Abstract
Because of changes in demography, non‐communicable diseases cause more deaths worldwide than infectious disease for the first time in history. One of the most prevalent of these maladies is diabetes mellitus, which resulted in 4·6 million deaths in 2011. There will be approximately 552 million people with diabetes worldwide by 2030. For these patients, one of the most common severe complications will be a foot wound. Patients with diabetes have at least a 25% lifetime risk of developing a foot ulcer. Many of these infections go on to amputation. Those patients have a 50% mortality rate in the 5 years following the initial amputation. Indeed, these problems are costly as well. In 2010, spending on diabetes was estimated to account for 11·6% of the total health care expenditure in the world. This review merges scientific evidence with expert experience to show the role of negative pressure wound therapy using reticulated open cell foam (V.A.C.® Therapy, KCI USA, Inc., San Antonio, TX) in limb preservation.
Keywords: Diabetic foot ulcer, Limb preservation, Negative pressure wound therapy, Venous leg ulcer
DIABETIC FOOT ULCERS
Diabetic foot ulcers (DFUs) are a challenging problem for clinicians 1, 2, 3, 4, 5, 6, 7, 8, 9. In the presence of neuropathy and the associated absence of pain, foot ulceration can be subjected unknowingly to repetitive pressure and shear on the sole of the foot 7, 10. The patient may be entirely unaware of the initial ulceration or its progression that occurs during daily walking. Redistribution of shear and pressure forces is a key aspect in the treatment approach to ulceration and surgical wounds in persons with diabetes. Unless these stresses are attenuated, healing wounds, already viscoelastically compromised by diabetes itself, are literally torn open by continued stress. This exposes the damaged integument which increases the risk for amputation – particularly when combined with peripheral arterial disease. One could liken this pathway to a stairway, where each factor on the step above contributes negatively to the aetiologic foundation below (Figure 1) (11).
Figure 1.
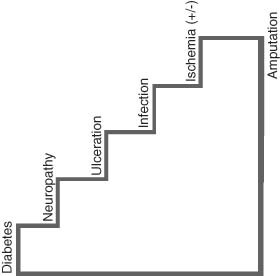
The stairway to amputation [Adapted from Ref 11].
To redistribute plantar pressure and unload ulcerative forces off of diabetic foot wounds, the gold standard method is the total contact cast (TCC) 12, 13, 14. In addition to redistributing pressure, it reduces the activity level of the patient, and because the TCC cannot be removed, it protects the foot from repetitive injury and bacterial contamination (15). In two randomised clinical trials TCCs, reapplied on a weekly basis, produced generally higher healing rates compared to other off‐loading modalities 13, 16. In addition, the proportion of patients in TCCs with healed wounds is better than those reported from clinical trials of wound healing with skin substitutes, electrical stimulation or other pressure reduction approaches. Critical downsides to the TCC technique are that it is time consuming, often not well tolerated by patients (especially the frail and elderly), and a technically challenging application that requires expertise and special supplies.
Several off‐the‐shelf commercially available devices, which simulate the TCC in pressure reduction are termed ‘removable cast walkers' (RCWs) (17). They are circumferential and attach (typically by Velcro) to the leg and extend from the foot to an area distal to the knee similar to a standard cast. Previous gait laboratory studies have suggested that certain RCWs offload the foot to a degree equivalent to that of TCCs (17).
MERGING EFFECTIVE PRESSURE OFFLOADING WITH NEGATIVE PRESSURE WOUND THERAPY (NPWT)
If RCWs reduce pressure to a degree equivalent to TCCs, why are they less effective in healing wounds? The answer may lie in the fact that these devices are (by their very name) removable. In a project conducted by our group using computerised activity monitoring systems, only 28% of the total activity undertaken by patients with diabetic foot wounds was conducted while wearing the RCW (18). It would stand to reason, therefore, that no advanced wound healing modality, such as NPWT, could stand up to this sort of repetitive stress or live up to its therapeutic potential. Therefore, we have proposed a simple solution: render the RCW irremovable by wrapping it with a cohesive bandage or a single layer of plaster of Paris. This has been termed an ‘instant total contact cast' (iTCC) (19).
Negative pressure wound therapy using reticulated open‐cell foam (NPWT/ROCF; V.A.C.® Therapy, KCI USA, Inc., San Antonio, TX) has become an increasingly used adjunct in treating the complex diabetic foot wound. This modality has been shown to both improve wound healing and reduce the risk for amputation 20, 21, 22. A multicentre randomised controlled trial (RCT) by Blume et al. (22) compared NPWT/ROCF with advanced moist wound therapy (AMWT) for the treatment of patients with DFUs. Within the 112‐day active treatment phase, a significantly greater number of DFUs closed with NPWT/ROCF compared to AMWT [73/169 (43·2%) versus 48/166 (28·9%), respectively; P = 0·007]. DFUs treated with NPWT/ROCF also had decreased time to closure, as indicated by the Kaplan–Meier median estimate for 100% ulcer closure [96 days (95% CI 75·0–114·0)] for NPWT versus not determinable for AMWT; P = 0·001). More importantly, there was a significant reduction in secondary amputations with NPWT/ROCF as compared to AMWT [7/169 (4·1%) versus 17/166 (10·2%), respectively; P = 0·035].
Newly available ultra‐lightweight, disposable, single‐patient use NPWT (SP‐NPWT) devices (V.A.C.Via™ Therapy System, KCI USA, Inc.) allow patients to take care of activities of daily living and still achieve the benefits of NPWT. However, walking unprotected on the NPWT hose/foam apparatus introduces potential risk for complications in a profoundly neuropathic foot. Therefore, current investigators have incorporated a bridging technique (See ‘Technical Pearls' below) to safely and effectively combine use of NPWT/ROCF with iTCC.
THE ROLE OF NPWT IN LIMB PRESERVATION
NPWT/ROCF has also become an important adjunctive technique in limb preservation, because this integrated wound care system has been shown to reduce incidence of infected DFUs, diabetic foot wounds and venous leg ulcers (VLUs), as well as subsequent lower extremity amputations 20, 23, 24, 25. An overarching goal of diabetic foot care and limb preservation is to move the patient as quickly, comfortably and safely as possible from an acute to a post‐acute setting. Resource utilisation data from the first large‐scale randomised trial of NPWT reported approximately 89% of total days of therapy were delivered whilst outpatient (26). This growing demand for outpatient usage has prompted industry to create smaller devices better geared towards portability, quiet operation and long battery life. This ‘bridge‐to‐home’ is seen as an important attribute of NPWT for years to come.
INITIATION CRITERIA AND WOUND BED PREPARATION
Initially, a thorough patient and wound assessment is important to determine treatment plans incorporating NPWT/ROCF with appropriate offloading techniques and good wound care practice (e.g. debridement and antibiotics) 27, 28. Other medical assessments include vascular and wound bed assessments to determine the optimal treatment plan for DFUs or VLUs. Debridement, infection and exudate management, and wound margin assessments should also be performed before initiation of NPWT/ROCF.
Managing the wound environment of a DFU is essential for proper wound healing. Several studies have shown successful use of NPWT/ROCF in preparing the wound bed for closure by promoting granulation tissue formation and decreasing the wound size 20, 22, 29, 30, 31.
TREATMENT GOALS
The main treatment goals for managing a DFU or VLU are to reduce the complexity and size of the ulcer. To that end, we prefer a ‘vertical’ and ‘horizontal’ wound healing strategy. The ‘vertical’ strategy involves filling in defects and covering vital structures with NPWT. The ‘horizontal’ philosophy involves skin grafting, assisting secondary healing through bioengineered tissue or other substances, and aggressive offloading. The ability of NPWT/ROCF to manage the wound environment and promote perfusion and granulation tissue formation allows for reduction of ulcer size and wound bed preparation for closure (32). This is crucial for preventing infections and amputations (33).
CONTRAINDICATIONS
Although there are no specific contraindications of NPWT/ROCF for DFUs or VLUs, those that would generally apply include malignancy in the wound, untreated osteomyelitis, and necrotic tissue with eschar present. Also, foam dressings should not come in direct contact with exposed vessels or organs. All contraindications and warnings can be found in the clinical guidelines (34).
TECHNICAL PEARLS: USE OF NPWT/ROCF WITH THE ITCC AND OTHER OFFLOADING MODALITIES ON PLANTAR WOUNDS
Using a technique known as ‘bridging,’ described by Greer and co‐workers (35), the foam remains on the plantar wound but the tubing and interface, which could potentially cause tissue injury and necrosis to the dorsum or side of the foot, are remote. Separated with a bridge of foam, the tubing and pressure‐sensing pad are moved to the dorsum of the foot or even more proximally up the anterior aspect of the removable cast boot or out the end of the device. This entire construct can then be wrapped in a cohesive bandage, allowing the patient to walk in a protected fashion with the device in place whilst ensuring adherence to pressure offloading (36). Dressings may be changed every other day (or three times per week). An available bridge dressing (V.A.C.® GranuFoam™ Bridge Dressing, KCI USA, Inc.) that allows concomitant use of NPWT with offloading devices or compression products is ideally suited for this type of technique in many cases.
A gait laboratory study performed by Armstrong and colleagues (36) evaluated the effect of bridging compared with standard offloading without applied NPWT in ten patients. While there was an increase (statistically significant) in plantar pressure [22 kPa (9·9%), Figure 2] with NPWT applied, the authors concluded that this increase in pressure does not add undue stress to the plantar aspect of the foot and still allows for the benefits of NPWT and sufficient offloading.
Figure 2.
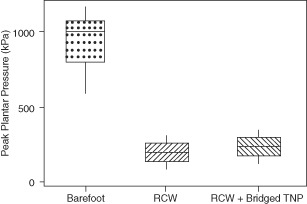
Plantar pressure in bridged topical negative pressure device [Adapted from Ref. 36] [RCW, removable cast walker; Bridged TNP, topical negative pressure (i.e. NPWT) device applied plantarly with foam dressing bridged to dorsum of foot.].
CLINICAL CASES
Case study 1
This 59‐year‐old patient shows the use of NPWT/ROCF on a typical wound following emergency debridement for a deep‐space diabetic foot infection. Initial wound shows progression from 24 hours following intraoperative debridement to 4 weeks following bridged NPWT with RCW protection to 3 more weeks in a TCC followed by healing after 9 weeks. Dressings were changed every other day (Figure 3).
Figure 3.
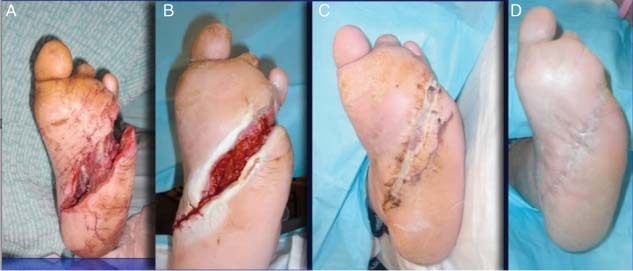
Case study 1: (A) Initial wound presentation following intraoperative debridement. (B) After 4 weeks of bridged negative pressure wound therapy using reticulated open cell foam. (C) Three weeks of total contact cast. (D) Wound healed after 9 weeks.
Case study 2
This 39‐year‐old diabetic male presented with a septic right foot. Plantar wound was debrided followed by NPWT/ROCF. The bridging technique was used to prepare the wound for skin grafting. Dressings were changed every other day (Figure 4).
Figure 4.
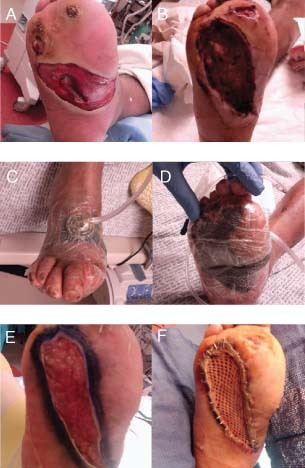
Case study 2: (A) Initial presentation of a plantar wound. (B) Debridement of wound. (C) Application of negative pressure wound therapy using reticulated open cell foam (NPWT/ROCF). (D) Bridging technique. (E) Granulation tissue formation after 3 weeks of NPWT/ROCF. (F) Application of split‐thickness skin graft.
Case study 3
Treatment of this 60‐year‐old male patient illustrates the use of a portable, SP‐NPWT (V.A.C.Via™ Therapy, KCI USA, Inc.) and a bridge dressing on a partial calcanectomy debridement with 4 weeks of outpatient therapy. Dressings were changed every other day (Figure 5).
Figure 5.
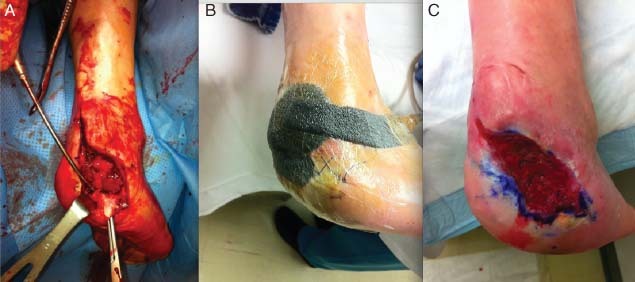
Case study 3: (A) Debridement of a partial calcanectomy. (B) Application of single‐patient use negative pressure wound therapy using the bridging technique for 4 weeks. (C) Wound after 4 weeks of therapy.
FUTURE DIRECTIONS AND ECONOMIC VALUE
There is enhanced optimism with these difficult DFU cases. By merging established and effective treatment modalities and bringing them to the amputation prevention team, the toll of diabetic foot ulceration can be further reduced. This has tremendous implications from an economical standpoint because the annual costs of DFUs and lower extremity amputations alone are estimated at $18·9 billion and $11·7 billion, respectively (2007 US dollars) (5). A multidisciplinary approach is vital for successful limb preservation (23); teams of doctors and nurses, in almost any permutation, help in reducing the risk for amputation 37, 38, 39, 40, 41, 42. Merging clinicians with interest in managing the structural components and specific aspects of lower extremity wound healing with those specialised in open and endovascular intervention appears to yield natural clinical outcomes. This is particularly true when this ‘toe and flow’11, 43 concept is surrounded by excellent general and specialist medical care for the patient with diabetes.
Wound care costs are substantial for the health care system, and cost‐effectiveness studies are necessary in selecting therapies that maximise clinical outcomes with budget limitations. For example, Apelqvist et al. evaluated economic costs for the treatment of diabetic foot wounds using NPWT/ROCF and standard moist wound therapy based on clinical outcomes from an RCT by Armstrong and Lavery and found that the average total cost to achieve healing was lower using NPWT/ROCF 20, 26. Other studies have also showed the cost effectiveness of NPWT/ROCF for the treatment of acute and chronic wounds 24, 32, 33, 44, 45. Further works in this area should focus on comparative effectiveness between technologies measuring not only time to healing and cost but also quality of life using standard and portable form factors.
ACKNOWLEDGEMENT
We would like to thank Julissa Ramos, PhD (KCI, Inc.) for assisting with preparation of the manuscript.
CONFLICTS OF INTEREST
Dr DA and Dr GA have Consulting agreements with Kinetic Concepts, Inc. This article is part of an educational supplement funded by Kinetic Concepts, Inc. to provide an overview of the V.A.C.® Therapy family of products for new users in developing markets. Targeted for distribution at the 2012 World Union of Wound Healing Societies (WUWHS) conference, this supplement article presents a brief literature review and clinical experience treating diabetic foot and leg ulcers with V.A.C.® Therapy.
REFERENCES
- 1. Murphy SL, Xu J, Kochanek KD. Deaths: preliminary data for 2010. National vital statistics reports; Vol. 60 No. 4. Hyattsville, MD: National Center for Health Statistics. 2012. Available from: http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_04.pdf
- 2. International Diabetes Federation. IDF Diabetes Atlas, 5th edn. Brussels: International Diabetes Federation (IDF), 2011. [Google Scholar]
- 3. Hogan P, Dall T, Nikolov P, American Diabetes Association. Economic costs of diabetes in the US in 2002. Diabetes Care 2003;26:917–32. [DOI] [PubMed] [Google Scholar]
- 4. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28. [DOI] [PubMed] [Google Scholar]
- 5. Rogers LC, Lavery LA, Armstrong DG. The right to bear legs–an amendment to healthcare: how preventing amputations can save billions for the US Health‐care System. J Am Podiatr Med Assoc 2008;98:166–8. [PubMed] [Google Scholar]
- 6. Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care 1990;13:513–21. [DOI] [PubMed] [Google Scholar]
- 7. Armstrong DG, Nguyen HC, Lavery LA, van Schie CH, Boulton AJ, Harkless LB. Off‐loading the diabetic foot wound: a randomized clinical trial. Diabetes Care 2001;24:1019–22. [DOI] [PubMed] [Google Scholar]
- 8. Armstrong DG, Wrobel J, Robbins JM. Guest Editorial: are diabetes‐related wounds and amputations worse than cancer? 2007;4:286–7. [DOI] [PubMed] [Google Scholar]
- 9. Zhang P, Zhang X, Brown JB, Vistisen D, Sicree RA, Shaw J, Nichols GA. Economic impact of diabetes. IDF Diabetes Atlas. 4th edn. Brussels: International Diabetes Federation (IDF), 2009. [Google Scholar]
- 10. Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ. Predictive value of foot pressure assessment as part of a population‐based diabetes disease management program. Diabetes Care 2003;26:1069–73. [DOI] [PubMed] [Google Scholar]
- 11. Rogers LC, Andros G, Caporusso J, Harkless LB, Mills JL Sr, Armstrong DG. Toe and flow: essential components and structure of the amputation prevention team. J Vasc Surg 2010;52(3 Suppl):23S–7S. [DOI] [PubMed] [Google Scholar]
- 12. Armstrong DG, Boulton AJ. Pressure offloading and “advanced” wound healing: isn't it finally time for an arranged marriage? Int J Low Extrem Wounds 2004;3:184–7. [DOI] [PubMed] [Google Scholar]
- 13. Bus SA, Valk GD, van Deursen RW, Armstrong DG, Caravaggi C, Hlavácek P, Bakker, K , Cavanagh PR. The effectiveness of footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in diabetes: a systematic review. Diabetes Metab Res Rev 2008;24(1 Suppl):S162–S180. [DOI] [PubMed] [Google Scholar]
- 14. Wu SC, Crews RT, Armstrong DG. The pivotal role of offloading in the management of neuropathic foot ulceration. Curr Diab Rep 2005;5:423–9. [DOI] [PubMed] [Google Scholar]
- 15. Mueller MJ, Diamond JE, Sinacore DR, Delitto A, Blair VP III, Drury DA, Rose SJ. Total contact casting in treatment of diabetic plantar ulcers. Controlled clinical trial. Diabetes Care 1989;12:384–8. [DOI] [PubMed] [Google Scholar]
- 16. Bus SA, Valk GD, van Deursen RW, Armstrong DG, Caravaggi C, Hlavácek P, Bakker, K , Cavanagh PR. Specific guidelines on footwear and offloading. Diabetes Metab Res Rev 2008;24(1 Suppl):S192–S193. [DOI] [PubMed] [Google Scholar]
- 17. Lavery LA, Vela SA, Lavery DC, Quebedeaux TL. Reducing dynamic foot pressures in high‐risk diabetic subjects with foot ulcerations. A comparison of treatments. Diabetes Care 1996;19:818–21. [DOI] [PubMed] [Google Scholar]
- 18. Armstrong DG, Lavery LA, Kimbriel HR, Nixon BP, Boulton AJ. Activity patterns of patients with diabetic foot ulceration: patients with active ulceration may not adhere to a standard pressure off‐loading regimen. Diabetes Care 2003;26:2595–7. [DOI] [PubMed] [Google Scholar]
- 19. Armstrong DG, Short B, Espensen EH, Abu‐Rumman PL, Nixon BP, Boulton AJ. Technique for fabrication of an “instant total‐contact cast” for treatment of neuropathic diabetic foot ulcers. J Am Podiatr Med Assoc 2002;92:405–8. [DOI] [PubMed] [Google Scholar]
- 20. Armstrong DG, Lavery LA, Diabetic Foot Study Consortium. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704–10. [DOI] [PubMed] [Google Scholar]
- 21. Armstrong DG, Lavery LA, Abu‐Rumman P, Espensen EH, Vazquez JR, Nixon BP, Boulton AJ. Outcomes of subatmospheric pressure dressing therapy on wounds of the diabetic foot. Ostomy Wound Manage 2002;48:64–8. [PubMed] [Google Scholar]
- 22. Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum‐assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care 2008;31:631–6. [DOI] [PubMed] [Google Scholar]
- 23. Sumpio BE, Driver VR, Gibbons GW, Holloway GA, Joseph WS, Lavery LA, McGuigan FX, Steinberg J, Anderson CA, Blume P, Attinger CE. A multidisciplinary approach to limb preservation: The role of V.A.C. therapy. Wounds 2009;21(9 Suppl 2):1–19. 25904579 [Google Scholar]
- 24. Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State‐of‐the‐art treatment of chronic leg ulcers: a randomized controlled trial comparing vacuum‐assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg 2006;44:1029–38. [DOI] [PubMed] [Google Scholar]
- 25. Kieser DC, Roake JA, Hammond C, Lewis DR. Negative pressure wound therapy as an adjunct to compression for healing chronic venous ulcers. J Wound Care 2011;20:35–7. [DOI] [PubMed] [Google Scholar]
- 26. Apelqvist J, Armstrong DG, Lavery LA, Boulton AJ. Resource utilization and economic costs of care based on a randomized trial of vacuum‐assisted closure therapy in the treatment of diabetic foot wounds. Am J Surg 2008;195:782–8. [DOI] [PubMed] [Google Scholar]
- 27. Harding K. Vacuum assisted closure: recommendations for use. A consensus document. Principles of Best Practice 2008;1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boulton A, Bowling F. Diabetic foot ulcers. In: Vinik AI, LeRoith D, editors. Controversies in treating diabetes. New York: Humana Press, 2008:229–35. [Google Scholar]
- 29. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76. [PubMed] [Google Scholar]
- 30. Eginton MT, Brown KR, Seabrook GR, Towne JB, Cambria RA. A prospective randomized evaluation of negative‐pressure wound dressings for diabetic foot wounds. Ann Vasc Surg 2003;17:645–9. [DOI] [PubMed] [Google Scholar]
- 31. Page JC, Newswander B, Schwenke DC, Hansen M, Ferguson J. Retrospective analysis of negative pressure wound therapy in open foot wounds with significant soft tissue defects. Adv Skin Wound Care 2004;17:354–64. [DOI] [PubMed] [Google Scholar]
- 32. Baharestani MM, Driver VR, De Leon JM, Gabriel A, Kaplan M, Lantis J, et al. Optimizing clinical and cost effectiveness with early intervention of V.A.C. therapy. Ostomy Wound Manage 2008;54(11 Suppl):1–15. [Google Scholar]
- 33. Driver VR, De Leon JM. Health economic implications for wound care and limb preservation. J Manag Care Med 2008;11:13–9. [Google Scholar]
- 34. V.A.C. Therapy clinical guidelines: a reference source for clinicians. San Antonio: Kinetic Concepts, Inc.,2010. [Google Scholar]
- 35. Greer SE, Duthie E, Cartolano B, Koehler KM, Maydick‐Youngberg D, Longaker MT. Techniques for applying subatmospheric pressure dressing to wounds in difficult regions of anatomy. J Wound Ostomy Continence Nurs 1999;26:250–3 [DOI] [PubMed] [Google Scholar]
- 36. Armstrong DG, Kunze K, Martin BR, Kimbriel HR, Nixon BP, Boulton AJ. Plantar pressure changes using a novel negative pressure wound therapy technique. J Am Podiatr Med Assoc 2004;94:456–60. [DOI] [PubMed] [Google Scholar]
- 37. Sumpio BE, Armstrong DG, Lavery LA, Andros G. The role of interdisciplinary team approach in the management of the diabetic foot: a joint statement from the Society for Vascular Surgery and the American Podiatric Medical Association. J Vasc Surg 2010;51:1504–6. [DOI] [PubMed] [Google Scholar]
- 38. Rogers LC, Bevilacqua NJ. Organized programs to prevent lower‐extremity amputations. J Am Podiatr Med Assoc 2010;100:101–4. [DOI] [PubMed] [Google Scholar]
- 39. Driver VR, Madsen J, Goodman RA. Reducing amputation rates in patients with diabetes at a military medical center: the limb preservation service model. Diabetes Care 2005;28:248–53. [DOI] [PubMed] [Google Scholar]
- 40. Alexandrescu V, Hubermont G, Coessens V, Philips Y, Guillaumie B, Ngongang C, Vincent G, Azdad K, Ledent G, De Marre C, Macoir C. Why a multidisciplinary team may represent a key factor for lowering the inferior limb loss rate in diabetic neuro‐ischaemic wounds: application in a departmental institution. Acta Chir Belg 2009;109 :694–700. [DOI] [PubMed] [Google Scholar]
- 41. Canavan RJ, Unwin NC, Kelly WF, Connolly VM. Diabetes‐ and nondiabetes‐related lower extremity amputation incidence before and after the introduction of better organized diabetes foot care: continuous longitudinal monitoring using a standard method. Diabetes Care 2008;31:459–63. [DOI] [PubMed] [Google Scholar]
- 42. Krishnan S, Nash F, Baker N, Fowler D, Rayman G. Reduction in diabetic amputations over 11 years in a defined U.K. population: benefits of multidisciplinary team work and continuous prospective audit. Diabetes Care 2008;31:99–101. [DOI] [PubMed] [Google Scholar]
- 43. Rogers LC, Armstrong DG. Podiatry Care. In: Cronenwett JL, Johnston KW, editors. Rutherford's vascular surgery, 7th edn. Philadelphia: Saunders Elsevier, 2010:1747–60. [Google Scholar]
- 44. Kaplan M, Daly D, Stemkowski S. Early intervention of negative pressure wound therapy utilizing vacuum assisted closure in trauma patients: impact on hospital length of stay and cost. Adv Skin Wound Care 2009;22:128–32. [DOI] [PubMed] [Google Scholar]
- 45. Schwien T, Gilbert J, Lang C. Pressure ulcer prevalence and the role of negative pressure wound therapy in home health quality outcomes. Ostomy Wound Manage 2005;51:47–60. [PubMed] [Google Scholar]


