza‐Katzer K. Hypertrophic scars and keloids – a review of their pathophysiology, risk factors and therapeutic management. Dermatol Surg 2009;35:171–81. [DOI] [PubMed] [Google Scholar]
- 4. Verhaegen PDHM, van der Wal MBA, Bloemen MCT, Dokter JAN, Melis P, Middelkoop E, van Zuijlen PPM. Sustainable effect of skin stretching for burn scar excision: long‐term results of a multicenter randomized controlled trial. Burns 2011;37:1222–8. [DOI] [PubMed] [Google Scholar]
- 5. Tziotzios C, Profyris C, Sterling J. Cutaneous scarring: pathophysiology, molecular mechanism, and scar reduction therapeutics. Part II. Strategies to reduce scar formation after dermatologic procedures. J Am Acad Dermatol 2012;66:13–24. [DOI] [PubMed] [Google Scholar]
- 6. Verhaegen PDHM, van Trier AJ, Jongen SJM, Vlig M, Nieuwenhuis MK, Middelkoop E, van Zuijlen PPM. Efficacy of skin stretching for burn scar excision: a multicenter randomized controlled trial. Plast Reconstr Surg 2011;127:1958–66. [DOI] [PubMed] [Google Scholar]
- 7. Melis P, van Noorden CJ, van der Horst CMAM. Long‐term results of wounds closed under a significant amount of tension. Plast Reconstr Surg 2006;117:259–65. [DOI] [PubMed] [Google Scholar]
- 8. Ibrahim AE, Dibo SA, Hayek SN, Atiyeh BS. Reverse tissue expansion by liposuction deflation for revision of post‐surgical thigh scars. Int Wound J 2011;8:622–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Millard DR. Scar repair by the double‐breasted vest principle. Plast Reconstr Surg 1970;45:616–9. [DOI] [PubMed] [Google Scholar]
- 10. Mostafapour SP, Murakami CS. Tissue expansion and serial excision in scar revision. Facial Plast Surg 2001;17:245–52. [DOI] [PubMed] [Google Scholar]
- 11. Stannard JP, Robinson JT, Anderson ER, McGwin G, Volgas DA, Alonso JE. Negative pressure wound therapy to treat hematomas and surgical incisions following high‐energy trauma. J Trauma 2006;60:1301–6. [DOI] [PubMed] [Google Scholar]
- 12. Atkins BZ, Wooten MK, Kistler J, Hurley K, Huges C, Wolfe WG. Does negative pressure wound therapy have a role in preventing poststernotomy wound complications? Surg Innov 2009;16:140–6. [DOI] [PubMed] [Google Scholar]
- 13. Gomoll AH, Lin A, Harris MB. Incisional vacuum‐assisted closure therapy. J Orthop Trauma 2006;20:705–9. [DOI] [PubMed] [Google Scholar]
- 14. Wilkes RP, Kilpadi DV, Zhao Y, Kazala R, McNulty A. Closed incision management with negative pressure wound therapy (CIM): biomechanics. Surg Innov 2012;19:67–75. [DOI] [PubMed] [Google Scholar]
- 15. Colli A. First experience with a negative pressure incision management system on surgical incisions after cardiac surgery in high risk patients. J Cardiothorac Surg 2011;6:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kilpadi DV, Cunningham MR. Evaluation of closed incision management with negative pressure wound therapy (CIM): hematomas/seroma and involvement of the lymphatic system. Wound Repair Regen 2011;19:588–96. [DOI] [PubMed] [Google Scholar]
- 17. Nahas FX, Ferreira LM, Ghelfod G. Does quilting suture prevent seroma in abdominoplasty? Plast Reconstr Surg 2007;119:1060–4. [DOI] [PubMed] [Google Scholar]
- 18. Paul M, Raz A, Leibovici L, Madar H, Holinger R, Rubinovitch B. Sternal wound infection after coronary artery bypass graft surgery: validation of existing risk factors. J Thorac Cardiovasc Surg 2007;133:397–403. [DOI] [PubMed] [Google Scholar]
- 19. Saleh H, Olson M, Resig S, Bershadsky B, Kuskowski M, Gioe T, Robinson H, Schmidt R, McElfresh E. Predictors of wound infection in hip and knee joint replacement: results from a 20 years surveillance program. J Orthop Res 2002;20:506–15. [DOI] [PubMed] [Google Scholar]
- 20. Grennblatt DY, Rajamanickam V, Mell MW. Predictors of surgical site infection after open lower extremity revascularization. J Vasc Surg 2011;54:433–9. [DOI] [PubMed] [Google Scholar]
- 21. Teshima H, Kawano H, Kashikie H, Nakamura K, Imada T, Oda T, Aoyagi S. A new hydrocolloid dressing prevents surgical site infection of median sternotomy wounds. Surg Today 2009;39:848–54. [DOI] [PubMed] [Google Scholar]
- 22. Kimura A, Ogata H, Yazawa M, Watanabe N, Mori T, Nakajima T. The effects of platelet‐rich plasma on cutaneous incisional wound healing in rats. J Dermatol Sci 2005;40:205–8. [DOI] [PubMed] [Google Scholar]
- 23. Ennis WJ, Foremann P, Mozen N, Massey J, Conner-Kerr T, Meneses P. Ultrasound therapy for recalcitrant diabetic foot ulcers: results of a randomized doubled‐blind, controlled, multicenter study. Ostomy Wound Manage 2005;51:24–39. [PubMed] [Google Scholar]
- 24. Pachowsky M, Gusinde J, Klein A, Lehr S, Schulz-Drost S, Schlechtweg P, Pauser J, Gelse K, Brem MH. Negative pressure wound therapy to prevent seromas and treat surgical incisions after total lip arthroplasty. Int Orthop 2012;36:719–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stannard JP, Atkins BZ, O'Malley D, Singh H, Bernstein B, Fahey M, Masden D, Attinger CE. Use of negative pressure therapy on closed surgical incisions: a case series. Ostomy Wound Manage 2009;55:58–66. [PubMed] [Google Scholar]
- 26. Driver VR. Silver dressing in clinical practice. Ostomy Wound Manage 2004;50(9A, Suppl):11S–5S. [PubMed] [Google Scholar]


