Abstract
Significant progress has been made in the development of in vitro‐engineered skin substitutes that mimic human skin, either to be used for the replacement of lost skin or for the establishment of in vitro skin research models. However, at the present time, there are no models of bioengineered skin that completely replicate the nature of uninjured skin. Obviously, there is still much room for improvement of the components of bioengineered skin and their interplay. This review summarises the important new discoveries in key elements of engineering of tissue‐engineered skin including cell sources, biomaterials and growth factors, etc. Furthermore, basic and clinical applications for engineered skin substitutes in cell therapy, tissue engineering, and biomedical research continue to drive design improvements premised on these structure and function‐based engineering paradigms.
Keywords: Biomaterials, Cell, Engineered skin substitutes, Growth factors, Model
Introduction
At present, a number of different bioengineered skin substitutes are available for clinical use, and their performance has been desirable in restoring the barrier function, initiating or accelerating wound healing, reducing pain in superficial burns and correcting conditions in healing (1, 2, 3). The basic premise of skin bioengineering is to combine the appropriate cells with a biomaterial to produce a skin equivalent that is both functional and durable and allows for integration and manipulation of the cell biology of host cells and the multitude of signals that control their behaviour (4). The complex interplay among biomaterial scaffolds, cell populations and growth factors has been consistently used to design constructs that attempt to promote these interactions to restore the original architecture and function of skin. Conventionally, most bioengineered skin exists as cells cultured in vitro and subsequently seeded with a porous scaffold, all of which fail to fulfil the criteria for fully functional skin (5). Recently, design and fabrication in skin bioengineering according to specific functional objectives have undergone significant advances through improving singular aspects within the overall approach, for example, in cell sources, material design or creating biomimic environments.
Over the past 10 years, the challenges of designing complex engineered skin have been aided by major breakthroughs in tissue engineering techniques, stem cell research and biomimetic rational design of biomaterials that are founded on the basic principles of regenerative medicine. These advances have set the framework for overcoming some of the enduring challenges in applying skin substitutes clinically to additional areas of biomedical research. This review covers the design principles being applied to engineer biomedical skin, focusing on efficient recuperation of nature skin cell components, as well as biophysical and biochemical manipulations of 3D (three dimensional) networks for controlling interfaces and cell fates. Here, we highlight the important role of structural and functional‐designed engineering concepts in advancing the development of customised skin substitutes that contain a set of desired properties based on building blocks for specific design.
Cell source
A major consideration when developing a bioengineered skin is to identify suitable sources of cells and to understand the mechanisms by which they can function and interact properly. In most cases, a host‐ or donor‐derived cell source is used in engineering the skin structure, except for some cases of ingrowth of the host cells into a scaffold following implantation (6). In Table 1, we list some cells used or that could be used in skin bioengineering and highlight their advantages and disadvantages. Early bioengineered skin products have focused on either allogeneic cell lines, such as foreskin‐derived fibroblasts and keratinocytes, or autologous differentiated cells, such as autologous keratinocytes for the bioengineering process (7). However, in some cases, the autologous primary cells are not accessible or are not in sufficient numbers and do not have proliferative capacity to be viable for skin bioengineering. Multipotent stem cells and progenitor cells hold great promise for addressing the need for viable cell sources, and most studies have shown them to have great promise in developing new engineered tissues.
Table 1.
Summaries of current available cells and potential cells in skin bioengineering
| Category | Cell types | Advantages | Disadvantages |
|---|---|---|---|
| Somatic cells | Autologous fibroblasts/keratinocytes | Little risk of rejection; reliable applications | Longer time required to expand; not accessible or not in sufficient numbers sometimes |
| Allogeneic fibroblasts/keratinocytes | Readily accessible; can be preserved for applications | Potential problems of rejection and disease transfer | |
| Stem cells | Adipose‐derived stem/stromal cells (ASCs) | Abundant and readily accessible; contribute to the production of hypodermis | Vary in metabolic activity; proliferation and differentiation depending on the location of the tissue depot and the age and gender of the patient |
| Hair follicle stem cells | Higher proliferative capacity; contribute to the production of epidermis and skin appendages | Not accessible or not in sufficient numbers sometimes | |
| Epidermal stem cells/Dermal stem cells | Contribute to the production of skin and skin appendages | Not in sufficient numbers; absence of controlled, efficient and reproducible differentiated manner | |
| Mesenchymal stem cells (MSC) | Relatively easy to obtain and readily expanded; capable of differentiating into various tissues and cells | Absence of controlled, efficient and reproducible differentiated manner | |
| Embryonic stem cells (ESC) | Totipotent; capable of differentiating into various tissues and cells | Ethical and moral objections | |
| Differentiated epidermal cells | Potential reversion to undifferentiated stem cells | Relevant mechanism remains unclear | |
| Induced pluripotent stem (iPS) cells | Avoiding immunological rejection and current ethical dilemmas surrounding human ESC | Viral vectors required; lower efficiency |
There are a number of different sources of cells that could be used for skin tissue engineering. Stem cell types can be derived from essentially three locations: local, systemic and progenitor cell populations. Multipotent skin stem cells sourced locally have been identified mainly in adipose tissue and the hair follicle bulge. Use of adipose‐derived stem/stromal cells for skin bioengineering is very attractive, considering that subcutaneous fat is abundant and readily accessible by lipoaspiration, a minimally invasive procedure. The greater availability of adipose tissue compared to dermis as a source of cells needed for amplification in culture would translate into a faster production of skin substitutes for severely burned patients. Researchers have proposed that adipose‐derived stem/stromal cells could usefully substitute dermal fibroblasts for skin reconstruction using the self‐assembly method (8).
At present, bioengineered skin is not fully functional in that it lacks hair follicles, sweat and sebaceous glands. When the skin experiences trauma such as burn injury or wounding, hair follicle stem cells are thought to migrate to the surface to aid in re‐epithelialisation. Actually, hair follicle‐derived stem cells are efficient candidates for sources of cells to seed in a skin substitute (9, 10, 11, 12). Larouche and co‐workers (10) designed a unique model of tissue‐engineered skin cultured with hair buds that grew into hairs after grafting on mice. In addition, the incorporation of hair follicles in tissue‐engineered skin may promote and/or guide nerve migration, with hairs establishing active targets for nerves. Moreover, it should greatly improve the recovery of the sense of touch, as hair follicles are sensory receptors (11). With hope, the regenerative role of bulge cells (or dissociated cells) from the skin is multiple; these cells are thought to not only contribute to the production of epidermis and hair follicles but also are key to the formation of sebaceous glands.
Epidermal stem cells and dermal stem cells are other stem cells residing in skin; they contribute to the maintenance of adult skin homeostasis and hair regeneration as well as participate in the repair and regeneration of injured skin. Nevertheless, these stem cells are as yet only insufficiently defined and it still has to be elucidated how insights in cutaneous stem cell biology gained in mice can be extrapolated to humans (12). Other studies have demonstrated the possibility of constructing pigmented tissue‐engineered skin with human melanocytes, which brings a promising method to make up for the deficiency of traditional tissue‐engineered skin and provides an alternative treatment for depigmentation diseases (13).
Systemic adult stem cells are populations of cells resident in the blood or bone‐marrow system, and an attractive example from a bioengineering viewpoint is use of mesenchymal stem cells (MSCs). There is significant interest in the clinical translation of an MSC‐based therapy to promote dermal regeneration. Several recent studies have provided overwhelming evidence that MSCs can accelerate wound closure by modulating the inflammatory environment, promoting the formation of a well‐vascularised granulation matrix, encouraging the migration of keratinocytes, and inhibiting apoptosis of wound healing (14, 15, 16, 17, 18). Recently, the successful construction of MSCs‐based cell sheets in vitro suggests that creating tissue‐engineered skin using is MSCs feasible (19). Interestingly, success in our study further suggests that MSCs incorporated with the microspheres‐based engineered skin may repair sweat glands and improve cutaneous wound healing after injury (20).
Progenitor cells, such as embryonic stem cells (ESCs), are totipotent and able to differentiate into many different cell types. ESCs represent an attractive and viable source for cell‐replacement therapy. However, many controversial ethical and technical problems need to be overcome before the full potential of this type of cell can be realised (21). Use of adult stem cells could resolve the potential problems of ESCs, namely, treating patients with their own cells negates the inherent problems of rejection while avoiding ethical and moral objections. Although stem cells and progenitors of various origins can differentiate into various cell types, the challenge is differentiating the progenitors and stem cells in a controlled, efficient and reproducible manner to result in terminally differentiated tissue structures.Thus, many areas of stem cell research and their potential clinical applications entail controversy (22). Bioengineered skin may provide a means to gain critical insight into the behaviour of stem cells, by facilitating the control of the stem cell environment both chemically and physically in the three dimensions. This, in turn, may lead to the development of new skin substitutes and replacements.
Besides the pluripotent stem cell types noted previously, another promising alternative in skin bioengineering is dedifferentiation research, likely to become a new focus in skin bioengineering because of its potential for inducing cell reversion to stem cells or stem cell‐like cells, and the achievements have offered new evidence of and insights into the dedifferentiation of human epidermal cells (23). Our initial study based on biopsies from human wounded skin treated with epidermal growth factor suggested that differentiated epidermal cells are involved in skin wound healing and play important roles in accelerating wound healing (24). Moreover, recent work on induced pluripotent stem (iPS) cells generated from somatic cells by genetic manipulation has brought into sharp focus the potential use of patient‐specific iPS cells as a new strategy for bioengineered skin, which eliminates the possibility of immunological rejection and current ethical dilemmas surrounding human ESC research (25). With so many options for cell sourcing, skin bioengineering processes are unlikely to be confined to a small group of cell types and origins.
Progress in progenitor and stem cell research and recognition of the unique properties of such cells may enable bioengineering design of replacement skin which allows regeneration to occur in vivo. There are, however, many controversial ethical and technical problems that need to be overcome before the full potential of this type of cell can be realised. Although adult somatic stem cells could resolve the ethical problems that ESCs potentially have, whether adult stem cells can transdifferentiate in vivo remains an argument. For example, MSCs can differentiate in culture into a variety of mature cells; however, such transitions are not thought to occur extensively in the adult. As seen during foetal wound repair mechanisms, changes brought about by cell‐signalling cascades allow progenitor cells access for efficient engraftment and subsequent differentiation. Perhaps the greatest challenge in stem cell biology is to uncover the extracellular and intracellular mechanisms that determine whether a daughter cell of a stem cell division self‐renews or commits to a particular pathway of differentiation. Optimistically, biomedical scientists would understand the subtleties involved in these processes in future to create an environment that will permit successful engineering of a new generation of skin substitute.
Scaffolds
Traditionally, cellular scaffolds, from the typical 2D surfaces to the first 3D constructs (natural or artificial), were intended as inter‐platforms that merely served as support for the cultured cells. Since then, more emphasis has been given to provide these matrices with suitable physical (e.g. stiffness and mass transfer) and chemical (e.g. employed material type and degradation rate) properties for skin tissue engineering. In some cases, isolated cells have limited capacity to maintain the tissue architecture, because they lack a template that guides restructuring. Moreover, transplantation of large volumes of tissues is impossible because of diffusion limitations that restrict interaction with the host environment for nutrients, gas exchange and elimination of waste products (26). Hence, an ideal scaffold with the capacity to act as a template both for a supporting structure for the control of cell behaviour and for the construction of neonatal skin is important in skin bioengineering. The main tenets of a successful scaffold include a highly porous structure and good mechanical stability. High porosity and optimal pore size provides structural space for cell accommodation and migration and enables exchange of nutrients between the scaffold and the environment (27). Similarly, to serve as scaffolds for bioengineered skin, the highly porous and well‐connected pore structures were very important to emulate certain advantageous features of the natural ECM for regenerative skin cells. In particular, as cellular response to biological stimuli depends on the geometry and mechanical strength of ECM, the therapeutic success of bioengineered skin will partly depend on the mechanical properties of the scaffolds.
Materials for skin bioengineering to date include those derived from naturally occurring materials and those manufactured synthetically. To fulfil the diverse needs in skin bioengineering, largely empirical approaches to biomaterials have been pursued in recent years. Biomaterials have been developed to play a pivotal role as scaffolds to provide 3D templates and to mimic certain advantageous features of natural ECM environments for facilitating cell recruiting/seeding, adhesion, proliferation, differentiation and neo‐tissue genesis. These biomaterials include synthesis to achieve certain compositions or properties similar to those of the ECM, novel processing technologies to achieve structural features mimicking the ECM at various levels, approaches to emulate cell–ECM interactions, and biologic delivery strategies to recapitulate a signalling cascade or developmental/wound‐healing programme. Early on in the development of tissue engineering, a highly porous scaffold was identified as being critical for the necessary nutrient and waste transport to support the growth of large pieces of tissue replacements. Currently, a number of other techniques have been borrowed, adapted and developed to create porous scaffolds with highly controlled porosity in terms of pore size, structure and volume within the biomaterial. Many investigators have applied approaches from engineering, such as microfabrication and nanotechnology, into the design principles to govern whether cells grow, move, die or differentiate into 3D tissue structures with characteristic forms, mechanical properties and biochemical activities. Nanostructured fibrous materials have been made more readily available than microfibres owing to the unique features, including more interconnected pores and larger surface‐to‐volume ratio, which enable such nanofibrous scaffolds to meet higher demands of numerous practical applications (Figure 1). At present, the concept of using an electrospinning array to form multi‐component nanofibrous membranes will lead to the creation of novel scaffolds for skin bioengineering applications. The application of nanofibrous composite substitutes with natural and synthetic materials might prospectively mimic the structure of natural ECM and have the potential to be used as 3D scaffolds of bioengineered skin. For example, the versatile method of electrospinning can produce 3D open‐porous structures that approximate the structure of collagenous dermis (27, 28). Moreover, the morphogens, growth factors and cytokines play a key role not only in morphogenesis, chemotaxis and axogenesis (29) but also during processes like wound healing or tissue homeostasis. Such gradients can also be introduced into 3D skin models, for instance, using the micropatterning techniques (30).
Figure 1.
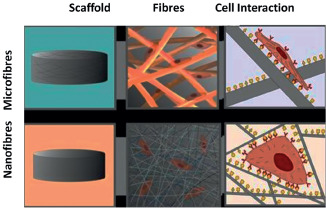
Schematic illustration of the use of micro‐ and nano‐structured fibrous materials as scaffolds and mode of cell interaction in 3D scaffolds.
On the basis of current biomimetic materials approaches, many investigators began to consider the merits of incorporating cell‐adhesion peptides known to be involved in cellular interactions into biomaterials during manufacturing (31). One of the best‐studied peptides in this aspect is the RGD peptide, a ubiquitous cell‐adhesion peptide found in fibernectin and laminin. Natural materials, such as collagen and chitosan, which have been extensively studied with regard to cell binding, can be spun on their own or together with synthetic polymers (32). Interest is strong in developing surface modifications to resist particular kinds of protein and cellular attachment or to select for specific cellular attachment, and the engineered peptides and their derivatives have high potential to be used for scaffold surface modification (33). Similarly, findings in the interactions between cell‐surface receptors and ECM ligands will continue to provide inspiration for biomimetic surface modification of scaffolds.
More recently, the biology of the scaffolds is gaining the attention of scientists, including signals that cells receive via adhesion to the material or directly from soluble factors in the microenvironment (34, 35). The emerging field of cell‐compatible hydrogel materials is therefore defined by design strategies focused on tuning the biological and physical attributes of hydrogels in order to achieve specific interactions and responses from cellular systems. Soluble biomolecules often show improved bioactivity when they are directly attached to the hydrogel network (36). In addition to improved stability, covalently immobilised growth factors can be used to spatially direct cell behaviour (e.g. chemotaxis or differentiation) (37). This research represented a major advance not only because it showed the profound influence of ECM mechanics on stem cell differentiation but also because it ignited a pursuit towards identifying other material properties that can potentially control cell fate.
Biomaterial strategies are bridging the gap in many scientific fields because they have become a necessary tool in tissue engineering or regenerative medicine. In fact, microfabrication, and more recently nanofabrication, is allowing the creation of suitable skin models where key factors may be studied from the nanometer to the supra‐millimeter length scale. Moreover, the ability of the new bioinspired materials to be tuned in a wide range of biophysical and biochemical features is also optimising the way scaffolds control the different biological properties of the cells.
Growth factors and their delivery systems
In addition to scaffolds and cells, molecular cues and biological signals such as growth factors or cytokines are a key component for cell function and tissue regeneration. As evidence presented above shows, the presence of growth factors is integral to the spatiotemporal coordination of cellular activities to ensure proper tissue formation during wound healing (38). Some of the important growth factors involved in various stages of the healing process are summarised in Table 2. When the damaged skin around the defect does not have the inherent potential to regenerate, skin regeneration therapy by tissue engineering technology cannot always be expected if only the scaffold is supplied. Regeneration is characterised by a constantly changing environment in which cells are exposed to a complex pattern of molecular cues and signals, which trigger a series of events that in combination control cell proliferation, differentiation and cell death and impart positional information necessary for correct development. As these molecules are often major components of early developmental pathways for cell specification, incorporating them into a tissue‐engineered skin repair product could produce major advancements in skin regeneration. Because of the sensitivity of cells to the concentration of cell‐signalling molecules and the short half‐lives of these molecules, the successful application of biological molecules in skin bioengineering critically depends on the delivery technologies (39, 40).
Table 2.
Wound healing with important growth factors
| Growth factor | Function involved in wound healing |
|---|---|
| bFGF | Proliferation of fibroblasts and epithelial cells; matrix deposition; wound contraction; angiogenesis; accelerates formation of granulation tissue |
| VEGF | Stimulates angiogenesis in granulation tissue; improves formation of collateral blood vessels in peripheral vascular disease |
| EGF | Differentiation, proliferation, migration and adhesion of keratinocytes |
| PDGF | Mitogenic for smooth muscle cells, endothelial cells and fibroblasts |
| TGF‐β | Mitogenic for fibroblasts and smooth muscle cells; chemotactic for macrophages; stimulates angiogenesis (indirect) and collagen metabolism |
| TGF‐α | Stimulates proliferation of epithelial cells and fibroblast; formation of granulation tissue |
| IL‐1 | Neutrophil chemotaxis; fibroblast proliferation |
| TNF | Fibroblast proliferation |
| HGF | Re‐epithelialisation; neovascularisation; formation of granulation tissue |
| IGF‐1 | Fibroblast proliferation |
| G‐CSF | Stimulates production of neutrophils; enhances function of neutrophils and monocytes; promotes proliferation of keratinocytes |
| GM‐CSF | Mediates proliferation of epidermal cells |
bFGF, basic fibroblast growth factors; VEGF, vascular endothelial growth factor; EGF, epidermal growth factor; PDGF, platelet‐derived growth factor; TGF‐β, transforming growth factor‐β; TGF‐α, transforming growth factor‐α; IL‐1, interleukin‐1; TNF, tumour necrosis factor; HGF, hepatocyte growth factor; IGF‐1, insulin‐like growth factor‐1; G‐CSF, granulocyte‐colony stimulating factor; GM‐CSF, granulocyte macrophage‐colony stimulating factor.
In skin bioengineering, releasing biological molecules within the scaffold in a controlled fashion is desirable. Growth factors could be directly added into a polymer solution or emulsion to fabricate scaffolds, or a scaffold could be modified with growth factors with use of certain coating techniques. These methods can achieve certain slow‐release characteristics, but the control over release kinetics is limited. Controlled release with microspheres is effective in retaining the bioactivities of various therapeutic agents. Recent technologies have been developed to immobilise nanospheres onto the pore surface of macroporous or nanofibrous scaffolds, which allow single or multiple growth factors to be released in a spatially and temporally controlled fashion. The release kinetics of each factor can be individually controlled by use of a specific nanosphere formulation. The number of growth factors that exist and are used in developmental and regenerative processes are obviously too numerous to catalogue in full. However, from the above approaches, incorporating growth factors and cell‐signalling molecules into a bioengineered material could be critical to creating a more functional skin replacement. Hence, of considerable importance is attempting to use matrix‐immobilised growth factors to mimic the release of growth factors from natural ECM; particularly the sequential release of multiple factors has been developed largely to optimise their effectiveness.
There is a wide range of possibilities for designing systems to deliver growth factors. Design considerations are varied according to the intended use and pursued goal. For instance, researchers interested in the study of cell migration and proliferation through given biomolecular gradients in vitro will possibly prefer the use of nanospheres or nanofibrous scaffolds to create their own patterns. On the contrary, those more interested in forming skin‐like tissue within scaffolds in vivo will probably choose hydrogels or microspheres that can be easily injected once implanted.
Summary and future trends
Besides the clinical application, currently, the in vitro‐reconstructed skin models will be widely used as promising tools in the laboratory to study all major principles in skin biology (41). As an alternative to animal experimentation, the bioengineered skin offers a way to not only concede to demands of regulatory authorities, animal welfare organisations, consumers and scientists but also to provide a means to improve and extend our knowledge of biological processes in the skin. Although the challenge of building a completely functional skin is ostensibly insurmountable, rapid progress in tissue engineering and technological advances to design a skin substitute including the use of stem cells, biomimic materials, may give us hope that such a product will be developed in the near future. Another exciting prospect is, such substitutes may be further engineered to offer the complete regeneration of functional skin, including all the skin appendages (hair follicles, sweat glands and sensory organs) and the establishment of a functional vascular and nerve network with the surrounding host tissue. Such integrated bioengineered skin should allow the cells to interact so as to regenerate all of the skin structures – such as that happens during embryonic development or adult regeneration. Indeed, as the technology advances and we gain new insights into the mechanisms that regulate cell–ECM interactions, we will be able to design more sophisticated and tailormade skin substitutes that may provide more effective therapies for patients.
Acknowledgements
This study was supported by the National Basic Science and Development Program (973 Program 2012CB518105), the Postdoctoral Science Foundation (20080440225, 21003777) and the National Natural Science Foundation of China (81121004, 81000843).
The authors declare that they have no competing financial interests.
Gang Lu and Sha Huang contributed equally to this work.
References
- 1. Pomahac B, Svensjö T, Yao F, Brown H, Eriksson E. Tissue engineering of skin. Crit Rev Oral Biol Med 1998;9:333–44. [DOI] [PubMed] [Google Scholar]
- 2. Falanga V, Sabolinski M. A bilayered living skin construct (Apligraf®) accelerates complete closure of hard‐to‐heal venous ulcers. Wound Repair Regen. 1999;7:201–7. [DOI] [PubMed] [Google Scholar]
- 3. Veves A, Falanga V, Armstrong DG, Sabolinski ML. Apligraf Diabetic Foot Ulcer Study. Graftskin, a human skin equivalent, is effective in management of non‐infected neuropathic diabetic foot ulcers. Diabetes Care. 2001;24:290–5. [DOI] [PubMed] [Google Scholar]
- 4. Boyce ST. Design principles for composition and performance of cultured skin substitutes. Burns 2001;27:523–33. [DOI] [PubMed] [Google Scholar]
- 5. Supp DM, Boyce ST. Engineered skin substitutes: practices and potentials. Clin Dermatol 2005;23:403–12. [DOI] [PubMed] [Google Scholar]
- 6. Ellis DL, Yannas IV. Recent advances in tissue synthesis in vivo by use of collagen‐glycosaminoglycan copolymers. Biomaterials 1996;17:291–9. [DOI] [PubMed] [Google Scholar]
- 7. Zanstra PW, Nagy A. Stem cell bioengineering. Annu Rev Biomed Eng 2001;3:275–305. [DOI] [PubMed] [Google Scholar]
- 8. Trottier V, Marceau‐Fortier G, Germain L, Vincent C, Fradette J. IFATS collection: using human adipose‐derived stem/stromal cells for the production of new skin substitutes. Stem Cells 2008;26:2713–23. [DOI] [PubMed] [Google Scholar]
- 9. Mahjour SB, Ghaffarpasand F, Wang H. Hair follicle regeneration in skin grafts: current concepts and future perspectives. Tissue Eng Part B Rev 2012;18:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Larouche D, Cuffley K, Paquet C, Germain L. Tissue‐engineered skin preserving the potential of epithelial cells to differentiate into hair after grafting. Tissue Eng Part A 2011;17:819–30. [DOI] [PubMed] [Google Scholar]
- 11. Gagnon V, Larouche D, Parenteau‐Bareil R, Gingras M, Germain L, Berthod F. Hair follicles guide nerve migration in vitro and in vivo in tissue‐engineered skin. J Invest Dermatol 2011;131:1375–8. [DOI] [PubMed] [Google Scholar]
- 12. Boehnke K, Falkowska‐Hansen B, Stark HJ, Boukamp P. Stem cells of the human epidermis and their niche: composition and function in epidermal regeneration and carcinogenesis. Carcinogenesis 2012;33:1247–58. [DOI] [PubMed] [Google Scholar]
- 13. Liu Y, Suwa F, Wang X, Takemura A, Fang YR, Li Y, Zhao Y, Jin Y. Reconstruction of a tissue‐engineered skin containing melanocytes. Cell Biol Int 2007;31:985–90. [DOI] [PubMed] [Google Scholar]
- 14. Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 2008;180:2581–7. [DOI] [PubMed] [Google Scholar]
- 15. Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol 2003;196:245–50. [DOI] [PubMed] [Google Scholar]
- 16. Chen L, Tredget EE, Liu C, Wu Y. Analysis of allogenicity of mesenchymal stem cells in engraftment and wound healing in mice. PLoS One 2009;4:e7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith AN, Willis E, Chan VT, Muffley LA, Isik FF, Gibran NS, Hocking AM. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res 2010;316:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, Isik F. Contribution of bone‐marrow‐derived cells to skin: collagen deposition and wund repair. Stem Cells 2004;2:812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Williams C, Xie AW, Emani S, Yamato M, Okano T, Emani SM, Wong. JY. A comparison of human smooth muscle and mesenchymal stem cells as potential cell sources for tissue‐engineered vascular patches. Tissue Eng Part A 2012;18:986–98. [DOI] [PubMed] [Google Scholar]
- 20. Huang S, Lu G, Wu Y, Jirigala E, Xu Y, Ma K, Fu X. Mesenchymal stem cells delivered in a microsphere‐based engineered skin contribute to cutaneous wound healing and sweat gland repair. J Dermatol Sci 2012;66:29–36. [DOI] [PubMed] [Google Scholar]
- 21. Peterson DA. Stem cells in brain plasticity and repair. Curr Opin Pharmacol 2002;2:34–42. [DOI] [PubMed] [Google Scholar]
- 22. McLaren A, Durcova‐Hills G. Germ cells and pluripotent stem cells in the mouse. Reprod Fertil Dev 2001;13:661–4. [DOI] [PubMed] [Google Scholar]
- 23. Cai S, Fu XB, Sheng ZY. Dedifferentiation: a new approach in stem cell research. BioScience 2007;57:655–62. [Google Scholar]
- 24. Fu XB, Sun XQ, Li XK, Sheng ZY. Dedifferentiation of epidermal cells to stem cells in vivo. Lancet 2001;358:1067–8. [DOI] [PubMed] [Google Scholar]
- 25. Nelson TJ, Martinez‐Fernandez A, Yamada S, Ikeda Y, Perez‐Terzic C, Terzic A. Induced pluripotent stem cells: advances to applications. Stem Cells Cloning 2010;3:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vacanti JP, Langer R, Upton J, Marler JJ. Transplantation of cells in matrices for tissue regeneration. Adv Drug Deliv Rev 1998;33:165–82. [DOI] [PubMed] [Google Scholar]
- 27. Kim G, Kim W. Highly porous 3D nanofiber scaffold using an electrospinning technique. J Biomed Mater Res B Appl Biomater 2007;81: 104–10. [DOI] [PubMed] [Google Scholar]
- 28. Noh HK, Lee SW, Kim JM, Oh JE, Kim KH, Chung CP, Choi SC, Park WH, Min BM. Electrospinning of chitin nanofibers: degradation behavior and cellular response to normal human keratinocytes and fibroblasts. Biomaterials 2006;27:3934–44. [DOI] [PubMed] [Google Scholar]
- 29. Choi NW, Cabodi M, Held B, Gleghorn JP, Bonassar LJ, Stroock AD. Microfluidic scaffolds for tissue engineering. Nat Mater 2007;6: 908–15. [DOI] [PubMed] [Google Scholar]
- 30. Wylie RG, Shoichet MS. Three‐dimensional spatial patterning of proteins in hydrogels. Biomacromolecules 2011;12:3789–96. [DOI] [PubMed] [Google Scholar]
- 31. Jeschke B, Meyer J, Jonczyk A, Kessler H, Adamietz P, Meenen NM, Kantlehner M, Goepfert C, Nies B. RGD‐peptides for tissue engineering of articular cartilage. Biomaterials 2002;23: 3455–63. [DOI] [PubMed] [Google Scholar]
- 32. Akiyama SK. Integrins in cell adhesion and signaling. Hum Cell 1996;3:181–6. [PubMed] [Google Scholar]
- 33. Morra M, Cassinelli C. Surface studies on a model cell resistant system. Langmuir 1999;15:4658–63. [Google Scholar]
- 34. Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater 2009;8:457–470. [DOI] [PubMed] [Google Scholar]
- 35. Kong HJ, Mooney DJ. Microenvironmental regulation of biomacromolecular therapies. Nat Rev Drug Discov. 2007;6:455–63. [DOI] [PubMed] [Google Scholar]
- 36. Shen YH, Shoichet MS, Radisic M. Vascular endothelial growth factor immobilized in collagen scaffold promotes penetration and proliferation of endothelial cells. Acta Biomater. 2008;4:477–89. [DOI] [PubMed] [Google Scholar]
- 37. Shoichet MS. Polymer scaffolds for biomaterials applications. Macromolecules 2010;43:581–91. [Google Scholar]
- 38. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic‐Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 2008;16:585. [DOI] [PubMed] [Google Scholar]
- 39. Saltzman WM, Olbricht WL. Building drug delivery into tissue engineering. Nat Rev Drug Discov 2002;1:177–86. [DOI] [PubMed] [Google Scholar]
- 40. Huang S, Fu X. Naturally derived materials‐based cell and drug delivery systems in skin regeneration. J Control Release 2010;142:149–59. [DOI] [PubMed] [Google Scholar]
- 41. Akomeah FK, Martin GP, Brown MB. Variability in human skin permeability in vitro: comparing penetrants with different physicochemical properties. J Pharm Sci 2007;96:824–34. [DOI] [PubMed] [Google Scholar]


