Abstract
This was an open‐label, prospective, multicentre, randomised controlled study to evaluate the efficacy and safety of human fibroblast‐derived dermal substitute (HFDS) plus four‐layer compression therapy compared with compression therapy alone in the treatment of venous leg ulcers. The primary outcome variable was the proportion of patients with completely healed study ulcers by 12 weeks. The number healed was further summarised by ulcer duration and baseline ulcer size. Sixty‐four (34%) of 186 patients in the HFDS group experienced healing by week 12 compared with 56 (31%) of 180 patients in the control group (P = 0·235). For ulcers ≤ 12 months duration, 49 (52%) of 94 patients in the HFDS group versus 36 (37%) of 97 patients in the control group healed at 12 weeks (P = 0·029). For ulcers ≤ 10 cm2, complete healing at week 12 was observed in 55 (47%) of 117 patients in the HFDS group compared with 47 (39%) of 120 patients in the control group (P = 0·223). The most common adverse events (AEs) were wound infection, cellulitis and skin ulcer. The frequency of AEs did not markedly differ between the treatment and control groups.
Keywords: Fibroblasts, Skin, Ulcer, Venous ulcer, Wound healing
Introduction
Venous leg ulcers (VLUs) have a lifetime prevalence of approximately 1% 1 and remain a challenging problem in clinical practice. In the majority of patients, the principal cause is ambulatory venous hypertension occurring as the result of valvular incompetence and venous reflux 2. Lower limb compression to counteract these effects is the standard of care for treatment 2, but, unfortunately, it often fails to achieve healing in a timely fashion. Success rates are reported to range from 30% to 65% after 24 weeks of therapy 3.
A complex series of cellular and growth factor deficiencies are present in the ulcer environment. Venous hypertension may cause blood proteins to leak in the extravascular space, trapping growth factors and cytokines needed for tissue repair. Leakage of fibrinogen from veins as well as deficiencies in fibrinolysis may also cause fibrin to build up around the vessels, preventing oxygen and nutrients from reaching cells 4. In a group of sequential biopsies taken from the margins of VLUs during their healing, a deficiency of fibronectin has been observed in granulation tissue compared with the surrounding normal dermis 5. In another series, venous ulcers were shown to be deficient in oxygen compared with normal tissue 6. As a consequence, the conditions of the VLU wound environment may not efficiently support the epidermal migration, attachment and proliferation of regenerating cells needed for healing.
Effective treatment of non healing VLUs may require, in addition to compression bandaging, the provision of a suitable substrate, specifically a dermal replacement capable of promoting epithelialisation of the wound. Dermagraft® (Shire Regenerative Medicine, San Diego, CA), a human fibroblast‐derived dermal substitute (HFDS), consists of dermal fibroblasts derived from newborn human foreskin tissue and cultured in vitro onto a bioresorbable polyglactin mesh. The fibroblasts secrete human dermal collagen, matrix proteins, growth factors and cytokines as they proliferate throughout the three‐dimensional mesh and become deposited in a self‐produced human dermal matrix. The result is a living, metabolically active dermal tissue.
The results of pilot studies with HFDS suggested higher rates of complete wound epithelialisation with the combination of HFDS (using various application regimens) and standard therapy compared with standard therapy alone. On the basis of the favourable results of this and other preliminary studies, including a study in which a four‐piece regimen of HFDS yielded a greater proportion of patients who achieved complete healing (38%) compared with compression alone (15%) 7, a pivotal randomised controlled study was conducted to evaluate the efficacy and safety of HFDS and standard therapy compared with standard therapy alone.
Compression bandaging techniques vary widely from country to country. For the purposes of standardisation, all patients received compression therapy using the Profore™ four‐layer compression system (Smith & Nephew, Hull, UK). This four‐layer system consists of a layer of orthopaedic padding to absorb wound exudate and protect bony prominences, a light conformable bandage, an elastic compression bandage and an elastic cohesive bandage to apply additional compression and hold all bandages in place. Four‐layer compression therapy has demonstrated effectiveness in healing some chronic wound ulcerations 8.
Methods
Patients or participants
Patients at least 18 years of age who were referred to participating hospital or community‐based VLU clinics in the UK, the USA or Canada were eligible for the study. Patients were required to have a VLU located between the knee and ankle (at the level of, and including, the lateral and medial malleolus) that was present for at least 2 months and no more than 5 years prior to screening. The patient's ulcer had to (i) be 3–25 cm2 in size; (ii) have a typical appearance of a VLU without exposure of muscle, tendon or bone and (iii) have clean, granulating base with minimal adherent slough, suitable to receive a skin graft. Ulcers that reduced in size (cm2) by less than 50% while under compression therapy during the 2‐week screening period of the study were eligible for randomisation into the study.
Sufficient circulation to the study leg to make wound healing possible was required. To be included in the study, patients had to have an ankle brachial pressure index between 0·8 and 1·2 and venous disease had to be confirmed by duplex ultrasonography to demonstrate reflux of >0·5 seconds in saphenous, calf perforator or popliteal veins. Patients whose ulcers were deemed by the investigator to be caused by a medical condition other than venous insufficiency were excluded. Patients who had evidence of sinus tracts within their ulcer or evidence of a wound infection (purulence and/or odour), cellulitis and/or confirmed osteomyelitis were not enrolled. Other key exclusion criteria included morbid obesity, skin diseases near study ulcer, malignant disease within 5 years, severe peripheral vascular disease or renal disease, congestive heart failure, cell anaemia, thalassemia or uncontrolled diabetes. Patients who had received immune suppressants, systemic corticosteroids, cytotoxic chemotherapy or topical steroids for more than 2 weeks and within 1 month of initial screening or who had a history of radiation at the ulcer site were not included in the study. Patients with a known allergy to bovine products or components of the compression bandage, or who could not tolerate compression bandage therapy, had received an investigational drug within 30 days of randomisation or had been previously treated with HFDS and/or other tissue‐engineered materials were also excluded.
Study design and treatment
This was an open‐label, prospective, multicentre, randomised controlled study that evaluated HFDS plus four‐layer compression therapy compared with conventional therapy (compression therapy alone) in the treatment of VLUs. The patients gave informed consent. This study was conducted under independent Ethics Committees and Institutional Review Boards, and it was performed in accordance with the guidelines of the Declaration of Helsinki (1964) and subsequent revisions.
During a 2‐week screening period, a study ulcer was identified for each patient. Only one ulcer was selected for the study; if a patient had more than one ulcer, the largest ulcer that met the inclusion criteria was selected. Wound tracing and ulcer area grid evaluations were performed to determine percentage of healing between screening and randomisation. During the screening period, all subjects were treated with a standard dressing regimen including four‐layer compression bandaging. Each wound was covered with a layer of non adherent dressing (Dermanet®, DeRoyal, Powell, TN) followed by the appropriately sized compression bandage, which was determined by measuring the circumference of the patient's ankle 1 inch above the tibial malleolus. For deeper ulcers, extra light packing (gauze) could be used on top of the non adherent dressing to ensure adequate contact with the base of the ulcer. For heavily exuding ulcers, a further absorbent dressing could be applied at the discretion of the investigator. During the 2‐week screening period, the wound was observed weekly to determine that the ulcer was free of necrotic tissue, clear of infection and had a vascular bed. If clinically indicated, the dressing could be changed earlier.
Prior to randomisation, surgical debridement of the study ulcer could be performed at the discretion of the investigator. After confirming the absence of clinical infection or necrotic tissue, the wound was rinsed with normal saline, the ulcer was traced and the area of the ulcer was calculated using planimetry analysis. Randomisation was conducted in blocks of either two or four to ensure that study personnel could not predict treatment allocation. Randomisation was stratified by study centre and by study ulcer area. The two strata for ulcer size were ulcers from ≥3 to ≤10 cm2 and from >10 to ≤25 cm2. Patients were randomised to receive HFDS plus the four‐layer compression bandage therapy (active) or the four‐layer compression bandage therapy alone (control). Patients randomised to the active treatment group received HFDS applied to the wound at weeks 0, 1, 4 and 8. Before implantation, HFDS was thawed, rinsed and prepared according to directions for use. The prepared HFDS was cut to fit the shape of the ulcer and to accommodate any epithelial islands, placed into the wound bed with no overlap onto the intact skin surrounding the ulcer and smoothed gently to ensure that the entire piece of HFDS was in contact with the wound surface. The wound‐dressing regimen was identical for both treatment groups and was identical to that used during the screening period.
Assessments
Efficacy assessment
The primary outcome variable was the proportion of patients with completely healed study ulcers by 12 weeks. Complete healing was defined by having a ‘closed wound’ for two consecutive weekly visits. A closed wound was defined as full epithelialisation of the wound with the absence of drainage (i.e. no exudate or scab). The primary outcome of complete healing was ascertained by the primary investigator by visual inspection. The primary outcome variable was also summarised by duration of current ulceration and baseline ulcer size (≤10 and >10 cm2).
Secondary outcome variables included time to healing, complete healing by week 24 (follow‐up endpoint) and percent reduction in ulcer area. The area of the study ulcer was calculated using planimetry analysis. After debridement (if required) and cleansing, ulcer tracings were obtained by placing a clear plastic bag over the patient's wound and making a direct tracing at the edge of the intact epithelium and around epithelial islands, if present. Whenever possible, the same person performed the tracing at every visit. Once the bag was removed from the wound and directionally marked, planimetry was performed on each tracing to calculate the ulcer area.
Safety assessment
Adverse events (AEs) were identified and grouped by system organ class using the Dictionary for Medical Drug Regulatory Activities (MedDRA) Version 7. The AEs were reported according to guidelines specific to the country where the treatment centres were located. Patients exhibiting serious and/or unexpected adverse drug reactions were withdrawn from the study at the discretion of the investigator.
Statistical analysis
Sample size analysis called for 166 patients in each treatment group to detect a 15% difference in the proportion of patients who achieve complete healing at week 12. The sample size calculation was based on a χ 2 test for the comparison of proportions of healing using a healing rate of 32% for control and 47% for HFDS with a 0·05 two‐sided significance level and at least 80% power. The intent‐to‐treat (ITT) population was defined as all patients receiving study treatment at baseline and having a follow‐up visit post‐baseline. The safety population was defined as all patients with a baseline visit.
Descriptive statistics were used to report baseline demographics and characteristics. The primary outcome variable, a binary measure of healed or not healed by 12 weeks, was assessed for the ITT population after adjusting for covariates including treatment, ulcer area at baseline and duration of current ulceration using an initial logistical regression model. A forward selection procedure was used for the addition of other baseline covariates with an F of 0·1. The 95% confidence interval (CI) (unadjusted for all covariates) for the difference between treatments in the percent healed by 12 weeks was generated along with the χ 2 test P value. The percent reduction in ulcer area per week was compared between treatment groups using the Mann–Whitney test. No multiplicity testing across secondary endpoints was performed to control for type 1 error. The number of patients with an AE was compared between treatment groups using a two‐sided Fisher's exact test. A P value of ≤0·05 was considered significant.
Results
Patients
Of the 573 patients screened, 207 failed screening (36% screen failure rate). The most common reasons for screen failure were study ulcers reducing in size by more than 50% during screening, study ulcers less than 3 cm2 at randomisation and subjects without evidence of venous reflux. The remaining 366 patients were randomised to receive treatment at a total of 25 centres: 19 in the UK, 1 in Canada and 5 in the USA. The ITT population included 186 patients in the HFDS group and 180 patients in the control group.
A total of 10% (19 of 186) of patients in the HFDS group discontinued the study early compared with 23% (41 of 180) of patients in the control group. The reasons for early discontinuation were AE (3% in the HFDS group versus 6% in the control group), patient's own request (2% versus 9%), patient lost to follow‐up (2% versus 3%) and ‘other’ (4% from each group).
The mean age of the ITT population was 68·5 years, 46% of patients were male, 54% were female and most were Caucasian (92%). The mean body mass index was 30·1 kg/m2 (Table 1).
Table 1.
Baseline demographics and characteristics
| Parameter | HFDS plus compression therapy (N = 186) | Compression therapy (N = 180) |
|---|---|---|
| Mean age, years (SD) | 67·9 (13·8) | 69·1 (12·4) |
| Sex, n (%) | ||
| Female | 100 (53·8) | 97 (53·9) |
| Male | 86 (46·2) | 83 (46·1) |
| Race, n (%) | ||
| White | 173 (93·0) | 164 (91·1) |
| Black | 5 (2·7) | 8 (4·4) |
| Asian | 3 (1·6) | 1 (0·6) |
| Other | 5 (2·7) | 7 (3·9) |
| Mean body mass index, kg/m2 (SD) | 30·0 (6·4) | 30·1 (6·8) |
| Median duration of current study ulcer, weeks (range) | 49·7 (8·9–262·1) | 45·3 (9·9–470·4) |
| Median ulcer size, cm2 (range) | 7·4 (2·4–28·2) | 7·2 (2·3–26·6) |
| Study ulcer has healed and recurred, n (%) | 104 (56) | 86 (48) |
| Median duration since the ulceration first appeared at the study site, weeks (range) | 158·7 (8·9–3121·9) | 125·4 (9·9–3122·0) |
HFDS, human fibroblast‐derived dermal substitute; SD, standard deviation.
The treatment groups were generally well balanced with regard to baseline characteristics, including key study ulcer characteristics known to be associated with ulcer healing. The median duration of the current ulcer was 49·7 weeks in the HFDS group and 45·3 weeks in the control group; the median study ulcer area was 7·4 cm2 in the HFDS group and 7·2 cm2 in the control group. The groups were also well balanced with regard to gender, height, study ulcer health state, percentage of unhealthy tissue and ankle circumference.
Assessments
Efficacy assessments
Primary endpoint
Sixty‐four (34%) of 186 patients in the HFDS group experienced healing by week 12 compared with 56 (31%) of 180 patients in the control group (P = 0·235, odds ratio = 1·40, 95% CI = 0·80, 2·41) (Figure 1). Evidence of a treatment by duration of the current study ulcer interaction was observed (odds ratio, ulcer duration HFDS: ulcer duration control = 0·99, 95% CI = 0·98, 1·00). Because the primary analysis assumed a consistent treatment effect across the baseline duration of the current study ulcer, rather than the inconsistent effect that was observed, the primary analysis did not hold. After identification of the treatment‐by‐ulcer interaction, a pre‐specified subgroup analysis evaluating complete healing by ulcer duration was performed. For the subgroup of patients with ulcer duration of 12 months or less, healing by week 12 was observed in significantly more patients in the HFDS group compared with the control group, 49 (52%) of 94 patients versus 36 (37%) of 97 patients, respectively (P = 0·029, odds ratio 2·37, 95% CI = 1·08, 5·14) (Figure 2).
Figure 1.
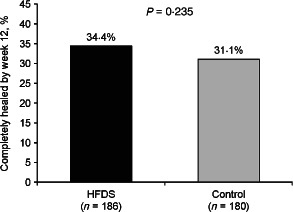
Percent of patients with complete healing of study ulcer by week 12 (overall intent‐to‐treat population). HFDS, human fibroblast‐derived dermal substitute.
Figure 2.
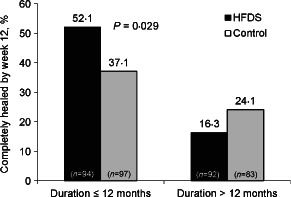
Effect of ulcer duration on percent of patients with complete healing of study ulcer by week 12 (intent‐to‐treat population). HFDS, human fibroblast‐derived dermal substitute.
In the subgroup of patients with ulcers 10 cm2 or less, complete healing at week 12 was observed in 55 (47%) of 117 patients in the HFDS group compared with 47 (39%) of 120 patients in the control group, but this difference did not reach statistical significance (Figure 3).
Figure 3.
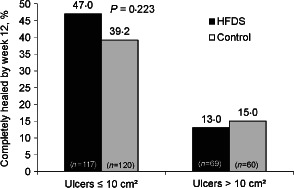
Effect of initial ulcer area on percent of patients with complete healing of study ulcer by week 12 (intent‐to‐treat population). HFDS, human fibroblast‐derived dermal substitute.
Secondary analyses
For the full ITT population, 96 (52%) of 186 patients in the HFDS group and 88 (49%) of 180 patients in the control group achieved complete healing by week 24. No significant difference between treatment groups was observed in the time to healing (P = 0·660, hazard ratio = 1·07, 95% CI = 0·80, 1·43). At week 12, the median percentage reduction in ulcer area was 83·7% in the HFDS group compared with 73·0% in the control group.
Safety assessments
The overall incidence and type of AEs were comparable between the HFDS and control groups. One hundred forty‐six (78%) of 187 patients in the HFDS group reported 444 AEs and 138 (77%) of 179 patients in the control group reported 472 AEs (P = 0·900).
The most common treatment‐emergent AEs were wound infection, cellulitis and skin ulcer (Table 2). These events were expected in this population and their frequencies did not markedly differ between the treatment and control group. A total of 39 AEs were reported in 21 patients (11% of all patients) assessed by the investigator as probably (4 patients) or possibly (35 patients) related to HFDS; none of which was serious. There were 24 serious/severe AEs in the HFDS group and 33 serious/severe AEs in the control group.
Table 2.
Most commonly reported treatment‐emergent adverse events (safety population)a
| Adverse event, n (%) | HFDS plus compression therapy (N = 187) | Compression therapy (N = 179) |
|---|---|---|
| Infections and infestations | ||
| Wound infection | 55 (29·4) | 43 (24·0) |
| Study site infection | 43 (23) | 46 (26) |
| Cellulitis | 12 (6·4) | 18 (10·1) |
| Nasopharyngitis | 12 (6·4) | 6 (3·4) |
| Skin and subcutaneous tissue disorders | ||
| Skin ulcer | 25 (13·4) | 39 (21·8) |
| Pruritus | 13 (7·0) | 6 (3·4) |
| Venous ulcer pain | 10 (5·3) | 9 (5·0) |
| Stasis dermatitis | 10 (5·3) | 6 (3·4) |
| Skin disorder | 8 (4·3) | 15 (8·4) |
| Injury, poisoning and procedural complications | ||
| Fall | 6 (3·2) | 11 (6·1) |
| General disorders and administration site conditions | ||
| Peripheral oedema | 13 (7·0) | 5 (2·8) |
| Musculoskeletal and connective tissue disorders | ||
| Pain in extremity | 9 (4·8) | 10 (5·6) |
HFDS, human fibroblast‐derived dermal substitute.
Adverse events reported in ≥5% in either treatment group.
There was no evidence of a difference between treatment groups in the numbers of patients experiencing a study site infection. During the course of the study, a study site infection was observed in 43 (23%) of 187 patients in the HFDS group and 46 (26%) of 179 patients in the control group (P = 0·62).
Of the study ulcers that healed, 15% recurred in the HFDS group and 23% recurred in the control group during the 24‐week study period.
Discussion
We compared HFDS plus compression to compression alone for the treatment of VLUs. Statistical significance was not reached for the primary efficacy endpoint of number of patients with study ulcers completely healed by 12 weeks. As a result of an observed treatment by duration of study ulcer interaction, a subanalysis was conducted for patients with duration of study ulcer < 12 months, which showed more patients healed at week 12 in the HFDS versus control group.
Other advanced therapies combined with compression have been investigated for the treatment of VLU. Studies of pentoxifylline and Apligraf® (Organogenesis, Canton, MA), another tissue‐engineered product, have both demonstrated a benefit when used in addition to standard compression therapy to treat leg ulcer populations with median or mean ulcer sizes less than 5 cm2 9, 10. Baseline ulcer area is a significant predictor of time to heal 3, 8, 11, 12. In one multicentre study conducted by Phillips et al., healing was observed in 72% of patients with a baseline ulcer area of <5 cm2 compared with 40% of subjects with a baseline area of >5 cm2 11. In a study of Apligraf, a benefit was observed in treating ulcers older than 12 months 10. However, the mean ulcer sizes in both the active and standard care groups of this study were smaller than 2 cm2. By contrast, the ulcers in the current HFDS study were larger and thus harder to heal, having a mean area of 6·6 cm2 in the subgroup of patients with ulcers of 12‐month duration or less 10.
Ulcer duration is a well‐established marker predicting the likelihood of healing 3, 8, 11, 12. In the study by Phillips et al., a healing rate of 64% was observed with ulcers of less than 1‐year duration compared with 48% with ulcers of 1‐ to 3‐year duration 11. Similarly, in our study, a significant effect of HFDS was observed in ulcers of 12‐month duration or less, but not in those of over 12‐month duration.
The failure to demonstrate a significant benefit in older ulcers (i.e. >12‐month duration) in this study may be attributed to changes over time in the biological environment of the wound that may make older ulcers more resistant to healing. Older ulcers may have higher levels of cellular senescence 13, matrix impairment 14 and bacterial burden that interfere with healing 15. Older ulcers may also require additional applications of HFDS over a longer period of time to achieve significant improvement in the rates of healing. However, further investigation is needed.
In conclusion, HFDS did not show a statistically significant improvement over compression therapy alone for overall healing by week 12. However, efficacy appears to improve as ulcer duration decreases, suggesting clinical merit for earlier use of adjuvant therapy. There were no differences between the groups in reported AEs. The safety profile of HFDS in the treatment of venous ulcers is comparable to that of standard therapy.
Acknowledgements
The authors take full responsibility for the contents of this manuscript and verify that they have met all of the journal's requirements for authorship. The authors thank The JB Ashtin Group, Inc., for assistance in preparing this manuscript for publication based on the authors' input and direction. Financial support for editorial assistance was provided by Smith & Nephew Wound Management, Hull, UK, and Shire Regenerative Medicine, San Diego, California, USA. Prof. KH discloses that the Wound Healing Research Unit is a self‐funded group in Cardiff University that receives funding from a variety of academic and commercial concerns. These include 3M, Convatec, Covidien, Frontier Therapeutics, KCI, Photopharmica, Shire Regenerative Medicine (formally Advanced BioHealing), Smith & Nephew and Tissue Therapies. Dr MS and Mr MC are full‐time employees of Shire Regenerative Medicine in San Diego, California, USA.
Harding K, Sumner M, Cardinal M. A prospective, multicentre, randomised controlled study of human fibroblast‐derived dermal substitute (Dermagraft) in patients with venous leg ulcers.
References
- 1. Nelzen O. Prevalence of venous leg ulcer: the importance of the data collection method. Phlebolymphology 2008;15:143–50. [Google Scholar]
- 2. Kunimoto B, Cooling M, Gulliver W, Houghton P, Orsted H, Sibbald RG. Best practices for the prevention and treatment of venous leg ulcers. Ostomy Wound Manage 2001;47:34–46 48–50. [PubMed] [Google Scholar]
- 3. Margolis DJ, Berlin JA, Strom BL. Risk factors associated with the failure of a venous leg ulcer to heal. Arch Dermatol 1999;135:920–6. [DOI] [PubMed] [Google Scholar]
- 4. Brem H, Kirsner RS, Falanga V. Protocol for the successful treatment of venous ulcers. Am J Surg 2004;188(1A Suppl):1–8. [DOI] [PubMed] [Google Scholar]
- 5. Herrick SE, Sloan P, McGurk M, Freak L, McCollum CN, Ferguson MW. Sequential changes in histologic pattern and extracellular matrix deposition during the healing of chronic venous ulcers. Am J Pathol 1992;141:1085–95. [PMC free article] [PubMed] [Google Scholar]
- 6. Falanga V, Moosa HH, Nemeth AJ, Alstadt SP, Eaglstein WH. Dermal pericapillary fibrin in venous disease and venous ulceration. Arch Dermatol 1987;123:620–3. [PubMed] [Google Scholar]
- 7. Krishnamoorthy L, Harding K, Griffiths D, Moore K, Leaper D, Poskitt K, Sibbald RG, Brassard A, Dolynchuk K, Adams J, Whyman M. The clinical and histological effects of Dermagraft® in the healing of chronic venous leg ulcers. Phlebology 2003;18:12–22. [Google Scholar]
- 8. Moffatt CJ, Franks PJ, Oldroyd M, Bosanquet N, Brown P, Greenhalgh RM, McCollum CN. Community clinics for leg ulcers and impact on healing. Br Med J 1992;305:1389–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Falanga V, Fujitani RM, Diaz C, Hunter G, Jorizzo J, Lawrence PF, Lee BY, Menzoian JO, Tretbar LL, Holloway GA, Hoballah J, Seabrook GR, McMillan DE, Wolf W. Systemic treatment of venous leg ulcers with high doses of pentoxifylline: efficacy in a randomized, placebo‐controlled trial. Wound Repair Regen 1999;7:208–13. [DOI] [PubMed] [Google Scholar]
- 10. Falanga V, Sabolinski M. A bilayered living skin construct (APLIGRAF) accelerates complete closure of hard‐to‐heal venous ulcers. Wound Repair Regen 1999;7:201–7. [DOI] [PubMed] [Google Scholar]
- 11. Phillips TJ, Machado F, Trout R, Porter J, Olin J, Falanga V. Prognostic indicators in venous ulcers. J Am Acad Dermatol 2000;43:627–30. [DOI] [PubMed] [Google Scholar]
- 12. Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Repair Regen 2004;12:163–8. [DOI] [PubMed] [Google Scholar]
- 13. Agren MS, Steenfos HH, Dabelsteen S, Hansen JB, Dabelsteen E. Proliferation and mitogenic response to PDGF‐BB of fibroblasts isolated from chronic venous leg ulcers is ulcer‐age dependent. J Invest Dermatol 1999;112:463–9. [DOI] [PubMed] [Google Scholar]
- 14. Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol 1998;111:850–7. [DOI] [PubMed] [Google Scholar]
- 15. Tuttle MS, Mostow E, Mukherjee P, Hu FZ, Melton‐Kreft R, Ehrlich GD, Dowd SE, Ghannoum MA. Characterization of bacterial communities in venous insufficiency wounds by use of conventional culture and molecular diagnostic methods. J Clin Microbiol 2011;49:3812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


