Abstract
Combining silver‐based dressings with negative pressure therapy after radical excision of chronically infected breast disease is a novel application of two technologies. One patient with complex, chronic, infected breast disease underwent radical excision of the affected area and was treated early with a combination of silver‐based dressings and topical negative pressure therapy. The wound was then assessed sequentially using clinical measurements of wound area and depth, pain severity scores and level of exudation. It is possible to combine accepted techniques with modern dressing technologies that result in a positive outcome. In this case, the combination of a silver‐based dressing with negative pressure therapy following radical excision proved safe and was well tolerated by the patient. Full epithelisation of the wound was achieved and there was no recurrence of the infection for the duration of the treatment.
Keywords: Breast abscess, Silver dressings, Topical antiseptic, Topical negative pressure therapy
INTRODUCTION
Cutaneous abscesses account for 2% of all emergency surgical referrals. In many cases, these are caused by Staphylococcus aureus infection and the majority are managed by surgical incision and drainage 1, 2. In addition to facilitating the drainage of pus, removal of the abscess wall both prevents epithelialisation and facilitates antibiotic entry. In most cases, the surrounding tissue is colonised by bacteria and therefore the wound is allowed to heal by secondary intention, with regular dressings to pack the wound with or without continued antibiotic cover. This approach is usually sufficient to allow healing, with a good cosmetic outcome. However, the time required to heal the wound may vary substantially, as it is dependent upon the general health of the patient and the size of the defect. In some cases the defect maybe large and subsequent wound care prolonged. Recurrent breast abscesses are common, can be difficult to treat causing frustration for the patient and the treating team, as well as a significant cause of morbidity 3, 4. We present a case of a complex breast abscess wound subsequently managed with a combination of topical negative pressure (TNP) therapy and silver dressings.
CASE
A 43‐year‐old female patient had had recurrent infections of the left breast, of unknown aetiology. She had had more than 12 previous incision and drainage operations over a 3‐year period. She developed a draining sinus on the medial aspect of the breast, with a second inferior‐medial sinus (Figure 1). A magnetic resonance imaging was performed to assess the extent of the inflammatory process. It showed an inferolateral abscess cavity that was not being drained by the sinus tracks. It was decided that further surgery would be required to remove the infected tissue and allow free drainage of pus. However, there was concern that the subsequent large wound would need management over many months, risking further wound infection.
Figure 1.
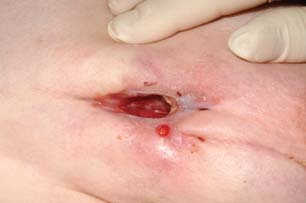
Draining medial sinus with second inferior sinus in left breast.
The patient underwent radical surgery to open and debride the cavity in the breast. At the operation all obviously inflamed tissue was excised and the wound bed thoroughly irrigated and curetted. The wound was initially packed with an alginate dressing (Kaltostat™, Convatec, Uxbridge, Middlesex, UK) for the first 48 postoperative hours to ensure haemostasis. After 48 hours the dressing was changed on the ward. Nitrous oxide analgesia was used, the alginate dressings were removed and the wound thoroughly irrigated with normal saline. The wound was then lined with a layer of silver dressing (Acticoat Flex™, Smith & Nephew, Hull, East Yorkshire, UK) (Figure 2) and then lightly packed with dampened gauze before application of the TNP therapy system (Renasys Go™, Smith & Nephew, Hull, East Yorkshire, UK) (Figure 3). The dressings were changed three times a week for 7 weeks. Over the 7‐week period, the negative pressure was used to maximise wound bed granulation (Figure 4). Following advice from a microbiologist, the patient remained on oral antibiotics throughout this entire period as the organism cultured, Gordonia araii, is both difficult to clear and a rare cause of breast infection. Once the wound was shallow enough, the TNP was stopped. The silver dressings were continued along with oral antibiotics and full epithelisation was achieved.
Figure 2.
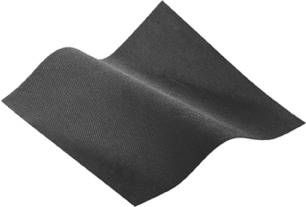
Acticoat Flex™ dressing.
Figure 3.

Renasys Go™ topical negative pressure therapy system.
Figure 4.
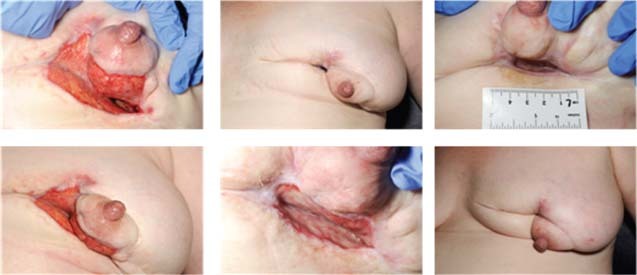
Sequential pictures showing improvement in wound bed and reduction in wound size.
DISCUSSION
TNP wound therapy is becoming an increasingly popular treatment option in ‘hard to heal wounds' (5). TNP therapy removes wound exudates including potentially harmful cytokines and proteases from the wound bed (6). In addition, it also reduces tissue oedema leading to increased localised blood flow, resulting in enhanced granulation tissue formation (7). Clinical improvements include reduction in wound size, stimulation of granulation tissue, increased rate of closure and reduction in wound bed non viable tissue 5, 8, 9, 10. TNP therapy has also been shown to reduce the pressure on the health care team and patient in terms of the number of dressing changes 5, 11 and positively change factors that have an impact on a patient's quality of life; such as improved general wound pain (5).
Although TNP can improve healing, there has been recent concern over its potential to promote wound infection. To date, 27 cases of serious infection with TNP have been reported to the Food and Drug Administration (FDA), necessitating further interventions, antibiotics and hospitalisation (12). Early studies on lower leg ulcers failed to show any increase in the risk of wound infection with TNP (13). Yet, when other wound types were studied, use of TNP was associated with an increase in bacterial bioburden, in some cases exceeding a concentration of 105 per gram tissue; resulting in a corresponding increase in wound infections 9, 14. Intriguingly, the growth of certain bacterial species such as gram negative rods appears to be inhibited (15). Overall, the use of TNP for infected wounds shows little advantage in clearing infection and in turn may promote micro‐organism growth (15). So while TNP may help healing, we were cautious of its use in our case; given that the wound had an infectious aetiology.
Dressings have a major role in modern wound management but must be combined with optimal local wound care. In wounds healing by secondary intention, one will always find bacteria colonising the wound surface. The resulting bacterial biofilms present a challenge to wound healing (16). Some of the bacteria will be potential pathogens and their presence and numbers needs to be controlled (6). The resistance of bacterial cells within biofilms to antibiotics has led to new approaches for their treatment, including the use of silver (16). Silver is toxic to multiple components of bacterial cell metabolism; including damage to the bacterial cell wall and membrane permeability leads to gross cellular structural changes, blockage of transport and enzyme systems such as the respiratory cytochromes, alteration of proteins and binding of microbial deoxyribonucleic acid and ribonucleic acid to prevent transcription and division (6); and therefore is less affected by the variations in the micro environment of the biofilm 17, 18. Silver is also known to destabilise the biofilm matrix by compromising the intermolecular forces and decreasing bacterial adhesion 17, 19, 20. The nanocrystalline structure provides a large surface area to volume ratio to allow rapid, effective, sustained release of Ag+ ions at bactericidal levels of 70–100 ppm 21, 22 (Figure 5). The release of the Ag+ ions is much the same as other silver containing devices, however, the surface topography created by the deposition of nanocrystalline silver on wound contact layers allows for a more rapid release and replenishment of silver ions compared with silver containing salts (22).
Figure 5.
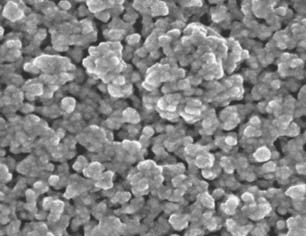
Surface of the Acticoat™ dressing showing the large surface area created by the Silcryst™ (nucryst, Fort Saskatchewan, Alberta, Canada) nanocrystals.
In this case, where surgery and oral antibiotics were used to treat a defined underlying infection, the normal course of treatment would have involved packing the wound with local antiseptic dressings to facilitate healing. To speed up healing, we have combined the use of topical antiseptic dressing and TNP, secure in fact that the infection risk could be controlled with the continued use of oral antibiotics and local antiseptic dressings. Thus for this patient, we have showed the successful use of TNP when combined with continued oral antibiotics and silver‐based dressings. This case‐report highlights the potential for using TNP, with caution, in combination with local antisepsis and oral antibiotics to treat patients for whom the primary focus on infection has been removed surgically; as is often the case with patients requiring incision and drainage.
REFERENCES
- 1. Meislin HW, Lerner SA, Graves MH, McGehee MD, Kocka FE, Morello JA, Rosen P. Cutaneous abscesses. Anaerobic and aerobic bacteriology and outpatient management. Ann Intern Med 1977;87:145–9. [DOI] [PubMed] [Google Scholar]
- 2. Meislin HW, McGehee MD, Rosen P. Management and microbiology of cutaneous abscesses. JACEP 1978;7:186–91. [DOI] [PubMed] [Google Scholar]
- 3. Maier WP, Au FC, Tang CK. Nonlactational breast infection. Am Surg 1994;60:247–50. [PubMed] [Google Scholar]
- 4. Gollapalli V, Liao J, Dudakovic A, Sugg SL, Scott‐Conner CEH, Weigel RJ. Risk factors for development and recurrence of primary breast abscesses. J Am Coll Surg 2010;211:41–8. [DOI] [PubMed] [Google Scholar]
- 5. Hurd T, Chadwick P, Cote J, Cockwill J, Mole TR, Smith JM. Impact of gauze‐based NPWT on the patient and nursing experience in the treatment of challenging wounds. Int Wound J 2010;7: 448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leaper DJ. Silver dressings: their role in wound management. Int Wound J 2006;3:282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76 discussion 577. [PubMed] [Google Scholar]
- 8. Campbell PE, Smith GS, Smith JM. Retrospective clinical evaluation of gauze‐based negative pressure wound therapy. Int Wound J 2008;5:280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Braakenburg A, Obdeijn MC, Feitz R, van Rooij IA, van Griethuysen AJ, Klinkenbijl JH. The clinical efficacy and cost effectiveness of the vacuum‐assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg 2006;118:390–7 discussion 398–400. [DOI] [PubMed] [Google Scholar]
- 10. Loree S, Dompmartin A, Penven K, Harel D, Leroy D. Is vacuum assisted closure a valid technique for debriding chronic leg ulcers? J Wound Care 2004;13:249–52. [DOI] [PubMed] [Google Scholar]
- 11. Ozturk E, Ozguc H, Yilmazlar T. The use of vacuum assisted closure therapy in the management of Fournier's gangrene. Am J Surg 2009;197:660–5. [DOI] [PubMed] [Google Scholar]
- 12. Mirsaidi N. Negative pressure wound therapy: use with care. 2010: [cited 12/02/2010]. Available from URL http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm190658.htm. [DOI] [PubMed]
- 13. Sadat U, Chang G, Noorani A, Walsh SR, Hayes PD, Varty K. Efficacy of TNP on lower limb wounds: a meta‐analysis. J Wound Care 2008;17: 45–8. [DOI] [PubMed] [Google Scholar]
- 14. Moues CM, van den Bemd GJ, Meerding WJ, Hovius SE. An economic evaluation of the use of TNP on full‐thickness wounds. J Wound Care 2005;14:224–7. [DOI] [PubMed] [Google Scholar]
- 15. Ubbink DT, Westerbos SJ, Nelson EA, Vermeulen H. A systematic review of topical negative pressure therapy for acute and chronic wounds. Br J Surg 2008;95:685–92. [DOI] [PubMed] [Google Scholar]
- 16. Rhoads DD, Wolcott RD, Percival SL. Biofilms in wounds: management strategies. J Wound Care 2008;17:502–8. [DOI] [PubMed] [Google Scholar]
- 17. Kostenko V, Lyczak J, Turner K, Martinuzzi RJ. The impact of silver‐containing wound dressings on biofilm bacteria viability and susceptibility to antibiotics during prolonged treatment. Antimicrob Agents Chemother 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bjarnsholt T, Kirketerp‐Møller K, Kristiansen S, Phipps R, Nielsen AK, Jensen PØ, Høiby N, Givskov M. Silver against Pseudomonas aeruginosa biofilms. APMIS 2007;115:921–8. [DOI] [PubMed] [Google Scholar]
- 19. Chaw KC, Manimaran M, Tay FE. Role of silver ions in destabilization of intermolecular adhesion forces measured by atomic force microscopy in Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother 2005;49:4853–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klueh U, Wagner V, Kelly S, Johnson A, Bryers JD. Efficacy of silver‐coated fabric to prevent bacterial colonization and subsequent device‐based biofilm formation. J Biomed Mater Res 2000;53: 621–31. [DOI] [PubMed] [Google Scholar]
- 21. Roberts CD. Product focus: nanocrystalline silver and diabetic foot management. Diabetic Foot J 2007;10:96–8. [Google Scholar]
- 22. Roberts CD. Use of interventional approaches to controlling healthcare‐acquired infections in wounds. The role of silver. J Wound Tech 2008;2: 58–60. [Google Scholar]


