Abstract
Tissue repair is a complex process, which may be favoured or inhibited by different factors. Potassium apigenin (AP) and other flavonoids present in verbena extract (PLX®) possess powerful anti‐inflammatory properties. The aim of this study was to evaluate the effects of topical treatment with AP and PLX gels on wounds inflicted on SKH‐1/CRL mice. Forty‐eight SKH‐1 mice were used (4 groups with 12 animals each), which were subjected to wound excision with a round scalpel, 4 mm in diameter, on the dorsal skin. The animals were divided into four groups: Group I received topical applications of apigenin gel; Group II received PLX gel; Group III received vehicle gel; Group IV acted as control. Wound contraction, reepithelialisation, inflammation and neovascularisation (by means of immunohistochemical staining with anti‐laminin) were recorded at study periods established at 2, 7 and 14 days. Reepithelialisation was faster in Groups I and II at 7 days (56·25% grade 3 and 43·75% grade 4) compared with the other groups. The degree of inflammation showed improvement with a tendency towards statistical significance in Groups I and II at 2 and 7 days. Anti‐laminin staining was more intense in the group treated with PLX at the 2‐ and 7‐day periods. Topical treatment with PLX gel improved the degree of reepithelialisation and inflammation, and favoured neo‐vascularisation of the wounds at 2 and 7 days following surgery.
Keywords: Apigenin, Excision wound, Flavonoids, Mice, Skin
Introduction
In recent years, the use of medicinal plants and herbs for the maintenance and improvement of health and for the treatment of various human conditions and diseases has increased all over the world. About 60% of the world's population and 60–90% of the populations in developing countries rely on traditional medicine for their primary health care 1, 2, 3, 4, 5.
Wound healing is an intricate multistage process that includes inflammation, cell proliferation, matrix deposition and remodelling phases. It is often associated with oxidative stress and consequent prolonged inflammation, resulting in impaired wound healing.
Wound healing occurs in three sequential phases: haemostasis and inflammation, proliferation and remodelling. Inflammation, the earliest phase, is considered as a critical period for wound healing because immune cells remove damaged tissues, foreign debris and remaining dead tissue. Wound healing would be delayed without inflammation and this phase is affected by antioxidation capacity 1, 3, 6, 7, 8, 9, 10, 11, 12.
Naturally occurring flavonoids have been shown to possess several biological properties including hepatoprotective, antithrombotic, anti‐inflammatory and antiviral activities, many of which may be related (at least partially) to their antioxidant and free radical scavenging ability 13, 14.
In particular, apigenin, present in a great number of plants (especially chamomile), has been observed to act as a natural anti‐inflammatory agent 14, 15, 16, 17. Its efficacy in the treatment of symptoms of gastritis, gastric ulcers and other mucosal inflammatory processes is due to the apigenin glycosides present in the plant. Some recent studies have demonstrated that apigenin could be effective in the treatment of skin inflammatory process induced by free radicals (such as UV, X or γ radiation, or chemical compounds) 15, 16, 17.
The hypothesis of this study is that flavonoids, because of their antioxidant effect, might modulate the wound healing process by altering the inflammatory response.
Lippia citriodora (Verbenaceae) is a herbal species mainly used as a spice and medicinal plant. It grows spontaneously in South America and is cultivated in North Africa and Southern Europe. The leaves of this species are reported to possess digestive, antispasmodic, antipyretic, sedative and stomachic properties. It has traditionally been used in infusions for the treatment of asthma, cold, fever, flatulence, colic, diarrhoea and indigestion. Previous studies on L. citriodora concerned its chemical characterisation and revealed the presence of several flavonoids and phenolic acids. However, there is no available information relating to the healing properties of this species 17, 18, 19, 20, 21, 22, 23, 24.
L. citriodora contain several phenolic compounds, namely phenylpropanoids and glycosilated flavones. Phenylpropanoids are the main class of compounds from this extract, verbascoside being the most abundant.
The objective of this study was to determine the effect of two preparations – apigenin and L. citriodora (PLX) – on incisional wound healing and to evaluate their therapeutic potential as topical applications to wounds in SKH‐1 mice.
Materials and methods
Study design
This study had a prospective, randomised, experimental design involving three groups of SKH‐1/CRL mice that were used to study skin wound progression with topical applications of apigenin, PLX, its vehicle and a control (Figure 1).
Figure 1.
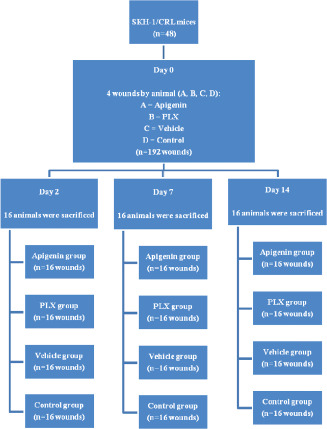
Study design.
Animals
The animals used in this study were obtained from the Faculty of Medicine of the University of Murcia (Spain). Forty‐eight adult (6 weeks of age) SKH‐1/CRL mice (males) with a mean weight of 25 g (range 20–35 g) were used, following a protocol approved by the Bioethics Committee of the University of Murcia. Housing and animal care were in accordance with Spanish National Research Council guidelines 25. The animals were housed individually in plastic cages in a monitored environment (21°C, 12:12 hours light cycle), with free access to drinking water and a standard laboratory mouse food pellet diet. The animals were monitored for signs of infection and discomfort before and after surgery, and up until euthanised.
Materials
Potassium apigenin (AP) and L. citriodora (Verbenaceae) extract were obtained and supplied by Nutrafur S.A. (Murcia, Spain). AP (0·2 g) was dissolved in 20 g deionised water. After total dissolution, 30 g of Arabic gum and 50 g of vegetal glycerine were added at room temperature to complete homogenisation for a final concentration of 2000 mg/kg (0·2% w/w) of the corresponding flavonoid. Similarly, L. citriodora water‐soluble extract (0·5 g) was dissolved in 20 g deionised water. After total dissolution, 30 g of Arabic gum and 50 g of vegetal glycerine were added at room temperature to complete homogenisation for a final concentration of 5000 mg/kg (0·5% w/w) of the corresponding extract. Both preparations were stored frozen until use.
Surgical procedure
The 48 mice were divided randomly into four groups (using a randomisation list, generated using specific software). The animals were anaesthetised with a mixture of ketamine and xylazine (50%). All incisions were made on the skin of the dorsal region by a single surgeon, using the same technique in all cases (perpendicular to the dorsal skin surface) making a circular excision using a 4‐mm punch (Stiefel GlaxoSmithKline GmbH & Co. KG, Bad Oldesloe, Germany). The edges of the wound were brought together mesially and distally with two simple 4/0 polypropylene sutures (Propilorc, Murcia, Spain).
Group I animals were treated immediately with topical applications of apigenin (5 mg/day per wound); Group II animals received topical PLX (5 mg/day per wound) until applications sacrifice; Group III were treated with the vehicle gel (5 mg/day per wound) and Group IV wounds (control group) did not receive any treatment. The wounds were not covered with gauze owing to the difficulty of keeping the dressing in place.
The mice in each group were euthanised after 2 (16 animals per study period, 4 per group), 7 (16 animals per study period, 4 per group) and 14 days following surgery (16 animals per study period, 4 per group) by means of CO2 inhalation. The varying degrees of reepithelialisation and inflammation in each group were compared over the three study periods. A biopsy was obtained using a 6‐mm punch. The samples were placed in individual containers with 10% formaldehyde solution, marked with the number assigned to each animal.
Wound contraction
Mice were photographed at the moment of wounding (day 0), before topical treatment, and again on days 2, 7 and 14 following wounding. Colour digital photographs of each animal beside a millimetre‐squared paper were taken, using a Canon EOS 500D camera. The wound surface area was calculated from the traced outline of each digital image using planimetry. The percentage of wound contraction was calculated as a percentage of the original wound size (day 0 = 100%). The wound was visually examined and scored by a single observer. Image analysis software was used to measure the non‐reepithelialised area – Leica Qwin V3.1 (Leica Microsystems GmbH, Wetzlar, Germany) – drawing a binary mask of the lesion areas, which were then measured individually.
Histopathological study
The biopsy specimens were embedded in paraffin and cut into 5–7 µm sections along a plane perpendicular to the incision. The sections were stained with haematoxylin and eosin and examined under magnifications of ×20, ×40 and ×100. All samples were studied by a single individual blinded to the study.
The criteria developed by Sinha and Gallagher 9 were used to measure the degree of reepithelialisation: grade 0 = reepithelialisation at the edge of the wound; grade 1 = reepithelialisation covering less than half of the wound; grade 2 = reepithelialisation covering more than half of the wound; grade 3 = reepithelialisation covering the entire wound with irregular thickness and grade 4 = reepithelialisation covering the entire wound with normal thickness.
The degree of inflammation was measured using the inflammation resolution phases described by Cotran et al. 26: grade 1 = acute inflammation (a pyogenic membrane is formed); grade 2 = predominance of diffuse acute inflammation (predominance of inflammatory cells); grade 3 = predominance of chronic inflammation (fibroblasts begin to proliferate) and grade 4 = resolution and healing (reduction or disappearance of chronic inflammation, although occasional round cells may persist).
Immunohistochemical technique
After dewaxing and blocking endogenous peroxidase with 3% hydrogen peroxide for 30 minutes, antigen in the samples was revealed using 0·1% protease (Sigma‐Aldrich, Madrid, Spain) at 37°C for 15 minutes, followed by washing in phosphate‐buffered saline (PBS). The tissues were then incubated with 10% normal goat serum (Millipore) for 1 hour at room temperature. The sections were then incubated with polyclonal primary antibodies to laninin (Dako, Barcelona, Spain) in a dilution of 1:2000, overnight at 4°C. Then, the samples were washed and incubated with goat secondary biotinylated anti‐rabbit antibody (Dako) for 1 hour at room temperature and finally incubated with avidin–biotin–peroxidase complex (Cultek, Madrid, Spain) in a dilution of 1:100 for 1 hour at room temperature. Immunostaining was performed using diaminobenzidine (Sigma‐Aldrich) for 3·5 minutes, staining samples with Mayer's haematoxylin.
Statistical analysis
Data were analysed using SPSS version 12.0 statistics program (SPSS® Inc., Chicago, IL). A descriptive study was made for each variable. The associations between the different qualitative variables were studied using Pearson's Chi‐square test. The Kolmogorov–Smirnov normality test and Levene's variance of homogeneity test were applied; as the data showed a skewed distribution, it was analysed using a non‐parametric ranking test. The Kruskal–Wallis test (for more than two samples) was used for quantitative variables. A P‐value of ≤0·05 was considered as significant.
Results
Figure 2 shows wound closure values, expressed as percentages of the total wound area, in which it can be seen that on day‐2 study period no significant differences occurred between groups. However, significant differences had developed by day 7‐(p< 0·001) and the 14‐day study period (P < 0·001) following wounding.
Figure 2.
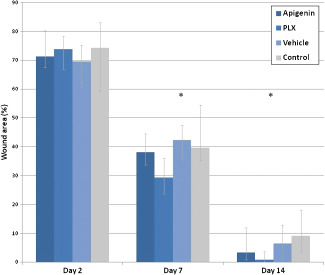
Wound closure in rats. The wound closure rate was expressed as the percentage of the total wound area immediately following wound excision (100%). Values are median (range). *Significant at P < 0·05.
Table 1 shows that at 2 days following wound induction, most of the samples presented incomplete reepithelialisation, in all three study groups; PLX showed 56·2% to be of grade 2 (P = 0·022). At 7 days, the apigenin group showed grade 4 (31·2%) and PLX 43·7% (P = 0·040). At 14 days, no significant differences in reepithelialisation were observed.
Table 1.
Grade of wound reepithelialisation 2, 7 and 14 days after surgical intervention (Pearson chi‐square test)
| Day | Group | Total | Histopathologic scale to evaluate reepithelialisation: n (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | P value | |||
| 2 | Apigenin | 16 | 1 (6·25) | 12 (75) | 3 (18·75) | 0 (0) | 0 (0) | 0·022 |
| PLX | 16 | 3 (18·75) | 3 (18·75) | 9 (56·25) | 1 (6·25) | 0 (0) | ||
| Vehicle | 16 | 6 (37·50) | 8 (50) | 2 (12·50) | 0 (0) | 0 (0) | ||
| Control | 16 | 5 (31·25) | 8 (50) | 3 (18·75) | 0 (0) | 0 (0) | ||
| 7 | Apigenin | 16 | 0 (0) | 0 (0) | 0 (0) | 11 (68·75) | 5 (31·25) | 0·040 |
| PLX | 16 | 0 (0) | 0 (0) | 0 (0) | 9 (56·25) | 7 (43·75) | ||
| Vehicle | 16 | 0 (0) | 0 (0) | 1 (6·25) | 14 (87·50) | 1 (6·25) | ||
| Control | 16 | 0 (0) | 0 (0) | 3 (18·75) | 11 (68·75) | 2 (12·50) | ||
| 14 | Apigenin | 16 | 0 (0) | 0 (0) | 0 (0) | 2 (12·50) | 14 (87·50) | 0·751 |
| PLX | 16 | 0 (0) | 0 (0) | 0 (0) | 2 (12·50) | 14 (87·50) | ||
| Vehicle | 16 | 0 (0) | 0 (0) | 0 (0) | 4 (25) | 12 (75) | ||
| Control | 16 | 0 (0) | 0 (0) | 0 (0) | 3 (18·75) | 13 (81·25) | ||
Grade 0, reepithelialisation at the edge of the wound; Grade 1, reepithelialisation covering less than half of the wound; Grade 2, reepithelialisation covering more than half of the wound; Grade 3, reepithelialisation covering the entire wound with irregular thickness; Grade 4, reepithelialisation covering the entire wound with normal thickness.
Table 2 shows that 2 days following wound induction, none of the groups showed complete resolution of the inflammatory process. After 7 days, 62·5% and 81·2% of the mice in Groups I and II (topical apigenin group and PLX group) showed grade 3; statistically significant differences were identified. After 14 days, grade 4 resolution was recorded in all groups (Figure 3).
Table 2.
Grade of wound inflammation 2, 7 and 14 days after surgical intervention (Pearson chi‐square test)
| Day | Group | Total | Histopathologic scale to evaluate inflammation: n (%) | ||||
|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | P value | |||
| 2 | Apigenin | 16 | 16 (100) | 0 (0) | 0 (0) | 0 (0) | 0·054 |
| PLX | 16 | 11 (68·75) | 5 (31·25) | 0 (0) | 0 (0) | ||
| Vehicle | 16 | 15 (93·75) | 1 (6·25) | 0 (0) | 0 (0) | ||
| Control | 16 | 13 (81·25) | 3 (18·75) | 0 (0) | 0 (0) | ||
| 7 | Apigenin | 16 | 0 (0) | 2 (12·50) | 10 (62·50) | 4 (25) | 0·051 |
| PLX | 16 | 0 (0) | 3 (18·75) | 13 (81·25) | 0 (0) | ||
| Vehicle | 16 | 0 (0) | 7 (43·75) | 7 (43·75) | 2 (12·50) | ||
| Control | 16 | 0 (0) | 8 (50) | 6 (37·50) | 2 (12·50) | ||
| 14 | Apigenin | 16 | 0 (0) | 0 (0) | 2 (12·50) | 14 (87·50) | 0·531 |
| PLX | 16 | 0 (0) | 0 (0) | 2 (12·50) | 14 (87·50) | ||
| Vehicle | 16 | 0 (0) | 0 (0) | 2 (12·50) | 14 (87·50) | ||
| Control | 16 | 0 (0) | 0 (0) | 0 (0) | 16 (100) | ||
Grade 1, acute inflammation (pyogenic membrane is formed); Grade 2, predominance of diffuse acute inflammation (predominance of granulation tissue); Grade 3, predominance of chronic inflammation (fibroblasts beginning to proliferate); Grade 4, resolution and healing (reduction or disappearance of chronic inflammation, although occasional round cells may persist); Vehicle, DMSO (dimethylsulphoxide).
Figure 3.
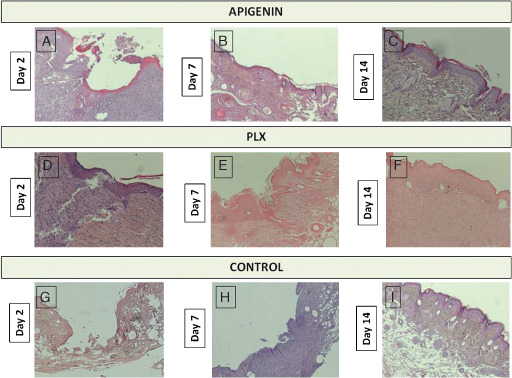
Grade of wound reepithelialisation. A = Grade 2, B = Grade 3, C = Grade 4, D = Grade 3, E = Grade 4, F = Grade 4, G = Grade 0, H = Grade 2, I = Grade 4. Grade 0 = reepithelialisation at the edge of the wound; Grade 1 = reepithelialisation covering less than half of the wound; Grade 2 = reepithelialisation covering more than half of the wound; Grade 3 = reepithelialisation covering the entire wound with irregular thickness; Grade 4 = reepithelialisation covering the entire wound with normal thickness.
Marking with anti‐laminin was more intense at 2 and 7 days in the group treated with PLX (Group II) (Table 3) (Figure 4).
Table 3.
Number of vessels on 2, 7 and 14 days after surgical intervention (Kruskal–Wallis test)
| Day | Number of vessels in 90 µm2: median (range) | ||||
|---|---|---|---|---|---|
| Apigenin (n = 16) | PLX (n = 16) | Vehicle (n = 16) | Control (n = 16) | P value | |
| 2 | 12·50 (5–20) | 22·50 (16–42) | 7·00 (5–10) | 6·50 (1–11) | 0·003 |
| 7 | 11·00 (9–18) | 22·00 (14–29) | 7·00 (6–9) | 8·00 (6–12) | 0·001 |
| 14 | 12·50 (10–15) | 13·00 (10–17) | 7·00 (5–10) | 8·50 (6–14) | 0·020 |
Figure 4.
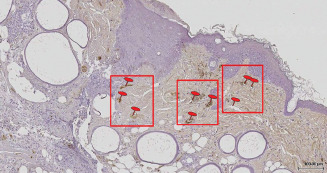
Image of laminin in the PLX group (Group II) at 14 days following wound excision.
Discussion
Wound healing is known to be a complex and dynamic process that usually involves distinct phases marking the healing stages and requires multiple cell types to complete a variety of cellular activities 2, 3. It is a multifarious procedure comprising systematic processes of events, which repair damaged tissue partially or completely. The process can be broadly categorised into three stages: inflammatory phase (consisting of the establishment of homeostasis and inflammation); proliferative phase (consisting of granulation, contraction and epithelialisation) and finally the remodelling phase, which ultimately determines the strength and appearance of the healed tissue 1, 2.
Plants are a source of compounds with antioxidant activity such as phenolic acids, flavonoids, anthocyanins, tannins and carotenoids, which can be used as pharmacologically active substances 20, 21, 22, 23, 24.
The main components of Verbena officinalis L. are the iridoids: hastatoside, verbenalin and aucubin. Verbenalin, the most abundant constituent of this species, has been shown to be responsible for the plant's pharmacological activity. Verbena is also known to contain polyphenols: verbascoside and various glycosylflavones (luteolin 7‐glucoside, apigenin 7‐galactoside, pedalitin 6‐glucoside, apigenin 7‐glucuronide, luteolin 7‐diglucuronide,6‐hydroxyluteolin, 6‐hydroxyapigenin or scutellarein glycoside, and small quantities of methylated aglycones) 19, 20, 21.
Apigenin has been shown to possess remarkable anti‐inflammatory, antioxidant and anti‐carcinogenic properties. In the last few years, significant progress has been made in studying the biological effects of apigenin at cellular and molecular levels, demonstrating apigenin's therapeutic potential as an anti‐inflammatory agent 17, 22.
The aim of wound management is to heal the wound as quickly as possible with minimal pain and scar formation. Agents that shorten the healing process are always required to facilitate rapid and better healing of wounds without scarring. Flavonoids are used for their various therapeutic effects such as antioxidant, anti‐inflammatory, antifungal and wound healing 5. Inhibition of lipid peroxidation effect by flavonoids is supposed to increase the viability of collagen fibrils by activating DNA synthesis and preventing cell damage 3. Flavonoids are also known to promote rapid wound healing owing to their antimicrobial and astringent properties 24. Therefore, the wound healing potential of chamomile may be attributed to the phytochemicals in the leaves, which may have an additive effect that accelerates progress (probably) during the proliferation phase of wound healing.
Various studies support the topical application of Verbacoside extracts as they possess an anti‐inflammatory agent, which can be used in the treatment of local inflammation 17, 18, 19, 20, 21, 22. In this study, skin wounds were induced on mouse skin and subsequently treated with topical applications of apigenin and PLX, showing faster reepithelialisation at the 7‐day study period than the other two treatment groups.
Calvo 18 reported that a topical preparation containing at least 3% V. officinalis methanolic extract possesses an anti‐inflammatory and analgesic effect, which can be used in the treatment of local inflammation. Phytochemical investigations of this plant showed the presence of iridoids and caffeoyl derivatives as well as flavonoid compounds 20, 21. Therefore, it is possible that both the anti‐nociceptive and anti‐inflammatory effects observed with this extract are, at least partially, attributable to them.
Deepak and Handa 22 in an attempt to locate the biologically active fraction(s) of the plant Verbena officinalis L. (Verbenaceae), performed a preliminary screening of successive petroleum ether, chloroform extracts and methanol extracts of aerial parts for anti‐inflammatory activity using a Carrageenan‐Induced Paw Edema model. All three extracts were found to exhibit anti‐inflammatory activity, with the chloroform extract being the most active.
Duarte et al. 16 studied the use of chamomile extract ointment for stimulated oral mucosa reepithelialisation and formation of collagen fibres after 10 days of treatment; chamomile did not, however, influence the degree of inflammation, fibroblast count or wound size. This study used the same reepithelialisation criteria as Sinha and Gallagher 9. According to Cotran et al. 26, cutaneous wound repair consists of a well‐defined series of events arbitrarily divided into three overlapping phases. The defining features of phase 1 are inflammation and resorption (0–7 days), with oedema as the earliest manifestation, followed by the infiltration of inflammatory cells (neutrophils, followed by macrophages and lymphocytes) that clear the wound of damaged cells and foreign material and, in turn, release growth factors and biochemical compounds that activate tissue regeneration. Neovascularisation and provisional matrix formation (3–14 days) define phase 2, with new blood vessels growing primarily from tissues subjacent to the wound. Provisional matrix is characterised first by proteoglycan secreted by connective tissue cells, followed by collagen synthesis by activated fibroblasts. The early collagen fibres are relatively thinner and aligned parallel to the blood vessels (perpendicular to the wound surface). During this time, and particularly in rodents, there is connective tissue contraction and epidermal growth over the wound surface. Phase 3 is a period of wound remodelling (10 days to 6 months) characterised by the obliteration of new blood vessels, reduction of proteoglycan matrix, and collagen remodelling with resorption of early collagen and the formation of new collagen fibres oriented along the stress lines.
According to the review published by Peplow et al. 27, rodents are attractive for wound healing studies because of their availability, low cost and easy handling characteristics. Murine models of excisional wound healing offer several advantages over models in other species, including the fact that mice are inexpensive, thus allowing studies to be performed with large sample sizes. Despite such advantages, however, rat skin healing does not perfectly mimic human skin wound healing, because the skin is morphologically different. Developing an animal model that has all the complexity of human chronic wounds may be an unattainable goal, because non‐healing and delayed wound healing in humans are often the result of combinations of impaired circulation, inadequate nutrition, age, limited physical activity and/or chronic physiological imbalances.
Several authors 18, 19, 20, 21, 22 have found that plant products have shown effects on one or more stages of the wound‐healing process, especially contributing to disinfection, debridement and providing a moist environment to encourage the establishment of an effective healing process. They provide a barrier against microbial attacks and protect the wounded area from various infections. The wound healing activity of the agents applied could be attributed to their antimicrobial effects, although this study did not include microbiological research into this possibility.
Conclusions
Many studies have investigated means of accelerating healing and whether the duration of wound healing can be shortened.
Results of this study suggest that 7 days after skin wounding, daily treatment with topical PLX produced wound reepithelialisation that was significantly more advanced than in other study groups. This shows the effectiveness of V. officinalis in wound healing. Nevertheless, further studies are needed to extrapolate the effectiveness of this topical application to wound repair in human skin.
Acknowledgements
We wish to thank Jose Victor Bolarin, technician at the department of Pathological Anatomy, and the Ministry of Science and Innovation for the Torres Quevedo research grant PTQ‐08‐03‐07880 who had made this study possible.
References
- 1. Kunwar RM, Shrestha KP, Bussmann RW. Traditional herbal medicine in far‐west Nepal: a pharmacological appraisal. J Ethnobiol Ethnomed 2010;13:6–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kondo T. Timing of skin wounds. Legal Med 2007;9:109–114. [DOI] [PubMed] [Google Scholar]
- 3. Shetty S, Udupa S, Udupa L. Evaluation of antioxidant and wound healing effects of alcoholic and aqueous extract of Ocimum sanctum Linn in rats. Evid Based Complement Alternat Med 2008;5:5–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother Res 2006;20:519–530. [DOI] [PubMed] [Google Scholar]
- 5. Okuda T. Systematics and health effects of chemically distinct tannins in medicinal plants. Phytochemistry 2005;66:2012–2031. [DOI] [PubMed] [Google Scholar]
- 6. Pogrel MA, Yen Ch‐K, Hansen LS. A comparison of carbon dioxide laser, liquid nitrogen cryosurgery, and scalpel wounds in healing. Oral Surg Oral Med Oral Pathol 1990;69:269–273. [DOI] [PubMed] [Google Scholar]
- 7. Carew JF, Ward RF, LaBruna A, Torzilli PA, Schley WS. Effects of scalpel, electrosurgery, and CO2 and KTP lasers on wound healing in rats tongues. Laringoscope 1998; 108:373–380. [DOI] [PubMed] [Google Scholar]
- 8. Woodruff LD, Bounkeo JM, Brannon WM Dawes KS, Barham CD, Waddell DL, Enwemeka CS. The efficacy of laser therapy in wound repair: a meta‐analysis of the literature. Photomed Laser Surg 2004;22:241–247. [DOI] [PubMed] [Google Scholar]
- 9. Sinha UK, Gallagher LA. Effects of steel scalpel, ultrasonic scalpel, CO2 laser, and monopolar and bipolar electrosurgery on wound healing in guinea pig oral mucosa. Laringoscope 2003;113:228–236. [DOI] [PubMed] [Google Scholar]
- 10. Camacho‐Alonso F, López‐Jornet P. Clinical‐pathological study of the healing of wounds provoked on the dorso‐lingual mucosa in 186 albino rats. Otolaryngol Head Neck Surg 2007;135:119–124. [DOI] [PubMed] [Google Scholar]
- 11. Sidhu GS, Singh AK, Thaloor D, Banaudha KK, Patnaik GK, Srimal RC, Maheshwari RK. Enhancement of wound healing by curcumin in animals. Wound Rep Regen 1998;6:167–177. [DOI] [PubMed] [Google Scholar]
- 12. Aggarwal BB, Takada Y, Oommen OV. From chemoprevention to chemotherapy: common targets and common goals. Expert Opin Investig Drugs 2004;13:1327–1338. [DOI] [PubMed] [Google Scholar]
- 13. Benavente Garcia O, Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti‐inflammatory activity. J Agric Food Chem 2008;56:6185–6205. [DOI] [PubMed] [Google Scholar]
- 14. Chen GM, Zhang JY, Hong GF, Liu HM. Determination of flavonoids content for Verbena officinalis . Chin J Mod Appl Pharm 2006;3: 798–799. [Google Scholar]
- 15. Chen GM, Zhang JY, Zang XP, Liu HM. Studies on chemical constituents of flavonoid from Verbena officinals . Zhong Yao Cai 2006;29:677–678. [PubMed] [Google Scholar]
- 16. Duarte CM, Quirino MR, Patrocínio MC, Anbinder AL. Effects of Chamomilla recutita (L.) on oral wound healing in rats. Med Oral Patol Oral Cir Bucal 2011;16:716–721. [PubMed] [Google Scholar]
- 17. Liu HM, Bao FY, Yan XB. Studies on chemical constituents of Verbena officinalis . Zhong Cao Yao 2002;33:492–494. [Google Scholar]
- 18. Calvo MI. Anti‐inflammatory and analgesic activity of the topical preparation of Verbena officinalis L. J Ethnopharmacol 2006;107: 380–382. [DOI] [PubMed] [Google Scholar]
- 19. Calvo MI, San Julian A, Fernandez M. Identification of the major compounds in extracts of Verbena officinalis L. (Verbenaceae) by HPLC with post‐column derivatization. Chromatographia 1997;46: 241–244. [Google Scholar]
- 20. Makboul AM. Chemical constituents of Verbena officinalis . Fitoterapia 1986;57:50–51. [Google Scholar]
- 21. Duan K, Yuan Z, Guo W, Meng Y, Cui Y, Kong D, Zhang L, Wang N. LC‐MS/MS determination and pharmacokinetic study of five flavone components after solvent extraction/acid hydrolysis in rat plasma after oral administration of Verbena officinalis L. extract. J Ethnopharmacol 2011;17:201–208. [DOI] [PubMed] [Google Scholar]
- 22. Deepak M, Handa SS. Anti‐inflammatory activity and chemical composition of extracts of Verbena officinalis . Phytother Res 2000;14: 463–465. [DOI] [PubMed] [Google Scholar]
- 23. Skaltsa H, Shammas G Flavonoids from Lippia citriodora . Planta Med 1988;54:465. [DOI] [PubMed] [Google Scholar]
- 24. Casanova E, García‐Mina JM, Calvo MI. Antioxidant and antifungal activity of Verbena officinalis L. leaves. Plant Foods Hum Nutr 2008;63:93–97. [DOI] [PubMed] [Google Scholar]
- 25. National Research Council . Guide for the care and use of laboratory animals. Spain: Official State Bulletin, 1995.
- 26. Cotran RS, Kumar V, Collins T. Reparación de los tejidos: regeneración celular y fibrosis. In: Robbins C, editor. Patología estructural y funcional, 6th edn.. Madrid: McGraw‐Hill Interamericana, 2000:112–117. [Google Scholar]
- 27. Peplow PV, Chung TY, Baxter GD. Laser photobiomodulation of wound healing: a review of experimental studies in mouse and rat animal models. Photomed Laser Surg 2010;28:291–325. [DOI] [PubMed] [Google Scholar]


