Abstract
A chronic wound fails to complete an orderly and timely reparative process and places patients at increased risk for wound complications that negatively impact quality of life and require greater health care expenditure. The role of extracellular matrix (ECM) is critical in normal and chronic wound repair. Not only is ECM the largest component of the dermal skin layer, but also ECM proteins provide structure and cell signalling that are necessary for successful tissue repair. Chronic wounds are characterised by their inflammatory and proteolytic environment, which degrades the ECM. Human acellular dermal matrices, which provide an ECM scaffold, therefore, are being used to treat chronic wounds. The ideal human acellular dermal wound matrix (HADWM) would support regenerative healing, providing a structure that could be repopulated by the body's cells. Experienced wound care investigators and clinicians discussed the function of ECM, the evidence related to a specific HADWM (Graftjacket® regenerative tissue matrix, Wright Medical Technology, Inc., licensed by KCI USA, Inc., San Antonio, TX), and their clinical experience with this scaffold. This article distills these discussions into an evidence‐based and practical overview for treating chronic lower extremity wounds with this HADWM.
Keywords: ECM, Extracellular matrix, Graftjacket RTM, Human acellular dermal matrix, Regenerative tissue matrix
Introduction
Normal wound healing is characterised by a well‐coordinated, progressive series of events designed to restore the barrier function and mechanical integrity of the skin. Similar to other developmental and reparative processes, wound healing involves interactions between cells and their microenvironment; the dermal extracellular matrix (ECM) is a primary component in the process of skin healing 1, 2, 3. In a process termed dynamic reciprocity 4, it is largely through interactions with ECM that cells are directed to differentiate or dedifferentiate, proliferate or remain quiescent, and assume the architecture and function of skin versus that of some other organ 2, 5. ECM is the largest element of the dermal layer and its components include proteoglycans, hyaluronic acid, collagen and elastin. Proteins contained in the ECM are important for wound healing, not solely because they provide structural support for cells but also because they provide signalling proteins 4.
In chronic wounds, the ECM is often dysfunctional, not only due to the inflammatory and proteolytic environment of chronic wounds, which breaks down ECM but also due to the relative deficiency in protease inhibitors, which normally regulate proteases 6, 7. In fact, a high ratio of protease to protease inhibitors has been found predictive of poor healing of chronic wounds 7. As ECM is often dysfunctional in chronic wounds, attempts have been conceptualised to correct or replace damaged ECM in order to stimulate healing. Using this paradigm, with the concept of exchanging the damaged human ECM and/or restoring a functional ECM, various approaches have been undertaken. These include the use of both cellular and acellular constructs, made up of biological, synthetic or composite materials. Biological material for these tissue matrices has been derived from a variety of allogeneic and xenogeneic sources (Table 1).
Table 1.
Composition of tissue matrices
| Category | Components |
|---|---|
| Biologic | Xenograft: Equine, bovine, porcine |
| Allograft: Human cadaveric tissue | |
| Plant‐derived: cellulose | |
| Synthetic | Fibres |
| Foams | |
| Composite | Manufactured mixture |
Current treatment strategies for skin wounds mostly aim to replace lost tissue rather than support intrinsic self‐healing mechanisms. However, new developments within the area of tissue‐engineered scaffolds are leading to an ultimate goal of tissue regeneration rather than replacement 8. The future of tissue engineered skin lies within the comprehension of intercellular biochemical communications, the engineering of scaffold structures on a micro‐ and nano‐dimension, and the integration of growth factors and stem cells into scaffolds to obtain a bioactive cocktail capable of active guidance in skin regeneration 8. The qualities of an ideal matrix are listed in Table 2.
Table 2.
Desirable features in a tissue matrix
| Biomimetic surface for cell attachment that: |
| Matches the elasticity and stiffness of dermis |
| Provides coverage for underlying structures |
| Replicates dense connective tissue properties |
| Regenerative: |
| Modulates the mechanotransduction properties of cells (i.e. produces appropriate ECM and growth factors) |
| Prevents hypertrophic scars or wound contractures |
| Durable: Matrix that persists (does not dissolve) until cellular infiltration is adequate |
| Non‐immunogenic: Degrades without producing inflammatory responses |
| One application sufficient (compared with serial applications of other dermal matrices) |
| Accepts: Epithelial covering |
ECM, extracellular matrix.
Acellular allografts or xenografts have both advantages and limitations. These grafts contain a framework (scaffolding) of insoluble molecules such as collagen, elastin and fibronectin. Such matrices may retain signals that promote attachment, proliferation and migration through retention of structure, attachment sites and matrix‐bound cytokines, signalling proteins and growth factors. Additionally, with the use of natural or biological materials, there is a remote chance of transmission of disease, and immunogenicity can also be a concern 9. Processing methods are thought to obviate these risks but those that fail to completely remove cellular components may not accomplish this or those that use chemicals that damage the collagen, or include cross‐linking to stabilise the matrix, can alter the collagen and other macromolecules, rendering them less capable of interacting with cells and, therefore, more likely to trigger an immune reaction in the host body.
Human acellular dermal wound matrix
Processed cadaveric dermis [human acellular dermal matrix (HADM); AlloDerm® Regenerative Tissue Matrix (RTM), LifeCell Corporation, Branchburg, NJ] has been used for the past 20 years 10 for burns 11 and surgical reconstruction 12. Recently, this technology has been adapted as a human acellular dermal wound matrix [HADWM; Graftjacket® RTM for wounds, Wright Medical Technology, Inc., licenced by KCI USA, Inc. San Antonio, TX], such that it may be applied to chronic wounds.
Processing
HADWM is processed from screened donated human skin, which is supplied from US tissue banks under the guidelines of the American Association of Tissue Banks (AATB) in accordance with regulations set forth by the US Food and Drug Administration (FDA). HADWM is regulated by FDA as human tissue for transplantation. Briefly, through a proprietary process, epidermal and dermal cells are removed while dermal structure is preserved. In doing so, an intact basement membrane complex is maintained and confirmed through histology and immunohistochemistry. Extensive microbiological cultures also confirm the absence of bacterial and fungal pathogens. The matrix is cryogenically preserved to ensure that there is no damage to the HADWM, which is packaged in a pouch.
Description and indications
HADWM is produced in both freeze‐dried sheet and cryofractured powder forms, which, when rehydrated, become soft and fully pliable sheets (HADWM) or a flowable paste [Graftjacket® Xpress flowable soft tissue scaffold (FSTS), Wright Medical Technology, Inc., licensed by KCI USA, Inc., San Antonio, TX]. HADWM was developed conceptually to provide a scaffold for the body's repair or replacement of damaged or inadequate integumental tissue [e.g. diabetic foot ulcers (DFUs), venous leg ulcers (VLUs), pressure ulcers (PrUs) or other homologous uses] 13. As mentioned, HADWM has both a dermal surface and an intact basement membrane; for optimal results, the dermal surface should be in contact with the wound bed to facilitate ingrowth of blood vessels through the empty vascular channels in the matrix. HADWM is also fenestrated to facilitate drainage of fluid from under the matrix.
As HADWM in micronised form, FSTS lacks basement membrane directionality but otherwise possesses the same natural biological components and, therefore, can support the body's repair of damaged or inadequate integumental tissue 14. The flowable nature of FSTS makes it suitable for placement into deep wounds such as deep DFUs or wound areas where tunnels and/or undermining are present, including sinus tracts, deep undermined PrUs and cavities. FSTS can conform to, and have maximum contact with, irregular wound surface areas. Both HADWM and FSTS are contraindicated for use in patients who are sensitive to polysorbate 20 or to any of the antibiotics used in product preparation 13, 14.
Scientific evidence
Animal studies have been conducted to determine the characteristics of HADM. (Correlation of these results to results in humans, however, has not been established.) While the majority of these studies relate to acute wounds (e.g. abdominal wall and breast reconstruction), they demonstrate the mechanisms of action that make HADWM suitable for use in chronic wounds as well. Truong et al. used a mouse model to evaluate both acellular and composite dermal matrices in terms of wound contraction reduction, histologic incorporation into the wound and epithelialisation 15. Compared with the control and synthetic implants at 28 days post surgery, HADM and a generic acellular dermal matrix (ADM) showed significantly less contraction, retaining larger percentages of the original wound area, 63 ± 14% (P < 0·01) and 57 ± 7% (P < 0·03), respectively. Histological analyses of HADM and ADM also reported little fibrosis associated with these implants. All the wounds achieved 80–99% epithelialisation 15.
Animal studies by Menon et al. 16, Campbell et al. 17 and Xu et al. 18 confirmed HADM vascularisation in abdominal wall defect models. Menon et al. created full‐thickness abdominal wall defects in 25 rabbits, which were randomly assigned to one of three closure groups: primary closure (n = 5) or abdominal wall reconstruction with either HADM (n = 10) or Gore‐Tex® Soft Tissue Patch (W. L. Gore and Associates, Inc., Newark, DE) (n = 10) 16. Visual inspection showed that both sides of the HADM implant appeared to be well incorporated into the surrounding tissue. They also confirmed perfusion in all of the HADM patches via fluorescein dye infusion and histological analysis 16. Campbell et al. used an acute ventral hernia model in guinea pigs to evaluate whether musculofascia or subcutaneous fat would provide greater cellular and vascular infiltration into HADM 17. The interface (lateral portion of HADM in direct contact with musculofascial edge of defect) demonstrated greater mean cell density than the centre portion of HADM (beneath subcutaneous fat) at week 1 (P = 0·01) and week 2 (P < 0·0001). At week 4 the interface zone of HADM had greater mean vessel density than the centre zone (P < 0·0001), indicating that musculofascia provided better cellular and vessel infiltration than subcutaneous fat. On the basis of these results, the authors recommended that HADM be positioned next to the best vascularising tissue 17. Finally, Xu et al. used a Vervet monkey abdominal wall reconstruction model to evaluate tissue remodelling after application of HADM, a primate acellular dermal matrix (PADM), and a human‐derived cellular dermal matrix (HCDM) 18. Both HADM and PADM incorporated well and after 35 days were indistinguishable 18.
The primate study by Xu et al. also evaluated host immune response, as antigenicity affects matrix incorporation 18. Both HADM and PADM were associated with a mild immune response at 1 month; however at 3 months only minimal inflammation was noted. At 6 months there were no signs of rejection or chronic inflammatory response in defects treated with HADM and PADM. In contrast to the two acellular matrices, histological analysis of HCDM at 90 days showed significantly higher levels of markers indicating sustained inflammation, which the authors attributed to the inactivated cellular content of the matrix. On the basis of these results, the authors reported that HADM contained ‘minimal antigenic components that induce an antibody response in non‐human primates’ and incorporated well into the host's abdominal wall tissue 18.
The effect of HADM basement membrane orientation on cell and vessel infiltration has been studied by both Eppley et al. (2001)19 and Campbell et al. (2012) 17. In a rabbit ear study, Eppley and colleagues reported that membrane orientation did not affect rate of matrix revascularisation 19. They tested four HADM configurations: single layer with membrane down, single layer with membrane up, rolled with membrane inside and rolled with membrane outside. Single layer grafts in both membrane orientation groups were completely revascularised within 14 days. The rolled grafts did not achieve full vascularisation in the 28‐day study period; however, the authors attributed this to slower ingrowth due to the thickness of the rolled matrix rather than to membrane orientation. In the 2012 guinea pig study, Campbell et al. reported that membrane position did affect the degree of ingrowth in their abdominal wall model 17. Excised HADM in which the basement membrane was oriented in (towards the peritoneum) had significantly greater cellular (P = 0·02) and vessel density (P = 0·0004) at week 4, compared with wounds in which the basement membrane was oriented out (away from the peritoneum). These findings confirmed the authors' hypothesis that the basement membrane inhibited cellular and vascular infiltration. Consequently, in ventral hernia repairs they commented that orienting the basement membrane towards the peritoneal cavity may improve matrix incorporation 17.
In these animal studies, HADM facilitated angiogenesis and vascular ingrowth with minimal inflammatory reaction. Research also indicated that orientation of the basement membrane may affect degree of vascularisation (particularly in abdominal wall defects).
Unanswered questions remain. Of interest are the degree to which the construct persists and whether paracrine effects exist for HADWM. Better understanding of HADWM effects in treated patients would provide greater insight into other potential mechanisms of action.
Clinical evidence
Three randomised controlled trials (RCTs) 20, 21, 22 and two retrospective studies 23, 24 report positive clinical outcomes with a single application of HADWM in DFUs. Complete wound healing (100% epithelialisation without drainage) was the primary endpoint for two RCTs, while the third RCT evaluated wound size reduction at 4 weeks as the primary endpoint. Results from a small retrospective study 25 also demonstrate the effectiveness of FSTS to treat complex tunneling DFUs and chronic ulcers.
A multicenter RCT by Reyzelman et al. compared HADWM to advanced moist wound therapy (AMWT), including alginates, foams, hydrocolloids and hydrogels in the treatment of DFUs 20. Of the 86 patients enroled, 47 patients received a single HADWM application (although 1 was not included in the analysis) and 39 were treated with AMWT based on physician discretion. At 12 weeks, significantly (P = 0·0289) more HADWM patients (32/46; 69·6%) achieved complete healing than AMWT patients (18/39; 46·2%). After adjusting for baseline ulcer size, the AMWT group's non‐healing rate was significantly higher (P = 0·0233), while the odds of healing were approximately two times higher for the HADWM group. No significant difference was observed between groups for mean time to complete healing (HADWM, 5·7 weeks versus control, 6·8 weeks) 20, which may impact cost efficacy assessment.
In an earlier single‐centre RCT, Brigido et al. (2006) compared HADWM to weekly sharp debridement with wound gel dressings (control group) 21. Twenty‐eight patients with uninfected, full‐thickness (Wagner grade 2), lower extremity wounds of greater than 6 weeks duration completed the 16‐week study. Significantly (P = 0·006) more HADWM patients (12/14; 85·71%) achieved complete wound healing compared with control patients (4/14; 28·57%). HADWM wounds also healed sooner (11·92 weeks) than control wounds (13·5 weeks), although the difference was not significant 21.
In the third RCT, Brigido et al. evaluated wound size reduction after 4 weeks in 40 diabetic patients receiving treatment with either one application of HADWM (n = 20) or a wound gel (Curasol® Healthpoint Biotherapeutics, Ft. Worth, TX) with gauze dressings (n = 20) 22. Both groups were evaluated weekly. At four weeks, HADWM wounds, compared with control wounds, had greater average weekly reductions in length, width, area and depth, respectively: length: 3·4 versus 1·0 mm, P < 0·001; width: 2·3 versus 1·0 mm, P < 0·001; area: 1·5 versus 0·5 cm2, P = 0·006 and depth: 1·9 versus 0·4 mm, P < 0·001). HADWM wounds, compared with control wounds, also had a greater percentage of wound closure at 4 weeks: length: 50·9% versus 15·4%, P < 0·001; width: 49·6% versus 22·9%, P < 0·001; area: 73·1% versus 34·2%, P < 0·001 and depth: 89·1% versus 25·0%, P < 0·001) 22. Of note, both studies were limited by use of wound gel dressings in the control arms.
Two retrospective studies using HADWM have also been published. In the larger retrospective study, Winters et al. reviewed the outcomes of 75 diabetic patients with 100 chronic full‐thickness lower extremity wounds that were treated with HADWM 23. In this difficult patient population, nearly half of wounds (47/100) were classified as grade 3 (having exposed bone or joint) according to the University of Texas (UT) Wound Classification System 26. The overall healing rate was 91%. For all wounds, mean times to matrix incorporation, 100% granulation and complete healing were 1·5 ± 0·90, 5·1 ± 3·5 and 13·8 ± 8·8 weeks, respectively. No matrix‐related adverse events were reported 23.
In a smaller retrospective study, Martin et al. evaluated the effectiveness of HADWM for UT grade 2A foot wounds 24. Records from 17 consecutive diabetic patients were reviewed to determine the time to complete wound closure and proportion of wounds healed within a 20‐week period. Patients were treated with debridement, a single application of HADWM, and use of moisture‐retentive dressings until complete epithelialisation. Average wound size was 4·5 ± 3·2 cm2 with an average duration prior to treatment of 29·8 ± 22·4 weeks. During the study period, 14 of 17 (82·4%) wounds healed in a mean time of 8·9 ± 2·7 weeks 24.
FSTS was evaluated in the 2009 study by Brigido et al. 25. Twelve patients with full‐thickness, uninfected sinus tract wounds (i.e. wounds with a depth greater than length and width) received a single 2 cc application of FSTS following wound debridement 25. Initial average baseline wound area was 342·7 ± 234·0 mm2 with wound depth of 13·8 ± 5·9 mm. At the end of the 12‐week study period, 10 of 12 patients (83·3%) achieved complete wound healing (100% epithelialisation without drainage). For all patients, average time to complete healing was 8·5 ± 2·0 weeks; however, those with complete healing achieved 100% depth reduction in 7·8 ± 2·2 weeks. The two patients who were non‐compliant with off‐loading had 95% and 98% closure rates with 99% reduction of volume in 12 weeks. All patients had 50% reduction in wound depth within first 14 days 25.
The body of evidence supporting use of HADWM in DFUs is small but includes several RCTs. These studies focus on diabetic patients with extremity wounds, including some surgical wounds as well as foot ulcers. The 91% healing rate (91/100 patients) in the large Winters retrospective study demonstrates the effectiveness of one application of HADWM in wounds representing all UT ulcer grades (from shallow to deep wounds with exposed joints and tendons) 23. In these RCTs and retrospective studies, a total of 149 of 177 (84%) diabetic foot wounds achieved complete healing. Limitations of these studies include small sample size and short follow‐up periods. Certainly, larger RCTs with longer follow‐up periods are needed; however, the current body of evidence does indicate favourable results using HADWM to treat DFUs. While the limited evidence supporting use of FSTS is favourable, additional research is needed.
Expectations of use of HADWM and FSTS
Chronic wounds (including neuropathic DFUs, PrUs and VLUs) impact patients and health care costs worldwide. A chronic wound fails to complete an orderly and timely reparative process and therefore does not produce anatomic and functional integrity at the injured site 27. This failure increases risk of wound complications that affect patients' quality of life and increase their need for health care resources. In fact, an estimated 1–2% of the population in developed countries will experience a chronic wound during their lifetime 28. In the USA, 6·5 million patients have chronic wounds 29 and costs to treat these wounds are estimated to be more than US$25 billion/year 30.
As an example, the worldwide increase in obesity and diabetes mellitus (DM) has contributed to increasing prevalence of diabetic neuropathic ulcers. In 2011, it was estimated that 366 million people worldwide had either type 1‐ or type 2‐DM, resulting in an estimated 4·6 million deaths per year and requiring $485 billion/year to treat the disease 31. The data presented above suggest a benefit of HADWM in treating DFUs; however, research still needs to be done on its use to treat PrUs and VLUs, the two other major categories of chronic wounds.
When to apply HADWM?
On the basis of the data presented, HADWM is used as an adjunct to standard care. Evidence suggests both superficial and deep ulcers are likely to benefit, as was the case in patients enroled in the RCTs presented. It is critical to continue standard care, which in the case of DFUs is offloading and debridement, as HADWM is not a replacement for standard care.
Prior to HADWM application
Adequate blood flow to the wound is critical, because HADWM develops new tissue by vascular growth into the matrix. As patients with DM may have varying degrees of peripheral arterial disease in the lower extremities, adequate perfusion should be confirmed and limb ischaemia addressed as needed.
Adequate debridement is also crucial to reduce bacterial bioburden, remove necrotic tissue, have responsive keratinocytes at the wound edge and provide a perfused wound bed prior to placement of HADWM. As a wound progresses through the continuum ranging from clean to infected, the growing types and numbers of bacteria increase the metabolic load. Many of these bacteria also produce biofilms that protect them from both the body's defences and antibiotics 32. The endotoxins and proteases produced by the bacteria stimulate the pro‐inflammatory wound environment that may stall healing 33. Necrotic tissue, which is unreceptive to growth factors or bioactive treatments 34, also acts as a physical barrier for growth factor‐receptor interaction 35. Debridement (removal of callus and necrotic tissue) reduces bacterial load in the wound and removes biofilms, necrotic tissue and phenotypically altered cells. With debridement, growth factors are stimulated, local stem cells are recruited and healing can begin.
How much and how often to debride are important considerations. Failure to adequately debride a wound may not remove sufficient bacteria, biofilms and necrotic tissue, thereby undermining treatment effectiveness. In 143 patients with DFUs, Saap and Falanga evaluated the adequacy of debridement (debridement performance index) and reported that debridement could be an independent predictor of wound healing 36. Ennis et al. studied 432 diabetic, venous, arterial and pressure ulcers and demonstrated that, when other factors were controlled, sharp debridement significantly increased healing in chronic wounds 37. In an RCT testing a topical growth factor in patients with 118 DFUs, Steed et al. reported that the extent of debridement impacted wound healing in both the control and treatment arms 38. A study by Cardinal et al. also noted that patients treated at serial debridement centres achieved a healing rate of 29% compared with 15% for patients at non‐serial debridement centres 39.
While there is some evidence that debridement supports wound healing, more rigorous evaluation would be helpful. In 2010 Lebrun et al. conducted a systematic literature review of current evidence regarding use and technique of surgical debridement to improve healing of DFUs 40. While five RCTs met the inclusion criteria, the studies did not focus on the role of debridement as a wound healing strategy for DFUs. Consequently, the degree to which the other therapies used with debridement (e.g. off‐loading and use of skin substitutes) contributed to successful wound healing was not clear. On the basis of their review, the authors concluded that a ‘focused, well‐designed study’ was needed to specifically evaluate the effect of debridement on DFU healing 40.
Along with debridement, other factors to address prior to HADWM application include infection and status of patient's general health. If the wound is infected, the extent of infection needs to be determined, the type of bacteria identified and appropriate treatment initiated. Issues related to the patient's health (including degree of compromised immunity, anticoagulants and nutritional status) also should be addressed to provide an optimal environment for HADWM application.
Negative pressure wound therapy (NPWT; V.A.C.® Therapy, KCI USA, Inc.) may also be used to prepare the wound bed prior to application of HADWM.
Rehydration and Application of HADWM
Prior to placement in the wound, HADWM is rehydrated in a two‐step process using either sterile normal saline or sterile lactated Ringer's solution 13. The matrix is submerged first in one sterile bowl for 5 minutes, and the paper backing is removed and discarded once it separates from the matrix. After being aseptically transferred to the second sterile bowl of solution, the matrix should soak for additional 5–10 minutes. Antibiotics may also be added to the second rehydration solution. Fully rehydrated matrix is soft and pliable throughout and must be used within 4 hours. (HADWM should not be used if it is discoloured, cracked or has passed the expiration date 13.)
When applying HADWM, ensuring that the dermal side (as opposed to the basement membrane side) is in contact with the wound bed is the critical point 13. The dermal side is shiny, smooth, white and absorbs blood, whereas the basement membrane side of the matrix is dull, rough, buff colour and repels blood. To help identify the dermal side, a sterile marker can be used to mark that side with an ‘X’ prior to rehydration. Another way to identify the dermal side is to use the patient's blood; dermal side will absorb the blood, while the basement membrane will repel the blood.
For deep wounds, HADWM should be measured and trimmed to overlap the wound to a greater degree to allow for contact with the deepest parts of the wound bed. Once positioned in the wound, the matrix can be securely fastened with skin staples or sutures circumferentially to the margin to enable vascular communication. Because HADWM is thicker than cellular constructs and persists longer, Steri‐Strips™ (Nexcare™ Products, St. Paul, MN) are not recommended to anchor the matrix.
Post HADWM application tips
Once placed in the wound, the HADWM should be covered with a non‐adherent layer [e.g. Adaptic® Non‐Adhering Dressing (Johnson & Johnson, New Brunswick, NJ) with mineral‐oil soaked gauze] in order to protect the matrix, maintain a moist wound healing environment and manage wound exudate. Appropriate off‐loading of the matrix site should also be prescribed. The initial dressing should remain in place 5–7 days with subsequent dressing changes weekly. For heavily exudating wounds, dressing changes should be done every 3–5 days, depending on amount of drainage.
A learning curve exists for clinicians to become familiar with use of HADWM, as they cannot remove the matrix to assess the progression of wound healing. Also, over time the outer surface of the matrix may resemble eschar (Figure 1). If so, the matrix must not be debrided or lifted because breaking the contact between HADWM and the wound bed may interrupt incorporation, resulting in matrix failure. Instead, moist dressings placed over HADWM will hydrate the matrix, so its colour becomes lighter.
Figure 1.
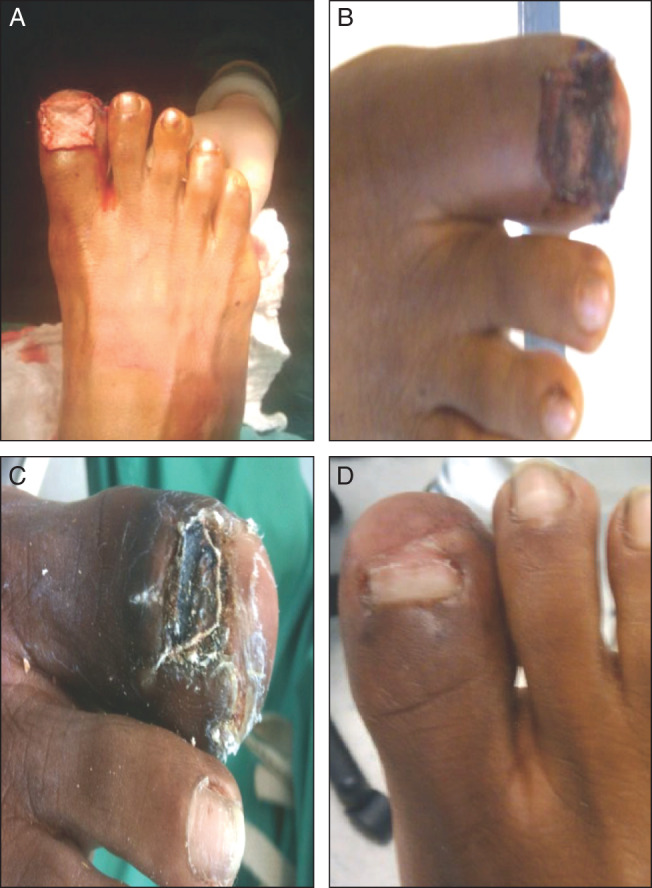
A 51‐year‐old woman presented with trauma to the great right toe with an open fracture and destruction to the nail bed. The trauma to the toenail left bone exposed: (A) initial placement of human acellular dermal wound matrix (HADWM); (B) 1 week after HADWM placement; (C) 1 month after HADWM placement. The wound healed within 6 weeks; (D) 3 months after HADWM placement a new nail developed on the great right toe. Patient data and photos reprinted with permission of Marie L. Williams, DPM.
NPWT may also be applied immediately post‐operatively to bolster the HADWM, using a wide meshed non‐adherent layer between HADWM and the reticulated open‐cell foam dressing (V.A.C.® GranuFoam™ Dressing, KCI USA, Inc) 41. A non‐adherent layer is not required if the white foam dressing (V.A.C.® WhiteFoam Dressing, KCI USA, Inc.) is used. NPWT should be left in place for approximately 4–5 days to ensure sufficient incorporation 41. Of note, clinical experience exists supporting use of NPWT as a bolster over HADWM, but rigorous study has not, to date, been performed.
As reported in the literature, a single application of HADWM is often sufficient to support wound closure 20, 21, 23. Also the 2004 RCT by Brigido demonstrated that a single application of HADWM resulted in good wound size reduction at 4 weeks 22, which is a time point often used to predict effectiveness of therapy in DFUs 42. Therefore, by 4 weeks after application, there should be signs indicating progress in healing. If the matrix is stuck (non‐mobile) but not infected, it can be left in place. However, it is important to monitor progress to preclude development of infection or osteomyelitis. If the wound does not appear to be making positive progress at 4 weeks, then a repeat application may be indicated.
Application of FSTS
FSTS is supplied as 2 cc of dried, acellular dermal particulate in a 5 cc syringe and, when reconstituted with 1·7 cc of sterile water, has been formulated to a consistency that will pass through an 18‐gauge OPTIVA® I.V. catheter (Smiths Medical, St. Paul, MN). When expressed into a properly debrided and irrigated tunnelling wound, FSTS fills the space, providing surface area contact of the product with irregular contours of the wound bed. In a 2009 retrospective study, Brigido et al. reported that FSTS was easy to prepare and use 25. They also stated that patient compliance with off‐loading was critical for good healing with FSTS 25.
Cost‐effectiveness of HADWM
The choice of advanced wound therapies weighs treatment benefit against cost of therapy. The increasing prevalence of chronic wounds (e.g. DFUs, VLUs and PrUs) has had a significant impact on the health care system, so measuring cost‐effectiveness of various treatment modalities is critical to the practice of responsible medicine. For example, the cost of failing to heal a DFU in a timely manner is a consideration, because DFU healing costs can range from approximately $8000 for an uncomplicated wound 43, $17 000 for an infected wound 44 to as much as $45 000 if amputation is required 43. Given the growing incidence and prevalence of DM and the economic impact of non‐healing DFUs, determining cost‐effectiveness is an important factor in clinical decision‐making when therapeutic need outweighs available resources or when no ‘gold standard’ exists.
Factors considered in assessing cost‐effectiveness
Measures of benefit, surrogate markers, measures of cost and assessment of product cost and effectiveness are the major factors used to determine cost‐effectiveness. Measures of benefit in wound healing include time to healing, time to recurrence (i.e. re‐ulceration), avoidance of infection and amputation‐free survival. Other utilities and surrogate endpoints include wound healing velocity, limb preservation and quality‐adjusted time without symptoms of disease or toxicity of treatment (Q‐TWIST), which is an endpoint of particular importance to the US government.
Analyses in wound healing have previously focused on direct costs; however, there are other types of cost that have not been as well addressed in the literature. Direct costs include hospital, doctor, medication, nursing care, dressings and supplies. Indirect costs (lost work or productivity), intangible costs (patient pain, lost companionship, secondary depression and ‘suffering’) and fixed versus variable costs also need to be considered.
Care setting is a critical cost driver. For example, placement of a skin substitute in the clinic is ten times less expensive than placement in the operating room. While the cost of components (e.g. advanced wound therapies) is often the focus for decision‐making, this is only one aspect of the overall cost of care. In fact, hospitalisation accounts for the majority of costs (70–80%) related to treatment of patients with lower extremity ulcers (LEUs) 45, 46.
Failure to heal chronic LEUs results in their extended duration and increases the chance of infection or other complications that can result in one or more hospitalisations. Consequently, the cost of failing to heal chronic ulcers needs to be considered when evaluating the cost‐effectiveness of an advanced therapy. Shearer et al. evaluated the cost impact of infection in chronic ulcers 47. They reported that the monthly cost to treat a uninfected ulcer was $775·55; however, an ulcer with cellulitis costs $2048·52 and one with osteomyelitis (the most expensive infectious event) costs $3798·27 47. Patients with infectious complications also have more outpatient health care provider visits as well as more hospitalisations 48. The additional health care expenditures that accrue with failure to achieve closure of a chronic wound provide a context for consideration of cost‐effectiveness of advanced therapies, such as skin substitutes.
Comparison of three skin substitutes
When no direct comparison of skin substitute products has been carried out, clinical evidence derived from published RCTs or retrospective studies can be used to develop hypothetical models that compare potential cost‐effectiveness of different skin substitutes. For example, Table 3 presents the number of product applications reported in RCTs in which HADWM 20, Graftskin 49 (Apligraf®, Organogenesis, Inc., Canton, MA), and human fibroblast‐derived dermal substitute (HFDS) 50 (Dermagraft®, Advanced Biohealing, Inc., La Jolla, CA) were used to treat patients with DFUs.
Table 3.
Comparison of data from RCTs using HADWM, Graftskin and HFDS
| Product and pivotal study | Study type | Number of patients (n) | DFU location | Study duration (weeks) | Age of wound (weeks) | Number of applications |
|---|---|---|---|---|---|---|
| HADWM, Reyzelman et al. 20 | Prospective RCT, 11 US centres | HADWM (n = 46) versus advanced moist wound therapy (alginates, foams, hydrocolloids or hydrogels) (n = 39) | Toe: 32·6%, foot: 32·6%, heel: 8·7% and other: 26·1% | 12 | 23·3 | 1·0 |
| Graftskin, Veves et al. 49 | Prospective RCT, 24 US centres | Graftskin (n = 112) versus saline‐moistened gauze* (n = 96) | Chronic plantar DFUs, (non‐infected and non‐ischaemic) | 12 | 50 | ≤5·0† |
| HFDS, Marston et al. 50 | Prospective RCT, 35 US centres | HFDS (n = 130) versus saline‐moistened gauze* (n = 115) | Plantar DFUs of forefoot or heel | 12 | 41 | ≤8·0† |
DFU, diabetic foot ulcers; HADWM, human acellular dermal wound matrix; HFDS, human fibroblast‐derived dermal substitute; RCTs, randomised controlled trials.
These studies were limited by use of saline moistened gauze in the control arms.
In these studies physicians were allowed up to a maximum number of five Graftskin applications and up to eight HFDS placements.
In these 12‐week, multicentre RCTs, the number of applications is of particular interest. HADWM was applied once compared with up to five applications for Graftskin and up to eight applications of HFDS (Table 3). These three skin substitutes have different mechanisms of action, which may account for the differences in number of applications required to achieve wound closure. The Graftskin and HFDS studies were also limited by use of saline moistened gauze in the control arms. As these studies were very different in design, the validity of comparing healing rate data is questionable; however, it should be noted that HADWM required fewer applications to achieve clinically acceptable healing rates, which might have potential cost benefit.
In summary, the cost drivers in wound care include healing rate, cost per application, cost of failure to heal, infection, hospitalisation and amputation. Use of early advanced care can be preventative, important and cost‐effective. Definitive conclusions await head‐to‐head studies in combination with rigorous attempts to capture all related cost data.
Conclusion
The rising global prevalence of chronic wounds (e.g. DFUs, PrUs and VLUs) has resulted in increased health care expenses, which demonstrate the cost of failing to heal a wound. Many factors contribute to delayed healing (e.g. patient comorbidities, bacterial quantity or quality and ischaemia). The challenge in treating chronic wounds is to identify and address both systemic factors and issues in the wound's microenvironment that have stalled the wound in the inflammatory stage of healing. Various substitutes have been developed to address what is lacking in a chronic wound's microenvironment and thereby reestablish the wound healing cascade. For example, both cellular and acellular matrices, which have different mechanisms of action, have been shown to stimulate wound healing. An acellular matrix like HADWM provides an ECM scaffold, which is the major component of the dermis and critical for wound healing. In fact, single application of HADWM has shown favourable results in several RCTs and retrospective studies to stimulate wound closure in lower extremity wounds. Clinical experience with HADWM and FSTS also supports the use of HADWM in treating chronic LEUs.
Acknowledgements
All authors have consulting agreements with KCI and participated in a KCI‐sponsored Graftjacket RTM/Graftjacket Xpress FSTS Advisory Panel on 30 November and 1 December 2012. The goal of the panel was to outline and provide content for an educational publication that would both review scientific and clinical evidence and discuss practical considerations related to the use of these products. The authors wish to acknowledge Cris Cadena, Alice Goodwin and Kristine Villarreal for their writing assistance and manuscript preparation. Graftjacket is a trademark of Wright Medical Technology, Inc.
References
- 1. Hynes RO. The extracellular matrix: not just pretty fibrils. Science 2009;326:1216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol 2006;22:287–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol 2002;14:608–16. [DOI] [PubMed] [Google Scholar]
- 4. Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen 2011;19:134–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: dynamic reciprocity via extra‐ and intra‐cellular matrices. Cancer Metastasis Rev 2009;28:167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schultz GS, Ladwig G, Wysocki A. Extracellular matrix: review of its roles in acute and chronic wounds. World Wide Wounds 2005:1–24. [Google Scholar]
- 7. Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen 1996;4:411–20. [DOI] [PubMed] [Google Scholar]
- 8. Yildirimer L, Thanh NT, Seifalian AM. Skin regeneration scaffolds: a multimodal bottom‐up approach. Trends Biotechnol 2012;30:638–48. [DOI] [PubMed] [Google Scholar]
- 9. Hodgkinson T, Bayat A. Dermal substitute‐assisted healing: enhancing stem cell therapy with novel biomaterial design. Arch Dermatol Res 2011;303:301–15. [DOI] [PubMed] [Google Scholar]
- 10.Lifecell's History. 2013. URL http://www.lifecell.com/corporate/lifecells‐history/ [accessed on 3 May 2013].
- 11. Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full‐thickness burns. Burns 1995;21:243–8. [DOI] [PubMed] [Google Scholar]
- 12. Buinewicz B, Rosen B. Acellular cadaveric dermis (AlloDerm): a new alternative for abdominal hernia repair. Ann Plast Surg 2004;52:188–94. [DOI] [PubMed] [Google Scholar]
- 13. KCI USA, Inc . Graftjacket regenerative tissue matrix for wounds: instructions for use. 173P0020 Rev B. San Antonio: KCI USA, Inc, 2012. [Google Scholar]
- 14. KCI USA, Inc . Graftjacket Xpress flowable soft tissue scaffold for wounds: instructions for use. Part No. 173P0024 Rev. A. San Antonio: KCI USA, Inc., 2012. [Google Scholar]
- 15. Truong AT, Kowal‐Vern A, Latenser BA, Wiley DE, Walter RJ. Comparison of dermal substitutes in wound healing utilizing a nude mouse model. J Burns Wounds 2005;4:72–82. [PMC free article] [PubMed] [Google Scholar]
- 16. Menon NG, Rodriguez ED, Byrnes CK, Girotto JA, Goldberg NH, Silverman RP. Revascularization of human acellular dermis in full‐thickness abdominal wall reconstruction in the rabbit model. Ann Plast Surg 2003;50:523–7. [DOI] [PubMed] [Google Scholar]
- 17. Campbell KT, Burns NK, Ensor J, Butler CE. Metrics of cellular and vascular infiltration of human acellular dermal matrix in ventral hernia repairs. Plast Reconstr Surg 2012;129:888–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu H, Wan H, Sandor M, Qi S, Ervin F, Harper JR, Silverman RP, McQuillan DJ. Host response to human acellular dermal matrix transplantation in a primate model of abdominal wall repair. Tissue Eng Part A 2008;14:2009–19. [DOI] [PubMed] [Google Scholar]
- 19. Eppley BL. Experimental assessment of the revascularization of acellular human dermis for soft‐tissue augmentation. Plast Reconstr Surg 2001;107:757–62. [DOI] [PubMed] [Google Scholar]
- 20. Reyzelman A, Crews RT, Moore JC, Moore L, Mukker JS, Offutt S, Tallis A, Turner WB, Vayser D, Winters C, Armstrong DG. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. Int Wound J 2009;6:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brigido SA. The use of an acellular dermal regenerative tissue matrix in the treatment of lower extremity wounds: a prospective 16‐week pilot study. Int Wound J 2006;3:181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brigido SA, Boc SF, Lopez RC. Effective management of major lower extremity wounds using an acellular regenerative tissue matrix: a pilot study. Orthopedics 2004;27:s145–9. [DOI] [PubMed] [Google Scholar]
- 23. Winters CL, Brigido SA, Liden BA, Simmons M, Hartman JF, Wright ML. A multicenter study involving the use of a human acellular dermal regenerative tissue matrix for the treatment of diabetic lower extremity wounds. Adv Skin Wound Care 2008;21:375–81. [DOI] [PubMed] [Google Scholar]
- 24. Martin BR, Sangalang M, Wu S, Armstrong DG. Outcomes of allogenic acellular matrix therapy in treatment of diabetic foot wounds: an initial experience. Int Wound J 2005;2:161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brigido SA, Schwartz E, McCarroll R, Hardin‐Young J. Use of an acellular flowable dermal replacement scaffold on lower extremity sinus tract wounds: a retrospective series. Foot Ankle Spec 2009;2:67–72. [DOI] [PubMed] [Google Scholar]
- 26. Lavery LA, Armstrong DG, Harkless LB. Classification of diabetic foot wounds. Ostomy Wound Manage 1997;43:44–53. [PubMed] [Google Scholar]
- 27. Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, Percoraro RE, Rodeheaver G, Robson MC. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen 1994;2:165–70. [DOI] [PubMed] [Google Scholar]
- 28. Wu SC, Driver VR, Wrobel JS, Armstrong DG. Foot ulcers in the diabetic patient, prevention and treatment. Vasc Health Risk Manag 2007;3:65–76. [PMC free article] [PubMed] [Google Scholar]
- 29. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–46. [DOI] [PubMed] [Google Scholar]
- 30. Brem H, Stojadinovic O, Diegelmann RF, Entero H, Lee B, Pastar I, Golinko M, Rosenberg H, Tomic‐Canic M. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol Med 2007;13:30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaslow R. Diabetes strikes “staggering” 366 million people. 2011. URL http://www.cbsnews.com/8301‐504763_162‐20105533‐10391704.html [accessed on 15 April 2013].
- 32. Davis SC, Martinez L, Kirsner R. The diabetic foot: the importance of biofilms and wound bed preparation. Curr Diab Rep 2006;6:439–45. [DOI] [PubMed] [Google Scholar]
- 33. Andersen A, Hill KE, Stephens P, Thomas DW, Jorgensen B, Krogfelt KA. Bacterial profiling using skin grafting, standard culture and molecular bacteriological methods. J Wound Care 2007;16:171–5. [DOI] [PubMed] [Google Scholar]
- 34. Falanga V. Wound bed preparation and the role of enzymes: a case for multiple actions of therapeutic agents. Wounds 2002;14:47–57. [Google Scholar]
- 35. Mulder GD, Vande Berg JS. Cellular senescence and matrix metalloproteinase activity in chronic wounds. Relevance to debridement and new technologies. J Am Podiatr Med Assoc 2002;92:34–7. [DOI] [PubMed] [Google Scholar]
- 36. Saap LJ, Falanga V. Debridement performance index and its correlation with complete closure of diabetic foot ulcers. Wound Repair Regen 2002;10:354–9. [DOI] [PubMed] [Google Scholar]
- 37. Ennis WJ, Meneses P. Managing wounds in a managed care environment: the integration concept. Ostomy Wound Manage 1998;44:22–39. [PubMed] [Google Scholar]
- 38. Steed DL, Donohoe D, Webster MW, Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. J Am Coll Surg 1996;183:61–4. [PubMed] [Google Scholar]
- 39. Cardinal M, Eisenbud DE, Armstrong DG, Zelen C, Driver V, Attinger C, Phillips T, Harding K. Serial surgical debridement: a retrospective study on clinical outcomes in chronic lower extremity wounds. Wound Repair Regen 2009;17:306–11. [DOI] [PubMed] [Google Scholar]
- 40. Lebrun E, Tomic‐Canic M, Kirsner RS. The role of surgical debridement in healing of diabetic foot ulcers. Wound Repair Regen 2010;18:433–8. [DOI] [PubMed] [Google Scholar]
- 41. Kinetic Concepts, Inc . V.A.C. Therapy Clinical Guidelines: a reference source for clinicians. San Antonio: Kinetic Concepts, Inc, 2012. [Google Scholar]
- 42. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4‐week period is a robust predictor of complete healing in a 12‐week prospective trial. Plast Reconstr Surg 2006;117:239S–44. [DOI] [PubMed] [Google Scholar]
- 43. Reiber GE, Boyko EJ, Smith DG. Lower extremity foot ulcers and amputations in diabetes. In: Harris MI, Cowie CC, Stern MP, editors. Diabetes in America. Bethesda: National Diabetes Data Group, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; NIH Publication No. 95–1468, 1995:409–28. [Google Scholar]
- 44. Siddiqui AR, Bernstein JM. Chronic wound infection: facts and controversies. Clin Dermatol 2010;28:519–26. [DOI] [PubMed] [Google Scholar]
- 45. Girod I, Valensi P, Laforet C, Moreau‐Defarges T, Guillon P, Baron F. An economic evaluation of the cost of diabetic foot ulcers: results of a retrospective study on 239 patients. Diabetes Metab 2003;29:269–77. [DOI] [PubMed] [Google Scholar]
- 46. Harrington C, Zagari MJ, Corea J, Klitenic J. A cost analysis of diabetic lower‐extremity ulcers. Diabetes Care 2000;23:1333–8. [DOI] [PubMed] [Google Scholar]
- 47. Shearer A, Scuffham P, Gordois A, Oglesby A. Predicted costs and outcomes from reduced vibration detection in people with diabetes in the U.S. Diabetes Care 2003;26:2305–10. [DOI] [PubMed] [Google Scholar]
- 48. Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg 2010;52:17S–22. [DOI] [PubMed] [Google Scholar]
- 49. Veves A, Falanga V, Armstrong DG, Sabolinski ML, Apligraf Diabetic Foot Ulcer Study . Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001;24:290–5. [DOI] [PubMed] [Google Scholar]
- 50. Marston WA, Hanft J, Norwood P, Pollak R, Dermagraft Diabetic Foot Ulcer Study Group . The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 2003;26:1701–5. [DOI] [PubMed] [Google Scholar]


