Abstract
Negative pressure wound therapy with instillation (NPWTi) is increasingly used as an adjunct therapy for a wide variety of infected wounds. However, the effect of NPWTi on mature biofilm in wounds has not been determined. This study assessed the effects of NPWTi using saline or various antimicrobial solutions on mature Pseudomonas aeruginosa biofilm using an ex vivo porcine skin explant biofilm model. Treatment consisted of six cycles with 10‐minute exposure to instillation solution followed by 4 hours of negative pressure at −125 mm Hg over a 24‐hour period. NPWTi using saline reduced bacterial levels by 1‐log (logarithmic) of 7‐log total colony‐forming units (CFUs). In contrast, instillation of 1% povidone iodine (2‐log), L‐solution (3‐log), 0·05% chlorhexidine gluconate (3‐log), 0·1% polyhexamethylene biguanide (4‐log), 0·2% polydiallyldimethylammonium chloride (4‐log) and 10% povidone iodine (5‐log), all significantly reduced (P < 0·001) total CFUs. Scanning electron micrographs showed disrupted exopolymeric matrix of biofilms and damaged bacterial cells that correlated with CFU levels. Compared with previous studies assessing microbicidal effects of topical antimicrobial dressings on biofilms cultured on porcine skin explants, these ex vivo model data suggest that NPWTi with delivery of active antimicrobial agents enhances the reduction of CFUs by increasing destruction and removal of biofilm bacteria. These results must be confirmed in human studies.
Keywords: Irrigation, pDADMAC, PHMB, PVP‐I, Vacuum‐assisted closure
Introduction
Negative pressure wound therapy (NPWT) has been reported to facilitate both acute and chronic wound closure by reducing localised oedema, promoting perfusion and wound contraction, removing inhibitory agents, stimulating formation of new granulation tissue and wound edge vascularity, maintaining a moist wound environment, bolstering skin grafts and reducing incidence of wound infection/reinfection 1, 2, 3, 4. A recent report analysing 97 wounds, including 69 that were infected (71%), found that NPWT was safe and effective in managing acute contaminated wounds, including patients meeting sepsis criteria 5. The practice of combining NPWT with periodic manual instillation of antiseptic or antibiotic solutions (NPWTi) was conceptualised in the clinic in 1996 6. Several successive improvements led to the development of the V.A.C. Instill® Wound Therapy (KCI USA, Inc.,San Antonio, TX) and the subsequent introduction of this system in 2003 6. NPWTi is reported to improve wound healing over NPWT alone, by the proposed mechanism of enhancing autolytic and mechanical debridement and reducing microbial bioburden 2, 7.
In the last 5 years, there has been increasing recognition that the presence of microbial biofilm is one of the main factors causing delayed wound healing 8, 9, 10, 11. However, the effects of NPWTi on mature microbial biofilms in patient's wounds are unknown. Compared with planktonic bacteria, bacteria in biofilms have considerably enhanced tolerance to exogenous antibiotics, antiseptics and disinfectants 9, 11, 12, 13, 14, 15. Therefore, microbicidal effects of solutions on planktonic bacteria do not consistently extrapolate to microbicidal effects on mature bacterial biofilm communities. To investigate the effects of NPWTi on mature biofilm attached to dermal tissue, we employed a modified ex vivo biofilm model 16 that uses fresh porcine skin explants (180 × 140 mm2) as the substrate for generating mature Pseudomonas aeruginosa biofilm.
Materials and methods
Preparation of sterile porcine skin explants
The porcine skin biofilm explants were prepared as previously described 16. Briefly, large sheets of freshly harvested porcine skin (approximately 30 × 30 cm2) were obtained from a local meat processing company, thoroughly cleaned, hair was shaved and the subcutaneous fat layer was trimmed to leave approximately 1‐ to 2‐mm thick fat pad (Figure 1). A single, central, partial‐thickness excision area measuring 5 cm wide, 10 cm long and 0·8 mm deep was made using an electric dermatome. Sheets of porcine skin were then cut to create rectangular explants measuring 18 × 14 cm2, submerged in phosphate‐buffered saline (PBS) containing 0·6% hypochlorous acid and 5 ml/l Tween‐80 for 5 minutes and then transferred to a gas chamber for two 45‐minute treatments with chlorine gas generated using a mixture of household bleach and acetic acid with repositioning of the explants between treatments for complete exposure. The gas‐exposed explants were submerged again in sterile PBS containing 0·6% hypochlorous acid and 5 µl/l Tween‐80 for 5 minutes. The sterile explants were then rinsed twice in sterile PBS and then transferred to 500 cm2 sterile bioassay dishes (Nunc 240835 with external dimensions of 245 × 245 × 25 mm3) with soft tryptic soy agar (TSA, 0·5% agar) supplemented with antimycotic (amphotericin B 2·5 µg/ml) and antibiotic solutions (gentamicin 50 µg/ml) to inhibit penetration of bacteria through the skin.
Figure 1.
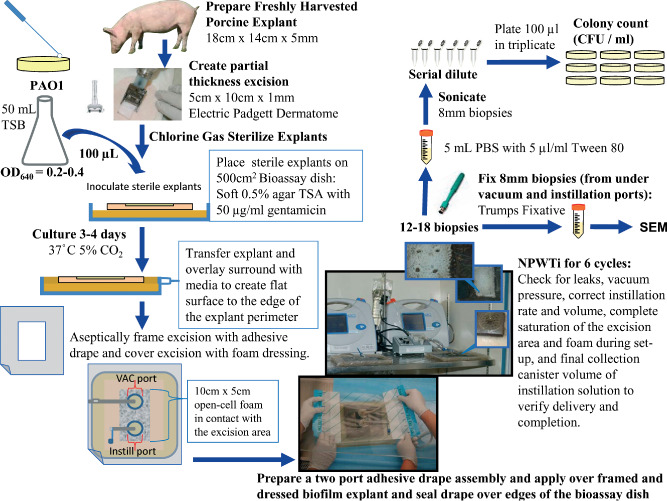
Schematic of negative pressure wound therapy with instillation (NPWTi) assay using an ex vivo biofilm on porcine skin model.
Growth of mature P. aeruginosa PAO1 biofilm on sterile porcine skin explants
The partial‐thickness excision area on each sterile porcine skin explant was inoculated with approximately 106 colony‐forming units (CFUs) per cm2 of P. aeruginosa PAO1 bacteria from an early logarithmic (log)‐phase (OD640nm = 0·2–0·6) planktonic culture. Inoculated explants were incubated for 3.5 days (84 hours) at 37°C and 5% CO2 with saturated humidity to generate mature biofilm and then explants were transferred into 245 × 245 × 18 mm3 sterile culture dishes (BD Falcon 351040) with a base of soft TSA (0·5% agar) containing gentamicin (50 µg/ml). Standard 1·5% TSA agar with gentamicin (50 µg/ml) was overlaid onto the soft agar base surrounding the explant to create a flat surface that was even with the surface of the explant to the edge of the culture dish (Figure 1).
Application of NPWTi
A large adhesive semi‐occlusive drape sheet with a 10 × 5 cm2 rectangle cut from the centre was positioned to frame the partial‐thickness excision area containing the biofilm, pressed onto the surface of the porcine explants and the edges of the drape were sealed over the sides of the culture dish (Figure 1). A single NPWTi reticulated open‐cell foam dressing (V.A.C. VeraFlo™ Dressing; KCI USA, Inc.) measuring 10 × 5 cm2 was placed onto the framed partial‐thickness excision area containing mature PAO1 biofilm. A second adhesive semi‐occlusive drape sheet with vacuum port assembly and an instillation port assembly separated by approximately 3·5 cm was centred over the reticulated open‐cell foam dressing, pressed firmly onto the back of the first semi‐occlusive drape sheet and sealed over the edge of the culture dish. The instillation port tubing was connected to the volumetric pump that delivered the instillation solution and the vacuum port tubing was connected to the pump unit (Prototype V.A.C.ULTA™ Therapy System; KCI USA, Inc.). NPWTi was applied for six cycles over 24 hours. Each 4‐hour cycle consisted of 30 seconds of instillation of approximately 50 ml of test solution, followed by 10 minutes of soak/dwell time and 4 hours of continuous negative pressure at −125 mm Hg. Following the treatment, the volume in the collection canister was recorded to ensure complete delivery of instillation solution.
Test groups were as follows: (i) untreated (no NPWT); (ii) NPWT alone (NPWTi without periodic instillation; continuous vacuum); (iii) NPWTi + saline (0·9% sodium chloride); (iv) NPWTi + L‐solution (KCI USA, Inc.); (v) NPWTi + S‐solution (KCI USA, Inc.); NPWTi + 10% PVP‐I (povidone iodine, undiluted 10% topical prep solution with 1% available iodine, Triadine); (vi) NPWTi + 1% PVP‐I (povidone iodine, 10‐fold dilution of PVP‐I in 0·85% saline, 0·1% available iodine, Triadine); (vii) NPWTi + 0·1% PHMB (Prontosan®, 0·1% polyhexamethylene biguanide/polyaminopropyl biguanide; BBraun Medical Inc., Bethlehem, PA); (viii) NPWTi + 0·2% pDADMAC (polydiallyldimethylammonium chloride dilution in 0·85% saline; Quickmed, Gainesville, FL); and (ix) NPWTi + 0·05% CHG (chlorhexidine gluconate, 80‐fold dilution of Hibiclens® in 0·9% saline; Molnlycke® Health Care, Norcross, GA).
Quantification of bacterial load
After 24 hours of treatment, the drape and dressing were removed, and eight 8‐mm diameter biopsies were collected from the vacuum port region, and eight biopsies were taken from the instillation port region of the partial‐thickness excision area from each explant (Figures 1 and 3). Additionally, one 8‐mm biopsy was taken from directly below the vacuum port and one 8‐mm biopsy was taken from directly below the instillation port and processed for scanning electron microscopy (SEM). The 16 biopsies used to determine CFUs were transferred to separate sterile 15‐ml conical centrifuge tubes containing 5 ml of sterile PBS with 5 µl/l Tween‐80 and then sonicated in a water bath for 5 × 1·5 minutes (with 1‐minute pauses) to disperse biofilm bacteria. Bacterial suspensions were serially diluted and plated in triplicate onto TSA plates to measure the total CFUs of bacteria (planktonic plus biofilm). Colonies were counted after 24 to 72‐hour culture at 37°C and reported as CFU/ml of sonicated biopsy bacterial suspension. Assessment for each antimicrobial instillation test solution was repeated in at least three separate experiments, and a saline instillation control and untreated control were included in every trial.
Figure 3.
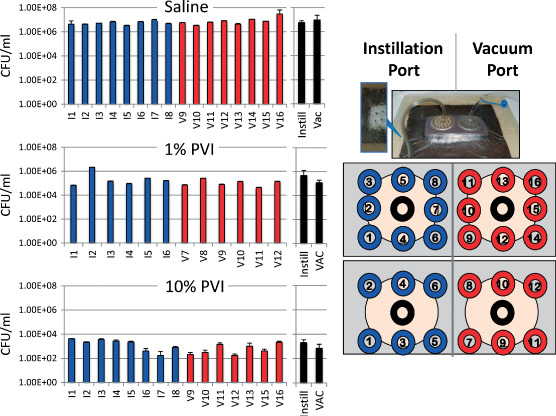
Bacterial load relative to biopsy position. Multiple biopsies from all trials were collected to determine if bacterial burden is influenced by position relative to the vacuum or instillation ports. Data from single experiments (trials) are shown here as representative examples. The data generated from a trial after 1% and 10% povidone iodine (PVP‐I) instillation is representative of this observed trend of slightly lower bacterial burden near the vacuum port, which was not observed with any of the saline instillation trials. The blue bars are results from biopsies collected under the instillation port and the red bars are results from biopsies collected under the vacuum port. The black bars are the means and standard deviations of the multiple samples collected from under the instillation port (Instill) or vacuum port (Vac).
Statistics
All bacterial counts (CFU/ml determined for each sonicated biopsy bacterial suspension) were log10‐transformed prior to analysis in order to normalise the sample distributions. All NPWTi with antimicrobial instillation samples were compared to saline instillation samples and untreated samples (no NPWTi control) after they were grouped by port position (instillation port or VAC port), producing two data sets (V and I groups) per treatment condition, as well as a single data set grouped by treatment condition alone. For each comparison, one‐way analysis of variance (ANOVA) was first performed on all samples to detect any treatment differences (P < 0·001), both relative to, and irrespective of, port position. Holm–Sidak multiple comparison post hoc tests for pairwise multiple independent comparisons were used to identify pairs with group mean differences (P < 0·001).
Results
Bacterial load after NPWT with or without periodic instillation
Combined results from multiple NPWTi experiments are shown in Figure 2. Treatment with NPWT alone (vacuum with no instillation, n = 3 experiments) reduced PAO1 bacterial load by less than 1‐log compared to ˜7‐log total CFUs in the untreated control group, which was not statistically significant (P = 0·221). NPWTi with periodic saline instillation (n = 9 experiments) significantly reduced the bacteria load (P = 0·011) ˜1‐log compared to untreated control group. We hypothesise that the 1‐log reduction observed with saline instillation compared to the bacterial load of 7‐log of the untreated group primarily reflects mechanical removal of unattached bacteria. In contrast, instillation with 10% PVP‐I (n = 3 experiments) resulted in the largest reduction of bacterial load, reducing CFUs ˜4‐log compared to instillation with saline or NPWT alone, and ˜5‐log reduction compared to total bacterial load. Although 10% PVP‐I is generally considered too cytotoxic to use as a routine wound cleansing solution, it was included in these experiments as a ‘positive control’ solution that would be expected to effectively reduce biofilm levels. The mean CFUs found after NPWTi with all the other antimicrobial agents were significantly different compared to saline instillation (P < 0·001), except for S‐solution (n = 3 experiments). NPWTi using 1% povidone iodine (n = 3 experiments) reduced total CFUs by ˜2‐log; instillation with L‐solution (n = 3 experiments) reduced total CFUs by ˜3‐log; instillation with 0·05% chlorhexidine gluconate (n = 3 experiments) reduced total CFUs by ˜3‐log; instillation with 0·1% polyhexamethylene biguanide (n = 6 experiments) reduced total CFUs by ˜4‐log; and instillation with 0·2% polydiallyldimethylammonium chloride (n = 3 experiments) reduced total CFUs by ˜4‐log.
Figure 2.
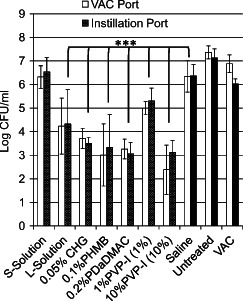
Effects of 24‐hour negative pressure wound therapy with instillation (NPWTi) (six cycles) on PAO1 biofilm attached to porcine dermis. NPWTi with antimicrobial solution instillation was compared to NPWTi with saline instillation and untreated (control). ***P < 0·001. The values are the mean and standard deviations of results from multiple experiments (trials) combined into one graph. The number of replicate experiments performed for each treatment group is presented in the Results section and ranged from three to nine replicate experiments.
Analysis of the uniformity of NPWTi effects across the treatment area
To assess uniformity of the microbicidal effects of the different test solutions delivered by peristaltic pump across the entire surface of the partial‐thickness excision under the reticulated open‐cell foam, 12 or 16 biopsies were collected from different locations as shown in Figure 3, and CFUs were measured for each individual biopsy. Overall, the level of bacterial reduction was uniform across the entire surface of the partial‐thickness excision for all eight instillation solutions. Although not statistically significant, we did observe a trend of reduced bacterial load near the vacuum port compared with the area near the instillation port when antimicrobial solutions were instilled (Figures 2 and 3). In contrast, there was no trend of reduced bacterial load relative to the vacuum or instillation ports observed when saline was instilled (Figures 2 and 3). We hypothesise that the trend of reduced bioburden under the vacuum port after NPWTi with antimicrobial solutions reflects mechanical debridement of bacteria/biofilm that were not firmly attached to the dermis owing to exposure to the antimicrobial agents.
SEM analysis
After 24 hours of NPWTi with saline or antimicrobial solutions, the tissue appeared clean and free of bacteria by gross visual examination, although high levels of planktonic and biofilm bacteria were present as determined by ultrasonic dispersal and culturing technique. Scanning electron micrographs of sterile porcine skin showed no attached bacteria or bacterial biofilm (Figure 4). In contrast, scanning electron micrographs of biopsies after NPWTi with saline instillation revealed abundant amounts of attached biofilm bacteria covering the surface of the partial‐thickness excision biopsy (Figure 5).
Figure 4.

Scanning electron micrograph of sterile porcine skin explant. Partial‐thickness excision area at 800× magnification. Note the absence of any bacteria, including Pseudomonas aeruginosa.
Figure 5.
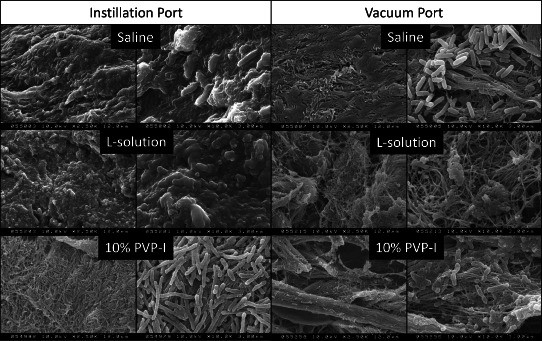
Scanning electron micrographs of PAO1 biofilm attached to porcine skin explants after negative pressure wound therapy (NPWT) with periodic instillation of saline, L‐solution or 10% PVP‐I. Left panel is 2500× magnification and right panel is 10 000× magnification of biopsies collected from directly under the central areas of the vacuum and instillation ports of the same piece of porcine skin treated with the listed instillation solutions.
SEM analysis was performed on biopsies collected from directly beneath the vacuum and instillation ports for solutions with the lowest (saline; 1‐log), intermediate (L‐solution; 2–3‐log) and highest (10% PVP‐I; 4–5‐log) reduction in viable bacterial load (Figure 5). Abundant extracellular polymeric substance (EPS) with embedded PAO1 bacteria was present on the surface of the biopsies harvested directly beneath the instillation port after either saline or L‐solution instillation. In contrast, the appearance of the biofilm colonies remaining after instillation with 10% PVP‐I was dramatically altered. The secreted EPS matrix that normally surrounds bacterial cells in biofilm communities was absent, but numerous rod‐shaped bacterial cells remained attached to the surface of the biopsies. However, the shapes of the PAO1 cells were highly distorted and elongated, which is consistent with cell cycle disruption and low viable CFU levels.
SEM analysis of biopsies collected from below the vacuum port were nearly devoid of EPS matrix after instillation with saline, L‐solution or 10% PVP‐I. However, a significant number of attached bacterial cells were still present on the surface below the vacuum port after saline instillation, whereas less surface bacteria were observed after L‐solution or 10% PVP‐I instillation. Furthermore, considerable bacterial cell damage was observed after L‐solution and 10% PVP‐I instillation. These observations were supported by the trend for lower CFU levels near the vacuum port compared with the instillation port for all the antimicrobial instillation solutions. Collectively, both SEM analyses and CFU levels indicate that NPWTi with antimicrobial solution can significantly reduce levels of viable P. aeruginosa bacteria in mature biofilm communities. The mechanism appears to be a combination of removing EPS matrix material and unattached/dispersed bacteria and periodic delivery of fresh antimicrobial solutions.
Discussion
The use of traditional, continuous NPWT as adjunctive treatment for infected wounds remains controversial. However, its use has expanded in recent years based on increasing evidence indicating significantly improved healing outcomes. A recent review reported that there is some evidence to suggest that NPWTi with periodic instillation on infected wounds shows no improvement with use of saline, but was effective with instillation of antiseptics 17. In 2009, an advisory panel reviewed evidence and generated practice recommendations regarding the usage of NPWT/NPWTi to manage infected wounds 2. More recently, guidelines were published for use of NPWT specifically addressing treatment variables 18 or specifically addressing use of NPWTi 19. However, NPWT/NPWTi is neither considered a replacement for thorough or repeated debridement nor recommended for use on untreated wounds, but rather as an adjunct therapy 2, 18, 19. Examples of solutions that have been previously reported to be effective in conjunction with NPWTi in patients are as follows: antibiotic solutions 6, 20; 0·5% silver nitrate solution 21; Microcyn® skin and wound cleanser (0·003% hypochlorous acid, 0·004% sodium hypochlorite and 0·023% sodium chloride, pH7·4; Oculus Innovative Sciences, Petaluma, CA) 22; taurolidine solution 23 and Lavasept® solution (0·04–0·2% PHMB; BBraun Medical Inc.) 24, 25, 26, 27, 28, 29, 30. Among these clinical reports, instillation (soak/dwell time) varied from 8 to 30 minutes with 1–6 hours negative pressure per cycle.
Despite case reports showing effective use of adjunctive NPWTi in managing infected wounds, literature review indicates the need for further assessment of NPWTi, particularly against microbial biofilm because of the increased tolerance of biofilm to antibiotic and antiseptic agents. During development of a biofilm, planktonic bacteria reversibly adhere to surfaces (i.e. open wounds), and in response to environmental signals, they replicate and become sessile (irreversibly attached), secrete a protective EPS matrix and then differentiate, forming microcolonies and maturing into complex three‐dimensional biofilms 12, 13, 31, 32. The substrate for attachment and nutritional availability are two factors that are known to contribute to the development and properties of bacterial biofilm 11. In this study, we used an ex vivo porcine skin explant mature biofilm model, which more closely replicates conditions in skin wounds than models that use biofilms grown on plastic or agar surfaces 16, to assess the capacity of periodic exposure to antimicrobial solutions combined with negative pressure to destroy and/or remove the mature biofilm attached to dermal tissue. Furthermore, studies have shown that our model organism, P. aeruginosa, preferentially binds to damaged epithelial cells 33. We have previously shown that the antimicrobial efficacy of topical application of antimicrobial agents or dressings using an ex vivo porcine biofilm model with P. aeruginosa PAO1 biofilm had results similar to those observed using an in vivo porcine model (34). However, this ex vivo model is a simplified system and does not assess possible contributions of other physiological processes that occur during wound healing, such as a patient's immunological systems and production of exudate containing other wound healing factors, in enhancing reduction of bacterial biofilm during NPWTi.
We found that NPWT alone or NPWTi with saline instillation was not effective in removing bacterial biofilm attached to partial‐thickness excision area of porcine skin, which suggests that mechanical debridement with NPWT/NPWTi is limited to unattached bacteria, debris and EPS. Interestingly, we found that instillation of PHMB, pDADMAC, PVP‐I, CHG or L‐solution significantly (P < 0·001) reduced the mean bacterial load from 6‐log total CFU to 1‐log to 4‐log compared with saline instillation. It is interesting to compare the effects of these antimicrobial solutions delivered with NPWTi compared to results of the same agents when applied topically for 24‐hour continuous exposure under static conditions (without NPWT). For example, application of 0·1% PHMB gel‐saturated gauze reduced the total bacterial load (planktonic and biofilm) by 1‐log from 8‐log total compared with the 4‐log reduction from 7‐log total bacterial load produced by NPWTi (unpublished). Similarly, application of 10% PVP‐I‐saturated gauze reduced the total bacterial load by 3‐log from 8‐log total compared with the 5‐log reduction from 7‐log total bacterial load produced by NPWTi (unpublished). These differences in destroying biofilm bacteria indicate that the antimicrobial efficacies of antiseptic agents were synergistically improved when applied for six cycles of 10 minutes of fresh agent exposure combined with negative pressure compared to continuous static exposure for 24 hours. There may be several reasons why NPWTi improved destruction of biofilm on dermis in this ex vivo porcine skin model. NPWTi cycling may remove non‐attached (planktonic) bacteria or loosely attached biofilm (e.g. saline instillation reduced total CFU by 1‐log). Macrodistortion and microdistortion forces produced by the reticulated open‐cell foam structure during negative pressure cycling may alter the biofilm EPS matrix structure sufficiently to enhance the penetration of the antimicrobial agents into the biofilm. Also, repeated instillation (six cycles) of fresh antiseptic solutions may result in detachment or dispersal of biofilm bacterial cells that are removed during subsequent negative pressure cycles.
It is clear that with the choice of instillation solution one must consider the degree of potential cytotoxic or other host responses that are specifically relevant to the type of wound and other patient considerations that can only be accurately assessed in vivo. For example, skin and soft tissue infection or osteomyelitis of a limb may have options of more aggressive instillation solutions with a higher threshold of cytoxicity than those options appropriate for abdominal lavage. Furthermore, these considerations would be adjusted to the degree and specific nature of the infection (e.g. biofilm, fungus and multidrug‐resistant bacteria). In contrast to continuous exposure of wound cells to agents in microbicidal dressings, NPWTi with periodic instillation of antimicrobial solutions could minimise exposure time, reducing potential cytotoxic and other negative host responses, while maximising antimicrobial and antibiofilm activities.
In summary, this ex vivo porcine model study indicates that: (i) NPWTi with volumetric pump instillation and reticulated open‐cell foam evenly delivered instillation solution to the entire partial‐thickness excision area over the entire course of treatment; (ii) mechanical disruption provided by NPWTi with saline was not sufficient to significantly remove bacteria biofilm firmly attached to dermis; (iii) NPWTi with instillation of antimicrobial solutions provided significantly more reduction in biofilm CFUs than continuous topical exposure to antimicrobial solution‐saturated dressings; and (iv) further clinical assessment of NPWTi with instillation of antiseptic solutions in patients with biofilm infections is needed.
Acknowledgements
Dr GSS presented as a faculty member during the 2012 International Surgical Wound Forum (ISWF), an annual educational event sponsored by Kinetic Concepts, Inc. (KCI). His article is part of a KCI‐funded educational supplement based on faculty presentations at 2012 and 2013 ISWF sessions related to wound care strategies with a focus on use of negative pressure wound therapy with instillation (i.e. V.A.C. Instill® Wound Therapy and V.A.C. VeraFlo™ Therapy; KCI, San Antonio, TX). KCI assisted with editorial review of the manuscript. Dr PLP and Ms QY state no conflict of interests or financial relationship with KCI. This study was funded by an unrestricted research grant from Kinetic Concepts, Inc. (KCI).
Phillips PL, Yang Q, Schultz GS. The effect of negative pressure wound therapy with periodic instillation using antimicrobial solutions on Pseudomonas aeruginosa biofilm on porcine skin explants.
References
- 1. Orgill DP, Bayer LR. Update on negative‐pressure wound therapy. Plast Reconstr Surg 2011;127:105S–15S. [DOI] [PubMed] [Google Scholar]
- 2. Gabriel A, Shores J, Bernstein B, De Leon J, Kamepalli R, Wolvos T, Baharestani MM, Gupta S. A clinical review of infected wound treatment with vacuum assisted closure (r) (vac (r)) therapy: experience and case series. Int Wound J 2009;6:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hinck D, Franke A, Gatzka F. Use of vacuum‐assisted closure negative pressure wound therapy in combat‐related injuries‐literature review. Mil Med 2010;175:173–81. [DOI] [PubMed] [Google Scholar]
- 4. Ichioka S, Watanabe H, Sekiya N, Shibata M, Nakatsuka T. A technique to visualize wound bed microcirculation and the acute effect of negative pressure. Wound Repair Regen 2008;16:460–5. [DOI] [PubMed] [Google Scholar]
- 5. Shweiki E, Gallagher KE. Negative pressure wound therapy in acute, contaminated wounds: documenting its safety and efficacy to support current global practice. Int Wound J 2013;10:13–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fleischmann W. The history of the vac instill (r) therapy. Infection 2009;37(1 Suppl):4. [Google Scholar]
- 7. Gabriel A, Shores J, Heinrich C, Baqai W, Kalina S, Sogioka N, Gupta S. Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J 2008;5:399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jones SG, Edwards R, Thomas DW. Inflammation and wound healing: the role of bacteria in the immuno‐regulation of wound healing. Int J Low Extrem Wounds 2004;3:201–8. [DOI] [PubMed] [Google Scholar]
- 9. Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis 2004;17:91–6. [DOI] [PubMed] [Google Scholar]
- 10. James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilms in chronic wounds. Wound Repair Regen 2008;16:37–44. [DOI] [PubMed] [Google Scholar]
- 11. Hall‐Stoodley L, Stoodley P, Kathju S, Hoiby N, Moser C, Costerton JW, Moter A, Bjarnsholt T. Towards diagnostic guidelines for biofilm‐associated infections. FEMS Immunol Med Microbiol 2012;65:127–45. [DOI] [PubMed] [Google Scholar]
- 12. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999;284:1318–22. [DOI] [PubMed] [Google Scholar]
- 13. Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 2002;15:167–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bowler PG, Davies BJ. The microbiology of infected and noninfected leg ulcers. Int J Dermatol 1999;38:573–8. [DOI] [PubMed] [Google Scholar]
- 15. Davies CE, Hill KE, Wilson MJ, Stephens P, Hill CM, Harding KG, Thomas DW. Use of 16s ribosomal DNA PCR and denaturing gradient gel electrophoresis for analysis of the microfloras of healing and nonhealing chronic venous leg ulcers. J Clin Microbiol 2004;42:3549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Phillips PL, Yang QP, Sampson EM, Schultz GS. Antimicrobial agents on an in vitro biofilm model of skin wounds. In: Sen CK, editor. Advances in wound care. New Rochelle: Mary Ann Liebert, Inc., 2010:299–304. [Google Scholar]
- 17. Murray CK, Obremskey WT, Hsu JR, Andersen RC, Calhoun JH, Clasper JC, Whitman TJ, Curry TK, Fleming ME, Wenke JC, Ficke JR, Infect PC‐R. Prevention of infections associated with combat‐related extremity injuries. J Trauma 2011;71:S235–57. [DOI] [PubMed] [Google Scholar]
- 18. Krug E, Berg L, Lee C, Hudson D, Birke‐Sorensen H, Depoorter M, Dunn R, Jeffery S, Duteille F, Bruhin A, Caravaggi C, Chariker M, Dowsett C, Ferreira F, Martinez JMF, Grudzien G, Ichioka S, Ingemansson R, Malmsjo M. Evidence‐based recommendations for the use of negative pressure wound therapy in traumatic wounds and reconstructive surgery: steps towards an international consensus. Injury 2011;42:S1–12. [DOI] [PubMed] [Google Scholar]
- 19. Gabriel A. Integrated negative pressure wound therapy system with volumetric automated fluid instillation in wounds at risk for compromised healing. Int Wound J 2012;9:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wiedeck C. Vac instill (r) treatment in severe septic abscess forming and perforating Ludwig's angina with large abscess formation in the neck and lower jawbone osteomyelitis due to dental caries with tooth root abscess – a case report. Infection 2009;37(1 Suppl):7–12. [Google Scholar]
- 21. Bernstein BH, Tam H. Combination of subatmospheric pressure dressing and gravity feed antibiotic instillation in the treatment of post‐surgical diabetic foot wounds: a case series. Wounds 2005;17:37–48. [Google Scholar]
- 22. Wolvos TA. Advanced wound care with stable, super‐oxidized water – a look at how combination therapy can optimize wound healing. Wounds 2006. Jan 1;18(1 Suppl):11–3. [Google Scholar]
- 23. Reith HB. The application of instillation combined with vacuum therapy in visceral surgery. Infection 2009;37(1 Suppl):41–2. [Google Scholar]
- 24. Brem MH, Blanke M, Olk A, Schmidt J, Mueller O, Hennig FF, Gusinde J. The vacuum‐assisted closure (vac) and instillation dressing. Limb salvage after 3 degrees open fracture with massive bone and soft tissue defect and superinfection. Unfallchirurg 2008;111:122–5. [DOI] [PubMed] [Google Scholar]
- 25. Timmers MS, Graafland N, Bernards AT, Nelissen RGHH, Van Dissel JT, Jukema GN. Negative pressure wound treatment with polyvinyl alcohol foam and polyhexanide antiseptic solution instillation in posttraumatic osteomyelitis. Wound Repair Regen 2009;17:278–86. [DOI] [PubMed] [Google Scholar]
- 26. Brem MH, Schulz‐Drost S, Gusinde J, Stuebinger A, Hennig FF, Olk A. Therapy options with vac instill (r) in the treatment of open, superinfected injuries of the lower limps – the erlangen algorithm. Infection 2009;37(1 Suppl):5–7. [Google Scholar]
- 27. Lehner B, Weiss S, Suda AJ, Witte D. Application of vac instill (r) therapy in case of periprosthetic infection in hip arthroplasty. Infection 2009;37(1 Suppl):13–7. [Google Scholar]
- 28. Koster G. Management of early periprosthetic infections in the knee using the vacuum‐instillation therapy. Infection 2009;37(1 Suppl):18–20. [Google Scholar]
- 29. Schintler MV, Prandl EC, Kreuzwirt G, Grohmann MR, Spendel S, Scharnagl E. The impact of vac instill (r) in severe soft tissue infections and necrotizing fasciitis. Infection 2009;37(1 Suppl):31–2. [Google Scholar]
- 30. Amtsberg G, Frank M, Lange J, Gondert M, Kramer A, Ekkernkamp A, Hinz P. Vacuum‐assisted closure and instillation dressing (vac instill (r)) in the treatment of open fractures. Infection 2009;37(1 Suppl):33–4. [Google Scholar]
- 31. Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. The involvement of cell‐to‐cell signals in the development of a bacterial biofilm. Science 1998;280:295–8. [DOI] [PubMed] [Google Scholar]
- 32. Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin‐Scott HM. Microbial biofilms. Annu Rev Microbiol 1995;49:711–45. [DOI] [PubMed] [Google Scholar]
- 33. Tsang KWT, Rutman A, Tanaka E, Lund V, Dewar A, Cole PJ, Wilson R. Interaction of Pseudomonas aeruginosa with human respiratory mucosa in‐vitro. Eur Respir J 1994;7:1746–53. [DOI] [PubMed] [Google Scholar]


