Abstract
Oncostatin M (OSM) is a multifunctional cytokine found in a variety of pathologic conditions, which leads to excessive collagen deposition. Current studies demonstrate that OSM is also a mitogen for fibroblasts and has an anti‐inflammatory action. It was therefore hypothesised that OSM may play an important role in healing of chronic wounds that usually involve decreased fibroblast function and persist in the inflammatory stage for a long time. In a previous in vitro study, the authors showed that OSM increased wound healing activities of diabetic dermal fibroblasts. However, wound healing in vivo is a complex process involving multiple factors. Thus, the purpose of this study was to evaluate the effect of OSM on diabetic wound healing in vivo.
Five diabetic mice were used in this study. Four full‐thickness round wounds were created on the back of each mouse (total 20 wounds). OSM was applied on the two left‐side wounds (n = 10) and phosphate‐buffered saline was applied on the two right‐side wounds (n = 10). After 10 days, unhealed wound areas of the OSM and control groups were compared using the stereoimage optical topometer system. Also, epithelialisation, wound contraction and reduction in wound volume in each group were compared. The OSM‐treated group showed superior results in all of the tested parameters. In particular, the unhealed wound area and the reduction in wound volume demonstrated statistically significant differences (P < 0·05). The results of this study indicate that topical application of OSM may have the potential to accelerate healing of diabetic wounds.
Keywords: Diabetic wound, Oncostatin M, Wound healing
Introduction
Optimum healing of wounds requires a well‐orchestrated integration of the complex biological and molecular events of cell migration, proliferation, extracellular matrix (ECM) deposition and remodelling (1). In chronic wounds, however, this balance is lost and the wound often remains in the inflammatory stage for a long period of time. In addition, dermal fibroblasts from chronic wounds commonly show decreased proliferative capacity, ECM and protein synthesis, and growth factor release (2).
Oncostatin M (OSM) is a multifunctional cytokine that belongs to the interleukin (IL)‐6 subfamily (3). Human OSM was originally isolated in 1986 from lymphoma cells. Current studies demonstrate that dysregulated production of OSM or its receptors plays a role in pulmonary fibrosis characterised by an increased deposition of ECM, being associated with proliferation and activation of subepithelial fibroblasts 4, 5. In addition, OSM is known to exhibit an anti‐inflammatory effect. In the study models of inflammation, OSM is produced late in the cytokine response and protects from lipopolysaccharide (LPS)‐induced toxicities, promoting reestablishment of homoeostasis by cooperating with proinflammatory cytokines and acute phase molecules to alter and attenuate the inflammatory response. Moreover, administration of OSM inhibits bacterial LPS‐induced production of tumour necrosis factor (TNF)‐α and septic lethality in a dose‐dependent manner (6).
Taken together, these results of ECM deposition and anti‐inflammatory effect suggest that OSM may play an important role in the healing process of chronic wounds with attenuated fibroblast activity and a prolonged inflammatory phase. Despite the theoretical potential of OSM in treating chronic wounds, there have been few studies addressing the effect of OSM on chronic wound healing.
In 2006, the authors demonstrated that OSM treatment could contribute to the acceleration of normal wound healing in vitro and the most effective concentration was found to be 100 ng/ml (7). Lin et al. also reported that OSM treatment increased wound healing activities of diabetic dermal fibroblasts including cell proliferation and synthesis of collagen and glycosaminoglycan (8). In contrast to in vitro experiments, however, wound healing in vivo is a complex process requiring multiple factors. Thus, the purpose of this study was to evaluate the effect of OSM on diabetic wound healing in vivo.
Materials and methods
The Institutional Review Board of Korea University Guro Hospital approved the study. All animals were treated humanely and all protocols were conducted according to the principles expressed in the Declaration of Helsinki.
Animals and wounding
Genetically diabetic 8‐ to 10‐week‐old male black mice (BKS, Cg‐m+/+Lepr db ; Korea Research Institute of Bioscience and Biotechnology, Daejeon, Korea), weighing 40–45 g were used. After the mice were anaesthetised with an intraperitoneal (i.p.) injection of pentobarbital sodium (0·04 mg/g), full‐thickness, 5‐mm punch wounds were created on the skin and in the panniculus carnosus on the dorsal surfaces (two wounds on the left and right sides) of each mouse. Five mice were used for this study (10 wounds per each group, i.e. a total of 20 wounds).
OSM treatment
To apply OSM on the wounds, a hydrofibre dressing (Aquacell; Convatec, Skillman, NJ) was used. These dressings were cut using a 5‐mm punch into 5‐mm‐diameter disks. The disks were soaked in 100 ng/ml of OSM solution. After the hydrofibre disks were loaded with OSM, they were applied on the two left‐side wounds. For the right‐side control wounds, hydrofibre disks, which were soaked in Dulbecco's phosphate‐buffered saline (DPBS) without Mg2+ and Ca2+ (Gibco, Grand Island, NY) solution, and OSM, were applied.
Thereafter, wounds were covered with Tegaderm (3 M, St. Paul, MN). The same dressing was applied on the second, fourth, sixth and eighth postoperative days (Figure 1). Ten days after treatment, the animals were euthanised with an i.p. injection of pentobarbital sodium (4 mg/g), and the wounds were examined.
Figure 1.
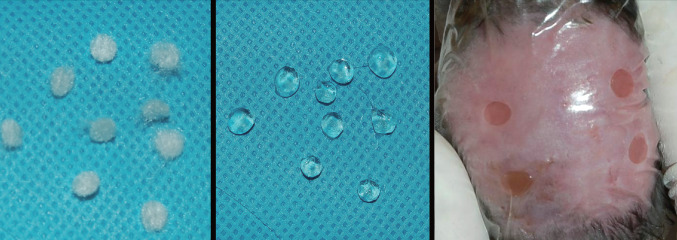
View of the hydrofibre disks used in the animal study (Left). The disks were loaded with 100 ng/ml of Oncostatin M (OSM) or DPBS solution without OSM (Centre). The loaded hydrofibre disks were applied on the wounds (OSM on the two left‐side wounds and DPBS on the two right‐side control wounds) (Right). Thereafter, the wounds were covered with a film dressing (Tegaderm).
Evaluation of wound healing
Unhealed wound areas of the OSM and control groups were measured to compare the amount of wound healing. In addition, epithelialisation, wound contraction and reduction in wound volume in each group were compared.
For quantification of the parameters, a stereoimage optical topometer (SOT) system was used. Briefly, photographs of each wound were taken using the SOT system at baseline and on the tenth postoperative day. The images were converted to digital data using a data acquisition board (PC Vision Plus®;, Adept Turnkey, Sydney, Australia) with two CCD cameras (TM440®;; Pulnix Co, CA). The stereo data were then analysed for the surface contours by matching 2 two‐dimensional images and calculating the three‐dimensional coordinates of the x‐, y‐ and z axes for each pixel 9, 10.
The unhealed wound area was directly measured by the SOT system. The epithelialised area, contracted area and reduction in wound volume were calculated as follows (11).
-
1
Epithelialised area = Total wound area − Unhealed wound area
-
2
Contracted area = Initial wound area − Total wound area
-
3
Reduction in wound volume = Initial wound volume − 10th day wound volume (Figure 2)
Figure 2.
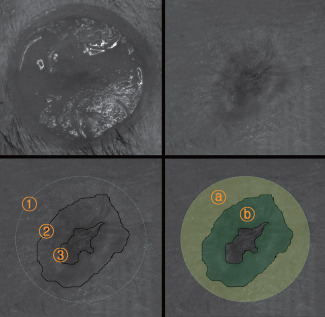
Photographs of a wound obtained by the stereoimage optical topometer (SOT) system. A full‐thickness round wound on the dorsum of a mouse created by a punch (Above, left). The wound 10 days after treatment (Above, right).  Initial wound area,
Initial wound area,  Total wound area,
Total wound area,  Unhealed wound area (Below, left).
Unhealed wound area (Below, left).  Contracted area (
Contracted area ( ),
),  Epithelialised area (
Epithelialised area ( ) (Below, right).
) (Below, right).
The initial wound area was measured immediately after creating the punch wound. The total wound area was measured on the tenth postoperative day including the epithelialised and unhealed wound areas. These areas and the wound volume were measured using MATLAB 7.0 software (Mathworks, Natick, MA) after taking photographs of each wound by the SOT system.
Statistical analyses
The results were expressed as mean ± SD and statistical comparisons were performed using the Mann–Whitney U test. Statistical significance was accepted at the 5% confidence level. The data were analysed using SPSS 13.0 software.
Results
By gross examination, a great difference was observed between the OSM treatment and control groups with respect to the unhealed wound areas. The wounds treated with OSM appeared to heal more rapidly than the control wounds (Figure 3).
Figure 3.
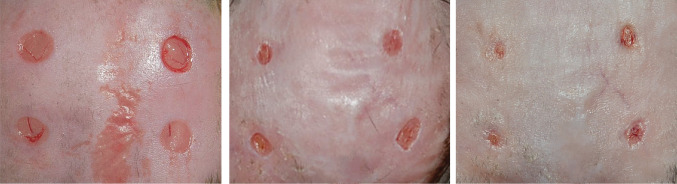
Gross findings of the wounds. Left‐side wounds of a mouse treated with Oncostatin M (OSM) showed superior results in comparison with the right‐side control wounds. Before treatment (Left). Six days after treatment (Center). Ten days after treatment (Right).
By the analyses using the SOT system, the OSM‐treated group was superior to the control group in the unhealed wound area (control: 1·1 ± 0·5 mm2; OSM: 0·2 ± 0·2 mm2; P < 0·05; Figure 4). The epithelialised area (control: 3·5 ± 1·4 mm2; OSM: 4·2 ± 1·0 mm2) and the contracted area (control: 11·2 ± 1·0 mm2; OSM: 11·6 ± 0·9 mm2) showed differences between the two groups, but these were not statistically significant (Figure 5). The OSM‐treated group also showed a superior result in the reduction of wound volume (control: 1156·6 ± 226·2 pixels; OSM: 1769·9 ± 257·6 pixels; P < 0·05; Figure 6).
Figure 4.
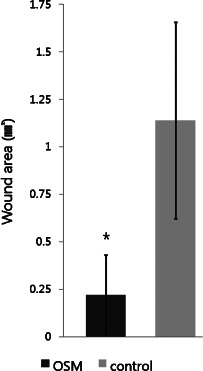
Results of the study comparing the unhealed wound areas (∗ P < 0·05).
Figure 5.
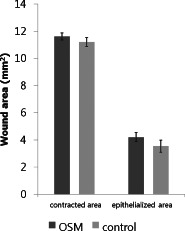
Results of the study comparing the amounts of the wound contraction and epithelialisation.
Figure 6.
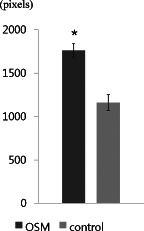
Results of the study comparing the reduction of wound volume (∗ P < 0·05).
There were no adverse events including wound infection and skin irritation in both groups during the study period.
Discussion
In normal wound healing, regeneration of the epithelial and mesenchymal tissues is affected by keratinocytes and fibroblasts, and is coordinated via complex cell–cell and cell–matrix interactions. However, these responses are altered in chronic wounds with prolonged inflammation and decreased activity of fibroblasts (12).
Wound inflammation is established to initiate and drive wound healing. However, wound inflammation has opposing effects in cases of diabetic wound healing. Diabetic wounds represent severely disturbed healing conditions that are associated with a dramatically exacerbated and prolonged inflammation at the wound site (13).
Fibroblasts play a pivotal role in mediating wound healing responses, ranging from the synthesis and remodelling of the ECM to the production of growth factors (14). However, fibroblasts isolated from diabetic wounds have lower proliferative potential and cause changes associated with cellular senescence because of high glucose concentrations (15). Similar studies regarding other types of chronic wounds are in agreement with these findings, showing a decreased response to growth factors and other cytokines (1). Impaired fibroblast activity could explain the lack of granulation tissue and ECM formation in chronic diabetic wounds (16).
As previously mentioned, OSM alters and attenuates the inflammatory response by protecting from LPS‐induced toxicities and promoting reestablishment of homoeostasis by cooperating with proinflammatory cytokines and acute‐phase molecules. In addition, OSM treatment increases wound healing activities of diabetic dermal fibroblasts including cell proliferation and synthesis of collagen and glycosaminoglycan. It can therefore be guessed that OSM played an important role in healing the diabetic wounds in this study due to its properties of mitogenic effect on fibroblasts and reduction of inflammatory phase. However, further study is needed to define exact mechanism of OSM on diabetic wound healing in vivo.
OSM is a 28 000‐Da glycoprotein that belongs to the IL‐6 cytokine family that includes IL‐6, leukaemia‐inhibitory factor (LIF), IL‐11, ciliary neurotrophic factor and cadiotrophin‐1 (17). This protein acts on a variety of cells and elicits diverse biological responses, suggesting potential roles in the regulation of cell survival, differentiation and proliferation (5). Recent studies have shown that OSM is elevated in the lungs of patients with fibrotic diseases characterised by an accumulation of ECM (4). In addition, OSM is known to induce endogenous IL‐6 synthesis in dermal fibroblasts that leads to the subsequent stimulation of mitogenesis of fibroblasts. Although OSM is structurally and functionally related to IL‐6, endogenous IL‐6 synthesis is not directly involved in the OSM‐induced mitogenic response of dermal fibroblasts. These results suggest that OSM may play a unique role in dermal fibrotic repair or fibrosis through specific OSM receptors (3). OSM is also known to regulate angiogenesis by stimulating the expression of bFGF, a well‐characterised angiogenic factor, at the RNA and protein levels in endothelial cells (18). Therefore, OSM may be involved in wound healing not only by the mitogenic effect on fibroblasts but also by the modulation of ECM synthesis and angiogenesis 19, 20.
Action mechanism of OSM has been well described previously 3, 4, 5, 17, 18, 19, 20. Cytokines of the IL‐6 family including OSM share the gp130 receptor subunit as a common signal transducer. OSM signals through two receptor complexes. The type I and II receptor are composed of gp130 and LIF receptor, and gp130 and OSM receptor, respectively. Common biological activities of LIF and OSM are mediated through the type I receptor and OSM‐specific activities through the type II receptor. Ligand binding to the receptor complex activates the Janus‐activated kinase/signal transducers and activators of transcription (JAK‐STAT) pathway, the mitogen‐activated protein kinase (MAPK) pathway and the phosphatidylinositol 3‐kinase (PI3K) pathway.
Polymorphonuclear neutrophils (PMNs) are a source of OSM and release OSM from pre‐formed stores 21, 22. OSM produced by PMNs in inflamed tissues acts as an anti‐inflammatory mediator, and inhibits IL‐1‐induced expression of IL‐8, known as a ‘neutrophil chemotactic factor’. This suggests that PMNs may limit their own recruitment to sites of local inflammation through OSM (23). OSM also functions to inhibit LPS‐induced TNF‐α production and up‐regulates IL‐6 and acute phase proteins. These data indicate that OSM may act therapeutically in the treatment of inflammatory diseases by regulating the spectrum of events that comprise a natural feedback loop to return active inflammation to homoeostasis.
These findings raise the hypothesis of a positive role of OSM in the healing of chronic wounds characterised by fibroblast dysfunction and a prolonged inflammatory phase. The direct effect of OSM on fibroblasts and epithelial cells, and the induction of protease inhibitors have not been reported with other anti‐inflammatory cytokines such as IL‐6, IL‐10 or IL‐11 (6). Thus, we hypothesised that OSM could be used in chronic wound healing due to its properties of mitogenic effect on fibroblasts and reduction of inflammatory phase.
The authors' previous in vitro study showed that OSM treatment increased wound healing activities of diabetic dermal fibroblasts (8). The results of the present in vivo study concur with those of the former in vitro study. Topical treatment with OSM accelerated diabetic wound healing by stimulating epithelialisation, wound contraction and reduction in wound volume, which are major contributing factors in wound healing. In addition, an even more profound difference was observed in wound volume reduction. The reduction in wound volume can reflect granulation tissue production. However, OSM treatment in the infected or critically colonised wound has the potential to exacerbate wound bioburden as it suppresses inflammation and has somewhat similar characteristics to growth factors, that is, stimulating fibroblast activities. Therefore, OSM should be used with caution when treating diabetic wounds that are at risk of infection.
Various methods have been used for the objective quantification of wound healing via animal studies. However, many drawbacks have been noticed, including the euthanasia of experimental animals, the impossibility of measuring changes of wound sizes, invasiveness, contamination of wounds and the complexity of the marker detection associated with the wound healing (9). To overcome these disadvantages, the SOT system was used in this study. The principle of stereoimaging involves the application of stereopsis principle of human vision. The technique can calculate the depth by measuring the changes in two‐dimensional images of the same object obtained by two video cameras and can interpret the disparity as a distance by a stereo‐matching process. Because information on the depth is included in the concept of this disparity, three‐dimensional information can be found. Therefore, it is possible to carry out a topographic study of the skin surface used an SOT to evaluate the wound volume objectively (24). According to Rho et al.(11), disparity can be considerably useful in quantitative result interpretation for evaluating the wound healing process. The SOT system can simultaneously measure the unhealed area, the epithelialised area, the contracted area and the granulation tissue formation. Moreover, the SOT system can serially measure these parameters without using invasive techniques and keep the wound surface clean.
However, there were some limitations in this study. First, the reduction in wound volume (granulation formation) was not measured directly, but calculated from the three‐dimensional data of each pixel on a disparity map. The reduction in wound volume includes not only the granulation formation but also the wound contraction. Therefore, the final reduction in wound volume may reflect a relative difference, rather than an absolute value of the granulation formation. Second, this study was focused only on the possibility of using OSM for diabetic wound healing in vivo. The mechanism of the action should be further investigated. Third, histological or biochemical analyses may be necessary to confirm the effect of OSM treatment. Fourth, OSM concentration of 100 ng/ml was used in this study based on the results of the previous in vitro study. It may be required to determine the most effective concentration of OSM for wound healing in vivo. Fifth, healing a human diabetic wound is a complex process and may be quite different from the experimental models. Further studies are needed to examine the role of OSM on diabetic wound healing in humans. Finally, the chemical and toxicological properties of OSM for human use have not been established yet even though there were no known hazards. More study is required for the safety issue.
Conclusion
The results of this study demonstrate that OSM treatment may have a potential to stimulate diabetic wound healing.
References
- 1. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005. ; 366 : 1736 – 43. [DOI] [PubMed] [Google Scholar]
- 2. Cha J , Kwak T , Butmarc J , Kim TA , Yufit T , Carson P , Kim SJ , Falanga V. Fibroblasts from non‐healing human chronic wounds show decreased expression of beta ig‐h3, a TGF‐beta inducible protein. J Dermatol Sci 2008. ; 50 : 15 – 23. [DOI] [PubMed] [Google Scholar]
- 3. Ihn H , Tamaki K. Oncostatin M stimulates the growth of dermal fibroblasts via a mitogen‐activated protein kinase‐dependent pathway. J Immunol 2000. ; 165 : 2149 – 55. [DOI] [PubMed] [Google Scholar]
- 4. Mozaffarian A , Brewer AW , Trueblood ES , Luzina IG , Todd NW , Atamas SP , Arnett HA. Mechanisms of oncostatin M‐induced pulmonary inflammation and fibrosis. J Immunol 2008. ; 181 : 7243 – 53. [DOI] [PubMed] [Google Scholar]
- 5. Scaffidi A , Mutsaers S , Moodley Y , McAnulty RJ , Laurent GJ , Thompson PJ , Knight DA. Oncostatin M stimulates proliferation, induces collagen production and inhibits apoptosis of human lung fibroblasts. Br J Pharmacol 2002. ; 136 : 793 – 801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wahl AF , Wallace PM. Oncostatin M in the anti‐inflammatory response. Ann Rheum Dis 2001. ; 60 ( 3 Suppl ): iii75 – iii80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chun K‐W , Han S‐K , Lee B‐I , Kim W‐K. Optimal concentration of OSM for wound healing activity of fibroblasts. J Korean Wound Care Soc 2006. ; 2 : 77 – 82. [Google Scholar]
- 8. Lim HW , Chun KW , Han S‐K , Kim W‐K. Effect of oncostatin M on wound healing activity of diabetic fibroblasts in vitro. J Korean Soc Plast Reconstr Surg 2008. ; 35 : 355 – 9. [Google Scholar]
- 9. Kim M‐G , Park S‐Y , Ha S‐H , Lee J‐D , Hong S‐H , Moon J‐S , Oh C‐H. Objective interpretation of severity of SLS induced edema by stereoimaging. J Dermatol Sci 2004. ; 35 : 125 – 31. [DOI] [PubMed] [Google Scholar]
- 10. Park S , Ha S‐H , Yu D‐S , Son S‐W , Kim D‐J , Kim I‐H , Moon J‐S , Kin M‐K , Oh C‐H. Quantitative evaluation of severity in psoriatic lesions using three‐dimensional morphometry. Exp Dermatol 2004. ; 13 : 223 – 8. [DOI] [PubMed] [Google Scholar]
- 11. Rho K‐H , Han S‐K , Kim W‐K. New measurement method of wound healing by stereoimage optical topometer system. J Korean Soc Plast Reconstr Surg 2008. ; 36 : 755 – 8. [Google Scholar]
- 12. Goren I , Kmpfer H , Mller E , Schiefelbein D , Pfeilschifter J , Frank S. Oncostatin M expression is functionally connected to neutrophils in the early inflammatory phase of skin repair: implications for normal and diabetes‐impaired wounds. J Invest Dermatol 2006. ; 126 : 628 – 37. [DOI] [PubMed] [Google Scholar]
- 13. Goren I , Linke A , Muller E , Pfeilschifter J , Frank S. The suppressor of cytokine signaling‐3 is upregulated in impaired skin repair: implications for keratinocyte proliferation. J Invest Dermatol 2006. ; 126 : 477 – 85. [DOI] [PubMed] [Google Scholar]
- 14. Cook H , Stephens P , Davies KJ , Harding KG , David WT. Defective extracellular matrix reorganization by chronic wound fibroblasts is associated with alterations in TIMP‐1, TIMP‐2, and MMP‐2 activity. J Invest Dermatol 2000. ; 115 : 225 – 33. [DOI] [PubMed] [Google Scholar]
- 15. Han S‐K , Choi K‐J , Kim W‐K. Clinical application of fresh fibroblast allografts for the treatment of diabetic foot ulcers: a pilot study. Plast Reconstr Surg 2004. ; 114 : 1783 – 9. [DOI] [PubMed] [Google Scholar]
- 16. Hehenberger K , Kratz G , Hansson A , Brismar K. Fibroblasts derived from human chronic diabetic wounds have a decreased proliferation rate, which is recovered by the addition of heparin. J Dermatol Sci 1998. ; 16 : 144 – 51. [DOI] [PubMed] [Google Scholar]
- 17. Gomez‐Lechon MJ. Oncostatin M: signal transduction and biological activity. Life Sci 1999. ; 65 : 2019 – 30. [DOI] [PubMed] [Google Scholar]
- 18. Wijelath ES , Carlsen B , Cole T , Chen J , Kothari S , Hammond WP. Oncostatin M induces basic fibroblast growth factor expression in endothelial cells and promotes endothelial cell proliferation, migration and spindle morphology. J Cell Sci 1997. ; 110 ( pt 7 ): 871 – 9. [DOI] [PubMed] [Google Scholar]
- 19. Richards CD , Shoyab M , Brown TJ , Gauldie J. Selective regulation of metalloproteinase inhibitor (TIMP‐1) by oncostatin M in fibroblasts in culture. J Immunol 1993. ; 150 : 5596 – 603. [PubMed] [Google Scholar]
- 20. Tanaka M , Miyajima A. Oncostatin M: a multifunctional cytokine. Rev Physiol Biochem Pharmacol 2003. ; 149 : 39 – 52. [DOI] [PubMed] [Google Scholar]
- 21. Aderka D , Le JM , Vilcek J. IL‐6 inhibits lipopolysaccharide‐induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol 1989. ; 143 : 3517 – 23. [PubMed] [Google Scholar]
- 22. Kerfoot SM , Raharjo E , Ho M , Kaur J , Serirom S , McCafferty DM , Burns AR , Patel KD , Kubes P. Exclusive neutrophil recruitment with oncostatin M in a human system. Am J Pathol 2001. ; 159 : 1531 – 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grenier A , Dehoux M , Boutten A , Vicioso MA , Durand G , Porcidalo MAG , Martin SC. Oncostatin M production and regulation by human polymorphonuclear neutrophils. Blood 1999. ; 93 : 1413 – 21. [PubMed] [Google Scholar]
- 24. Lee O , Lee G , Oh J , Kim M , Oh C. An optimized in vivo multiple‐baseline stereo imaging system for skin wrinkles. Opt Commun 2010. ; 283 : 4840 – 5. [Google Scholar]


