Abstract
Postoperative wound healing plays a significant role in facilitating a patient's recovery and rehabilitation. Surgical wound dehiscence (SWD) impacts on mortality and morbidity rates and significantly contributes to prolonged hospital stays and associated psychosocial stressors on individuals and their families. A narrative review of SWD was undertaken on English‐only studies between 1945 and 2012 using three electronic databases Ovid CINHAL, Ovid Medline and Pubmed. The aim of this review was to identify predisposing factors for SWD and assessment tools to assist in the identification of at‐risk patients. Key findings from the included 15 papers out of a search of 1045 revealed the most common risk factors associated with SWD including obesity and wound infection, particularly in the case of abdominal surgery. There is limited reporting of variables associated with SWD across other surgical domains and a lack of risk assessment tools. Furthermore, there was a lack of clarity in the definition of SWD in the literature. This review provides an overview of the available research and provides a basis for more rigorous analysis of factors that contribute to SWD.
Keywords: Surgical site infection, Surgical wound breakdown, Surgical wound dehiscence
Introduction
Timely and sustained postoperative wound healing plays a significant role in optimising a patient's postoperative recovery and rehabilitation. It has been established that surgical wound dehiscence (SWD) contributes to increased morbidity and mortality rates, and implicit and explicit costs for individuals and health care providers 1, 2, 3, 4, 5. Explicit costs result from prolonged hospitalisation, the need for community nursing and support services and the use of wound management consumables 6, 7, 8, 9, 10. Social costs include delay in return to employment, reduced ability to self‐care and limitations on returning to previous social roles in the community including family support. SWD is defined as the rupture or splitting open of a previously closed surgical incision site. According to the Centre for Disease Control (CDC), a SWD can be classified as either superficial or deep 11.
A review of the literature for factors associated with SWD was conducted in response to an identified increase in SWD referrals to a community nursing service in Western Australia, following either a cardiothoracic, orthopaedic, vascular or abdominal surgical procedure. The aim of this review was to identify predisposing factors for SWD and assessment tools to assist in the identification of at‐risk patients.
Wound dehiscence is a possible complication following any surgical procedure; however, most authors 1, 2, 3, 9, 10, 12, 13, 14 report the occurrence following orthopaedic, abdominal, cardiothoracic and vascular surgery. The literature outlines some associations between SWD and patient comorbidities and the type of surgical wound closure 5, 6, 12, 15, 16, 17, 18, 19. However, the validation of these associations as effective diagnostic predictors for SWD risk has been poorly studied across most surgical domains.
Methods
A narrative review of the literature was carried out in‐line with the ‘patient, phenomenon, outcome (PPO)’ search strategy established by the Oxford Centre for Evidence‐Based Medicine (http://www.cebm.net/index.aspx?o=1900, last accessed 6 March 2012). Electronic searches were carried out on PubMed, Ovid MEDLINE (1945–2012) and Ovid CINHAL (1986–2012) using the following key terms: patients* and surgical* wound* or wound breakdown* surgical wound dehiscence* or surgical site infection*. Studies were classified into the Oxford CEBM Levels of Evidence: level 1 (systematic review of RCTs, individual RCT and prospective cohort with good follow‐up); level 2 (systematic review with homogeneity of cohort studies and retrospective cohort studies) and level 3 (case–control studies) (http://www.cebm.net/index.aspx?o=1025, last accessed 6 March 2012). Inclusion criteria were studies that had evidence levels 1–3, had defined SWD and studies that were in the following surgical domains: cardiothoracic, orthopaedic, vascular or abdominal. Exclusion criteria were as follows: those studies beyond the level of evidence 3 and no definition of surgical wound dehiscence (CDC or other recognised definitions). Studies were restricted to the English language as access to translation of other languages was restricted, and once deemed suitable (see Figure 1) these studies were hand sorted for cross referencing.
Figure 1.
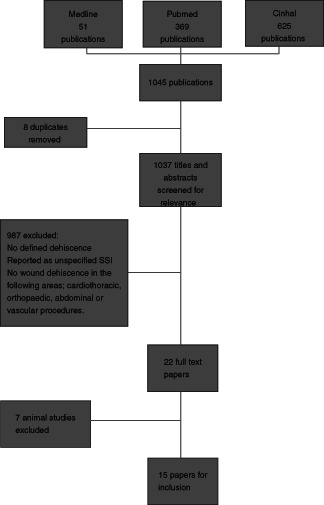
Flow chart of study inclusion.
Results
Of the initial 1045 papers related to SWD based on the previously described search criteria, eight duplicates were removed and 987 were excluded as they failed to fit the inclusion criteria of the surgical domains within the scope of the analysis and the lack of definition of the type of surgical site infection (SSI; superficial or deep) with no alignment to CDC classification or a similar classification system for SSI or SWD. Of the remaining 22 papers, 7 were excluded as they were animal studies. The remaining 15 papers were classified into levels of evidence.
Prevalence and incidence of SWD
The occurrence of SWD following different surgical procedures has been reported as ranging between 1·3 and 9·3% (Table 1). Amongst these studies, incidence data have been reported in accordance with the CDC SSI classification guidelines. The studies within the scope of the analysis were categorised into abdominal wound dehiscence, cardiothoracic, orthopaedic and vascular. For the purposes of this review, SWD is defined as the rupturing or splitting apart of the margins of a wound closure 20. Wound dehiscence can be a superficial or deep tissue injury and according to the CDC 21 wound dehiscence can be associated with SSI.
Table 1.
Incidence of surgical wound dehiscence
| Procedure | Study |
|---|---|
| Abdominal surgery—superficial dehiscence 2% and deep dehiscence 0·3% | Hadar et al. 17 |
| Abdominal 1·3–4·7% | Wounds West prevalence data (2007–2011) |
| Caesarean section 3% | De Vivo et al. 18 |
| Sternal wound 3% | John 72 |
| Hip prosthesis 3% | Smith et al. 16 |
| Saphenous vein graft 9·3% (10/108 patients) | Biancari and Tiozzo 38 |
Abdominal wound dehiscence
Abdominal wound dehiscence is the most widely reported dehiscence and features prominently in the literature. It is a severe postoperative complication with mortality rates reportedly as high as 45% 22 and the incidence ranges from 0·4% to 3·5% 1, 2, 13, 23, 24, 25, 26 (Wounds West). SWD risk assessment tools have been developed for abdominal wound dehiscence 1, 2. A prognostic risk model for SWD was developed in the USA by Webster et al. 2 following a retrospective audit of medical notes. The authors then conducted a prospective validation of their prognostic risk model on patients who underwent laparotomies. The authors 2 determined a percentage risk prediction value for patients and have suggested that this prognostic model can be used in a perioperative setting.
Van Ramshorst et al. 1 reported on the development and testing of a risk validation tool following a retrospective case investigation of abdominal SWDs. The authors 1 identified several risk factors such as age, gender, emergency surgery, the type of surgery, postoperative coughing and wound infection as contributing risk factors to SWD. Their findings were similar to those of the analysis of Webster et al. 2. The van Ramshorst risk prediction tool demonstrated high predictive values for patients with tested risk factors of SWD and the fit of the model by the Hosmer and Lemeshow test (P = 0·79), and ROC analysis of 0·91 showed high predictive value of the risk score.
When comparing the findings of Webster et al. with those of Ramshorst et al., the former revealed an increased risk of SWD after abdominal surgery when the operative time was greater than 6 hours, a fourth‐year postgraduate resident performed the surgery in lieu of a more experienced surgeon, the wound was a clean wound classification, a confirmed presence of wound infection and extended time on a ventilator as highly significant factors (see Table 3). Analogies drawn between the two studies may be limited because of the lack of homogeneity between the studies' sample populations. Webster's et al. cohort were patients who underwent laparotomies performed at 132 Veterans Affairs Medical Centres, average age 60 years (Neumeyer, personal communication, 16 April 2012), whereas the sample populations of van Ramshorst et al. were recruited from the general surgical population.
Table 3.
Incidence of SSI in Eastern Australia (2000) 41
| Surgical domain | Surgical procedure | Incidence of SSI* (%) |
|---|---|---|
| Cardiac | Coronary artery bypass graft (CABG) | 2·1 |
| Obstetrics | Caesarean section | 2·4 |
| Vascular | Abdominal aortic aneurysm repair | 7·3 |
| Orthopaedics | Hip prosthesis | 2 |
| Knee prosthesis | 9·8 | |
| Colorectal | Procedure not specified | 12·7 |
SSI, surgical site infection.
The type of closure method used has been identified as a risk factor for abdominal wound complications by some authors 15, 27. Rucinski et al. 27 conducted a meta‐analysis of the literature published in 2001 to determine that continuous mass (all‐layer) closure with absorbable monofilament sutures to be the optimal closure technique after laparotomy. The review paper of Ceydeli et al. 15 supports this finding and the authors concluded that the optimal method of closure following a vertical midline laparotomy incision was a mass closure using a simple running technique having #1 or #2 absorbable monofilament suture with a suture length to wound length ratio of 4:1. Hollinsky and Sandberg 28 conducted a study on cadavers to determine if a reinforced tension line (RTL) technique for abdominal wall closure was able to withstand the following tensile forces in the epigastrum of 110 Newtons (N), and the umbilicus and hypogastrium of 120 and 100 N, respectively (2007:124) compared with non‐reinforced sides. In 77% of the non‐reinforced sites, sutures tore away from the tissues at a median load of 60·7 N, which was a much lower force than tolerated by the reinforced sides. Similarly, Agarwal 29 who used the RTL continuous technique in patients who underwent emergency midline laparotomies found that this technique resulted in no burst sutures. Furthermore, in 100 patients who were closed using a non‐RTL continuous suture wounds resulted in SWD (P = 0·009). Perhaps, it is that increased abdominal wall force, because of rises in intraabdominal pressure and the chosen method of closure, may play a role in the occurrence of SWD, and this has been observed by several authors 1, 30, 31.
Sternal wound dehiscence
Sternal wound dehiscence can result in lengthy hospital stays and increased morbidity and mortality rates in patients. Incidence of infection of median sternotomy wounds is reported in Europe as between 0·3% and 5% 32. The most commonly reported predisposing factors identified in the literature for sternal wound dehiscence include diabetes, female gender, breast size, bilateral IMA procedure and prolonged postoperative ventilation (see Table 3). Buja 33 reported that there are several risk scales for the prediction of deep sternal wound infection (DSWI) involving confirmed infection; however, there remains to be a risk tool for prediction of non‐microbial dehiscence. In a retrospective review by Ridderstolpe et al. 6 sternal wound complications were recorded for 9·7% of the study population. Of those, 6·4% were related to superficial infections, 1·6% were deep sternal infections and 1·7% of the patients contracted postoperative mediastinitis. Risk factors were divided into groups of preoperative, intraoperative and postoperative factors and of the total 42 variables identified, 32 were associated with increased risk across all the three groups. The authors found that the major independent predictors of sternal wound complications were identified as age over 75 years, obesity, insulin‐dependent diabetes, smoking, peripheral vascular disease and prolonged ventilator support. The authors stated that with diligent follow‐up more sternal wound complications could be prevented.
Tekumit et al. 34 in a retrospective review compared ‘figure‐of‐eight’ and simple wire technique methods used in the closure of the sternum following coronary artery bypass graft (CABG) procedures. The findings revealed that there was no increased association with either closure technique or sternal wound dehiscence. However, patients undergoing CABG that is complicated by infection, results in triple the costs to the health care system 8. Graf et al. 8 conducted a case–control analysis of patients who contracted DSWI following a CABG, and reported the median overall cost per patient was €36 261 compared with €13 356 for the control patient. The costs for those patients with DSWI comprised ward care costs (24·7%), surgery costs (19%), ICU care (27·7%), laboratory tests (15%) and other costs not specified (13·6%). Few would disagree with Graf et al. 8 who argued for the need for appropriate infection control measures for the prevention of DSWI.
Orthopaedic wound dehiscence
As is the case with other wound types, orthopaedic surgery complications such as infection and SWD can lead to extended hospital stays, increased patient morbidity and an excess fiscal burden for patients and the health care system. Numerous studies have investigated the use of staples versus sutures and the relationship of this closure technique to wound complications 16, 34, 35, 36, 37. Smith et al. 16 and Shetty et al. 36 reported an increase in superficial wound infection occurrence with the use of staples when compared with suture in hip or knee procedures. Interestingly, other research has demonstrated statistically significant higher risk of developing infection following hip surgery when patients have been closed with staples compared with sutures (P = 0·02) 16. Further research by Newman et al. 37 reported significantly fewer complications occurred with staples than sutures after total knee replacement, and similar findings were reported by Khan et al. 34 following hip replacement. Whilst associations between wound closure methods and wound complications following orthopaedic surgery have been reported, there appears to be little research investigating associations between patient comorbidities, behavioural factors and orthopaedic SWD.
Vascular wound dehiscence
A Cochrane review by Biancari and Tiozzo reported 38 the incidence of SWD following saphenous vein harvesting to be 9·3% (10 of 108) in patients that have been closed with staples compared with sutures with an incidence of 8·8%. The incidence of SSI following this procedure was also reported to be 10·8% when the wound was closed with staples and 8% when sutures were used 38. Biancari and Tiozzo 38 stated that all these trials had suboptimal methodological quality and were at risk of bias; the reviewers called for more stringent research to be carried out. Furthermore, the authors commented that there is a lack of data on risk factors associated with leg wound complications. No vascular risk assessment tool for SWD or confounding variables for SWD following a vascular procedure was found when undertaking the literature review.
Surgical site infection
The precursor to SWD is frequently perceived to be SSI 39. In the UK, SSI constitutes 20% of all hospital health care‐associated infections and it is reported that at least 5% of patients will develop an SSI 40. The high economic cost is in part owing to prolonged hospital stays or readmission costs, which were just under £90 000 per patient in 2000 9. In North America, the estimated costs of SSI are reportedly $US10 billion annually in direct and indirect medical costs 7. Furthermore, Urban identified that superficial SSIs amount to $US400 per case, whereas organ or tissue space infections can amount to $US30 000 per patient. In Europe it has been determined that the costs attributable to SSI range from €1·47 to 19·1 billion 4. Leaper et al. 4 suggest that this considerable variance is owing to inconsistencies in the data collection methods, surveillance criteria and variations in the surgical procedures (2004:247). In Australia, the cost of SSI is reportedly $A60 million per year 5, 41. However, further implicit costs associated with delays in healing and reduced quality of life for the patient, family and the wider community are difficult to ascertain from a fiscal point of view.
In the Australian context, the Hospital Infection Standardised Surveillance (HISS) 41 program reviewed ten hospitals in New South Wales and reported SSI rates following a CABG to be 2·1%. These infections resulted in an estimated additional cost of $5892 per patient for an extended length of stay of an average of 12 days. Wound infection following colorectal surgery was rated 12·7% and led to an extended patient stay of 16 days on average, with a cost of $8066 per patient 41. Orthopaedic procedures such as a total hip replacement had a reported infection rate of 2%, an extended patient stay of 7 days and additional costs of $3767 allocated per patient 41. Total knee replacement SSI rates were reported to be 9·8% with an extended stay of 13·5 days and resulted in a total cost of $6520 per patient 41. However, as all of these data were obtained from inpatient surveillance solely and did not include post‐discharge follow‐up, it is possible that the findings outlined above could be an underestimate of the total fiscal burden and incidence of SWD.
An Australian report published in 2003 by the Australian Commission into Safety and Quality in Health Care (ACSQHC) 42 stated that between 2% and 13% of patients suffer from SSI whilst in a hospital environment (see Table 2).
Table 2.
Surgical wound dehiscence articles reviewed
| Author | Title | Year | Evidence level/study design | Outcome |
|---|---|---|---|---|
| Riou et al. | Factors influencing wound dehiscence | 1992 | Level 2: Multivariate analysis of abdominal wound dehiscence patients with identified risk factors | Factors were assessed independently, 22 were identified and include age over 65, wound infection, pulmonary disease, haemodynamic instability and ostomies in the incision. Compared to control group, 30% of patients with at least five risk factors developed dehiscence, and patients with more than eight dehisced. Overall mortality was 29%, all patients with more than ten risk factors died. |
| Gislason et al. | Burst abdomen and incisional hernia after major gastrointestinal operations—comparison of three closure techniques | 1995 | Level 1: RCT of 599 patients comparing three closing techniques with polyglyconate suture (Maxon) and polyglactin 910 (Vicryl) | Patients were randomised into three groups for abdominal wound closure: continuous mass double suture with loop (Maxon), continuous mass polyglactin 910 (Vicryl) and interrupted polyglactin 910 (Vicryl). 14% patients developed wound infection, incidence of wound dehiscence 2% and incisional hernia 7%. No differences in rates of dehiscence across the groups. Gislason et al. stated that the wound complications were not associated with the closure technique and that continuous closure is as safe as the interrupted technique. Also stated that most important factor in reducing wound infection is to minimise contamination of the operating field. |
| Weiland et al. | Choosing the best abdominal closure method by meta‐analysis | 1998 | Level 3: Meta‐analysis of 12 249 patients with abdominal wound closures | Continuous closures with non‐absorbable sutures are recommended. If infection is anticipated interrupted absorbable sutures are preferred, mass closure is superior to layered closures. Interrupted non‐absorbable sutures showed higher incidence of hernias and dehiscence. Did not discuss specific suture materials. |
| Niggebrugge et al. | Influence of abdominal wall closure technique on complications after surgery: a randomised study | 1999 | Level 1: RCT testing closure methods | Tested the animal model in a group of patients and found that the CDLC is not an optimal method of closure. The authors state that there is less compliance with the abdominal wall and may lead to rupture. |
| Ridderstolpe et al. | Superficial and deep sternal wound complications: incidence, risk factors and mortality | 2001 | Level 2: Retrospective study of 3008 patients who underwent cardiac surgery from 1996 to 1999 at Linkoping University Hospital, Sweden | Risk factors for superficial and deep sternal wound infections were identified and similar. Major risk factors for sternal wound complications were obesity, diabetes, smoking, peripheral vascular disease and prolonged ventilator support. |
| Webster et al. | Prognostic models of abdominal wound dehiscence after laparotomy | 2003 | Level 2: Retrospective case note review of 17 044 laparotomies (1996–1998), of which 587 had dehiscence and were used to identify factors, and 17 763 laparotomies (1998–2000), resulting in 562 SWDs were used to validate the model | Webster et al. identified the following as major risk factors associated with SWD following laparotomy: CVA, history of COPD, current pneumonia, emergency procedure, operative time greater than 2·5 hours, postgraduate year (PGY) level 4 of 6 resident as surgeon, clean wound classification, superficial or deep wound infection, failure to wean from ventilator and return to operating room. Patients with wound dehiscence had significantly lower preoperative serum albumin levels than the non‐dehiscence group. |
| Gottrup et al. | An overview of surgical site infections: aetiology, incidence and risk factors | 2005 | Level 2: Review paper | Paper discusses factors that influence surgical wound healing, preventative measures and classification systems of operative wounds, SSIs and wound assessment. |
| Ceydeli et al. | Finding the best abdominal closure: an evidence‐based review of the literature | 2005 | Level 2: Literature review of the evidence | Specifically discussed is the identification of evidence‐based conclusions in regard to the most effective method of midline abdominal fascial closure. Ceydeli et al. reveal that a consistent conclusion can be made in regard to a preferred method of closure; use of mass closure in a simple running technique using #1 or #2 absorbable monofilament with a suture length to wound length ratio of 4:1. |
| Waqar et al. | Frequency and risk factors for wound dehiscence/burst abdomen in midline laparotomies | 2005 | Level 2: Prospective cohort trial over 1 year, n = 117 patients undergoing laparotomies with midline incision. Follow‐up was wound examination from day 3 onwards to assess healing | Seven of 117 (5·9%) patients had a wound dehiscence, 5 of which were from emergency (4·2%) and 2 were elective patients (1·7%). Waqar et al. propose that ‘peritonitis, wound infection and failure to close the abdominal wall properly are the most important causes of wound dehiscence’ (2005: 73) |
| Singh et al. | Closure of hip wound, clips or subcuticular sutures: does it make a difference? | 2006 | Level 1: Prospective trial, two groups with cohort follow‐up. Group A had clips and group B had sutures. N = 71 | Thirty‐seven patients underwent fixation with a dynamic hip screw and 34 underwent a hemi or total hip arthroplasty. Wounds inspected at days 2, 5, 7, 10 and 14 for clinical signs of infection. Statistically significant greater amount of wound discharge (P < 0·002) and redness (P < 0·009) in the clip group when compared with the suture (vicryl) group. |
| Tekumit et al. | Comparison of figure‐of eight and simple wire sternal closure techniques in patients with non microbial sternal dehiscence | 2009 | Level 2: Retrospective case note review of 6211 patients that underwent cardiac operations (median sternotomy) between 2002 and 2008 was investigated for the development of non microbial sternal dehiscence. Fishers t and Student's t‐test comparing surgical closure methods; figure‐of‐eight or simple wire technique. Survival analysis with Kaplan–Meier test. | Figure‐of‐eight and simple wire techniques were associated with similar rates of dehiscence (1·46% and 1·43%, respectively). The authors concluded that findings suggest that both closing techniques may have similar outcomes in terms of the development of non‐microbial dehiscence. |
| Graf et al. | Economic aspects of deep sternal wound infections | 2010 | Level 2: Case–control study analysing cases of deep surgical wound infection following coronary artery bypass graft (CABG) surgery. Classified according to CDC criteria. | Median overall costs of a CABG case were €36 261 compared with €13 356 per control patient without infection (P < 0·01). Median overall length of stay was 34·4 days compared with 16·5 for the control group (P = 0·0006). The authors concluded that deep sternal wound infection represents an important economic factor for the hospital and that appropriate infection control measures should be enforced. |
| van Ramshorst et al. | Abdominal wound dehiscence in adults: development and validation of a risk model | 2010 | Level 2: Retrospective case– control with multivariate stepwise logistic regression, prospective risk model validation | van Ramshorst et al. identified the following major independent risk factors for SWD: age, gender, CPD, ascites, jaundice, anaemia, emergency surgery, type of surgery, postoperative coughing and wound infection. |
| Cevasco et al. | Positive predictive value of the AHRQ Patient Safety Indicator ‘Postoperative Wound Dehiscence’. | 2011 | Level 2: Retrospective cross‐sectional study of 112 medical records identifying patients with abdominopelvic wound dehiscence 2003–2007. | Study revealed that across 28 selected hospitals in the USA between 2003 and 2007 the observed rate of wound dehiscence was 6·4 per 1000 discharges. According to Cevasco et al., the patient safety indicator (PSI) has predictive ability to determine at‐risk patients. |
| Phan et al. | Failure of secondary wound closure after sternal wound infection following failed initial operative treatment: causes and treatment | 2012 | Level 3: Retrospective study | Female patients following CABG with large sternal wounds infected with gram‐negative bacteria and candida have an 85% risk of wound dehiscence after flap coverage. Reasons for wound dehiscence were wound size and >4 species of bacteria present in the wound. Not significantly correlated was smoking, obesity, atherosclerosis, renal insufficiency or type of closure. |
SWD, surgical wound dehiscence; SSI, surgical site infection.
The report also observed that there are difficulties encountered in recording the occurrence of SWD and SSI because of lack of reliable standardised data sets and discrepancies in recording methodologies. In light of the concurrent association between SSI and SWD, it is possible that some practitioners record SWD as SSI or vice versa. Thus, it has proven difficult to identify the percentage of cases that are SWD independent of SSI. Regardless, the costs associated with SSI have been reported to be significantly high by several authors 4, 5, 9, 10, 41, 42.
Comorbidities associated with SWD
Several authors 1, 2, 39, 43, 44, 45, 46 have identified various factors associated with SWD, such as age, gender, ascites, jaundice, cardiovascular disease, pneumonia and infection (see Table 3), and have sought to identify associations between patient comorbidities and SWD across specific surgical domains. van Ramshorst et al. 1 and Webster et al. 2 identified a suite of comorbidities associated with abdominal SWD. Webster et al. ranked the level of identified predisposing factors and developed a prognostic risk model for surgical patients.
van Ramshorst et al. 1 examined a number of variables in a small population of patients who were to undergo abdominal surgery and also devised a risk score for SWD. Variables that they proved to be significant were age, gender, an emergency surgical procedure, type of surgical procedure, the presence of ascites, chronic pulmonary disease, coughing and wound infection. In the field of cardiothoracic research, workers have identified potential causes and risk factors for SWD, which include age, gender, obesity, chronic obstructive pulmonary disease (COPD) and procedure‐related factors such as duration of surgery, use of bilateral mammary graft and reoperation for control of bleeding 6, 47, 48. However, the direct correlation and significance to SWD remains to be demonstrated as these risk factors were recorded in association with an undefined classification of SSI; therefore, it is difficult to ascertain whether the factors are associated with SWD or SSI. Baskett et al. 43 found that COPD was the only variable that was identified as a risk factor for deep sternal wound infection (DSWI) and they stated that strict adherence to perioperative aseptic technique, attention to haemostasis and precise sternal closure can result in a low incidence of mediastinitis. Floros et al. reported that diabetes and high body mass index (BMI) were associated with an increased risk of DSWI 44. Similarly, other workers 6, 49 reported that a high BMI and diabetes were some of the several associated risk factors for SWD following a cardiothoracic procedure (Table 4).
Table 4.
Summary of identified risk factors for SWD
| Author | Surgical domain—wound dehiscence and/or wound infection | Variables of significance listed as a risk factor, statistical analysis method used (P value where reported) |
|---|---|---|
| McDonald et al. 69 | Cardiothoracic surgery—median sternotomy infection and dehiscence | Multivariate analysis: female gender (P = 0·03), obesity (P = 0·002), diabetes (P = 0·01) and prolonged postoperative ventilation (P = 0·006) |
| Webster et al. 2, * | Abdominal surgery—abdominal wound dehiscence | Logistic regression P < 0·05, COPD (0·002), postgraduate year of surgeon (PGY4) (0·003), operative time (0·013), emergency procedure (<0·0001), clean wound classification (0·0031), superficial wound infection (0·0048), deep wound infection (<0·0001), failure to wean from ventilator (<0·0001) and current pneumonia (0·04) |
| Baskett et al. 43 | Cardiothoracic surgery—surgical wound infection | COPD (0·01) |
| Borger et al. 70 | Cardiothoracic surgery—deep sternal wound infection | Diabetes, male, bilateral internal thoracic artery grafting maybe contraindicated in diabetic patients |
| Paletta et al. 71 | Vascular surgery—leg complications | Multivariate analysis: female gender (0·001) and peripheral vascular disease (0·001) |
| Ridderstolpe et al. 6 | Cardiothoracic surgery—superficial and deep sternal wound complications | Superficial wound complications: univariate with ROC analysis: age ≥ 65 (P = 0·006), age ≥ 75 (P = 0·020), BMI ≥ 30 (P = 0·001), diabetes (P = 0·008), ventilator support (P = 0·008). Deep sternal infections/mediastinitis: BMI ≥ 30 (P = 0·001), diabetes (P = 0·001), smoking (P = 0·001), COPD (P = 0·001), PVD (P = 0·001), reoperation—bleeding (P = 0·08), red blood cells—units (P = 0·02) and ventilator support (P = 0·004) |
| Salehi Omran et al. 49 | Cardiothoracic surgery—superficial and deep sternal wound infection following CABG | Multivariate analysis: female gender (<0·05), preoperative hypertension (<0·05), diabetes (0·05), obesity (0·05), prolonged intubation time (>48 hours) (0·05), re‐exploration for bleeding (<0·05) and hypertension (<0·05) |
| Schimmer et al. 46 | Cardiothoracic surgery—sternal dehiscence and infection | Odds ratio, P value: body mass indices greater than 30 kg/m2 (P = 0·05), New York Heart Association class more than III (P = 0·07), impaired renal function (P = 0·07), peripheral arterial disease (P = 0·001), immunosuppressant state (P = 0·001), sternal closure performed by an assistant doctor (P = 0·004), postoperative bleeding (P = 0·03), transfusion of more than 5 red blood cell units (P = 0·03), re‐exploration for bleeding (P = 0·001) and postoperative delirium (P = 0·01) |
| Sharma et al. 45 | Vascular surgery—leg complications | Forward stepwise logistic regression: female gender (0·008), renal insufficiency (<0·001), diabetes (<0·001), BMI ≥ 30 kg/m2 (<0·001), peripheral vascular disease (0·09) and ICU stay < 72 hours (0·009) |
| van Ramshorst et al. 1, * | Abdominal surgery—abdominal wound dehiscence | Multivariate stepwise logistic regression with backwards elimination, P < 0·05, age overall P value (0·02), male gender (<0·001), ascites (<0·01), wound infection (<0·001), emergency surgery (0·001), CPD (<0·001), type of surgery overall P value (0·001) and coughing (<0·001) |
| Floros et al. 44 | Cardiothoracic surgery—deep sternal wound infection | Fisher's exact test P value (<0·05), previous cardiac surgery (0·03), BMI ≥ 30 (0·041), left ventricular ejection fraction (LVEF) ≤ 30 (0·01) and homologous blood usage (<0·01) |
BMI, body mass index; CABG, coronary artery bypass graft; SWD, surgical wound dehiscence.
Risk tool/prognostic models tested.
Smoking
Smoking is well documented to affect wound healing, in particular the occurrence of wound complications and delayed healing are higher in smokers than in non‐smokers 50, 51, 52, 53, 54, 55, 56, 57 Reduced tissue oxygenation has a detrimental effect on the reparative process during healing and neutrophil defence in the presence of pathogens 57, 58, 59, 60.
Research has shown that smoking cessation by patients prior to surgery compared to those patients who continue to smoke has an improved healing outcome and less wound complications 50, 55, 61. Ridderstolpe et al. 6 identified smoking to be a significant factor associated with patients who had DSWIs following cardiothoracic surgery (P = 0·001). van Ramshorst et al. 1 were unable to report on this behavioural factor because of the lack of reporting on this in the retrospective case note audit. Of the studies included in the review, the study performed by Ridderstolpe et al. was the sole research that investigated this behavioural variable and measured the impact on healing outcome in relation to surgical wound dehiscence. Ridderstolpe et al. 6 reported that in the study population that had DSWI following surgery, smoking was a significant risk preoperative factor (>0·05) associated with wound complications.
Discussion
SWD is a significant problem for patients, clinicians and the wider community. Management of these wound complications poses a continuous challenge. This review was carried out to identify studies that described validated risk assessments on patients with established factors that lead to SWD. This review reinforces the importance of the need for more research. It is clear there is a potential under‐reporting of SWD, and a lack of clarity around the definition, as SSI does not translate directly into dehiscence. It is reported that the most common pathogens associated with superficial SSI are Staphylococcus aureus and the flora associated with the skin 62. This review revealed that there was a lack of reported pathogens associated with SWD; therefore, more rigorous investigation is required if one is to determine a causal link to pathogens as being a catalyst for deep dehiscence. Discussion in the literature on the impact of biofilms on wound healing is considerable 63, 64, 65, 66; however, research into biofilms is still in its formative years. James et al. 67 demonstrated that only 6% of acute wounds had biofilms compared with chronic wounds (60%).
Speculatively, biofilms could be present if the surgical procedure is a second or third surgical attempt at closure or if the patient is undergoing multiple revision procedures. An association between biofilms and wound dehiscence is deserving of further investigation.
Separation of cardiothoracic wound margins is most likely to be associated with a failure of surgical technique; however, evidence to support this assertion is limited. Tekumit et al. 68 compared two different internal fixation techniques used in cardiothoracic surgery and reported that the rates of dehiscence are similar between the two methods. Wound dehiscence may be attributed to SSI as discussed by Graf et al. 8 and Phan et al. 3 who both reported that microbial presence as a contributing factor to wound dehiscence. One could suggest that less than optimal surgical closure and infection when combined could be doubly problematic.
Identified intrinsic risk factors such as uncontrolled patient comorbidities may contribute to delayed healing and subsequent dehiscence. Of those that were found to be commonly reported across different surgical procedures were high BMI 6, 44, 45, 46, 49 and diabetes 6, 45, 46, 49, 69. Other associated factors that span surgical domains and are associated with SWD include age 1, 6, gender 1, 49, 69, 70, 71 and prolonged ventilator use 6, 69. In cardiothoracic surgery in particular the focus has been links with SSI and a number of risk scores have been identified such as the Toronto Risk Index, the Euroscore and the Nosocomial Infection Surveillance Risk Index and the Sternal Wound Infection Prediction Scale‐R (SWIPS and SWIPS‐R). Identification of patient‐specific factors for vascular and orthopaedic surgery could not be ascertained as there was a shortfall in the published research.
Although commonalities were found to exist for comorbidities and behavioural factors across the surgical groups, further analysis is required to demonstrate causal links in the identification of ‘at‐risk’ patients. Furthermore, the authors of this article seek to encourage future researchers who present studies on SSI to specify whether dehiscence occurred or not. Equally important is the need to identify the aetiology of wound dehiscence amongst cases.
Concluding comment
This review identified a need for rigorous prospective validation of risk assessment tools for selected surgical procedures to assist clinicians to identify ‘at‐risk’ patients prior to surgery. Such tools are required to assist clinicians to identify ‘at‐risk’ patients and to determine the effectiveness of new therapies or technologies for sustained wound healing. In light of this, the authors are currently investigating the risk factors for SWD and plan to develop a validated risk assessment tool that will facilitate identification of ‘at‐risk’ patients and guide clinical decisions for preventative measures and to improve healing outcomes.
Acknowledgements
This work is funded by a Curtin University Co‐Operative Research Centre (CRC) Wound Innovation Grant, Project Number 3·13. The authors would like to thank Wounds West for their kind contribution of wound data for Western Australia.
References
- 1. van Ramshorst GH, Nieuwenhuizen J, Hop WC, Arends P, Boom J, Jeekel J, Lange JF. Abdominal wound dehiscence in adults: development and validation of a risk model. World J Surg 2010;34:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Webster C, Neumayer L, Smout R, Horn S, Daley J, Henderson W, Khuri S, Qua NVAS. Prognostic models of abdominal wound dehiscence after laparotomy. J Surg Res 2003;109:130–7. [DOI] [PubMed] [Google Scholar]
- 3. Phan TQ, Theodorou P, Depner C, Lefering R, Perbix W, Spilker G, Weinand C. Failure of secondary wound closure after sternal wound infection following failed initial operative treatment: causes and treatment. Ann Plast Surg 2013;70:216–21. [DOI] [PubMed] [Google Scholar]
- 4. Leaper DJ, van Goor H, Reilly J, Petrosillo N, Geiss HK, Torres AJ, Berger A. Surgical site infection—a European perspective of incidence and economic burden. Int Wound J 2004;1:247–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mclaws ML, Irwig LM, Mock P, Berry G, Gold J. Predictors of surgical wound‐infection in Australia—a National Study. Med J Aust 1988;149(11–12):591–5. [DOI] [PubMed] [Google Scholar]
- 6. Ridderstolpe L, Gill H, Granfeldt H, Ahlfeldt H, Rutberg H. Superficial and deep sternal wound complications: incidence, risk factors and mortality. Eur J Cardiothorac Surg 2001;20:1168–75. [DOI] [PubMed] [Google Scholar]
- 7. Urban JA. Cost analysis of surgical site infections. Surg Infect (Larchmt) 2006;7(Suppl 1):S19–22. [DOI] [PubMed] [Google Scholar]
- 8. Graf K, Ott E, Vonberg RP, Kuehn C, Haverich A, Chaberny IF. Economic aspects of deep sternal wound infections. Eur J Cardiothorac Surg 2010;37:893–6. [DOI] [PubMed] [Google Scholar]
- 9. Reilly J, Twaddle S, McIntosh J, Kean L. An economic analysis of surgical wound infection. J Hosp Infect 2001;49:245–9. [DOI] [PubMed] [Google Scholar]
- 10. de Lissovoy G, Fraemean K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control 2008;37:387–97. [DOI] [PubMed] [Google Scholar]
- 11. Mangram AJ, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol 1999;20:247–78. [DOI] [PubMed] [Google Scholar]
- 12. Weiland DE, Bay RC, Del Sordi S. Choosing the best abdominal closure by meta‐analysis. Am J Surg 1998;176:666–70. [DOI] [PubMed] [Google Scholar]
- 13. Waqar SH, Malik ZI, Razzaq A, Abdullah MT, Shaima A, Zahid MA. Frequency and risk factors for wound dehiscence/burst abdomen in midline laparotomies. J Ayub Med Coll Abbottabad. 2005. [PubMed] [Google Scholar]
- 14. Cevasco M, Borzecki AM, Chen Q, Zrelak PA, Shin M, Romano PS, Itani KM, Rosen AK. Positive predictive value of the AHRQ Patient Safety Indicator “Postoperative Sepsis”: implications for practice and policy. J Am Coll Surg 2011;212:954–61. [DOI] [PubMed] [Google Scholar]
- 15. Ceydeli A, Rucinksi J, Wise L. Finding the best abdominal closure: an evidenced base review of the literature. Curr Surg 2005;62:220–5. [DOI] [PubMed] [Google Scholar]
- 16. Smith TO, Sexton D, Mann C, Donell S. Sutures versus staples for skin closure in orthopaedic surgery: meta‐analysis. BMJ 2010;340:c1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hadar E, Melamed N, Tzadikevitch‐Geffen K, Yogev Y. Timing and risk factors of maternal complications of cesarean section. Arch Gynecol Obstet 2011;283:735–41. [DOI] [PubMed] [Google Scholar]
- 18. De Vivo A, Mancuso A, Giacobbe A, Priolo AM, De Dominici R, Savasta LM. Wound length and corticosteroid administration as risk factors for surgical‐site complications following cesarean section. Acta Obstet Gynecol 2010;89:355–9. [DOI] [PubMed] [Google Scholar]
- 19. Iavazzo C, Gkegkes ID, Vouloumanou EK, Mamais I, Peppas G, Falagas ME. Sutures versus staples for the management of surgical wounds: a meta‐analysis of randomized controlled trials. Am Surg 2011;77:1206–21. [PubMed] [Google Scholar]
- 20. Mosby Inc. , Mosbys Medical Dictionary, 8th edn. St Louis, MO: Mosby, 2009.
- 21. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care‐associated infection and criteria for specific types of infections in the acute care setting. 2013. URL http://www.cdc.gov/nhsn/PDFs/pscManual/17pscNosInfDef_current.pdf [accessed on 3 April 2012]. [DOI] [PubMed]
- 22. Carlson MA. Acute wound failure. Surg Clin Nor Am 1997;77:607–36. [DOI] [PubMed] [Google Scholar]
- 23. Niggebrugge A, Arthur HP, Trimbos JB, Hermans J, Steup W‐H, Van De Velde CJH. Influence of abdominal wall closure technique on complications after surgery: a randomised study. Lancet 1999;353:1563–7. [DOI] [PubMed] [Google Scholar]
- 24. Riou JP, Cohen JR, Johnson H Jr. Factors influencing wound dehiscence. Am J Surg 1992;163:324–30. [DOI] [PubMed] [Google Scholar]
- 25. Spiliotis J, Tsiveriotis K, Datsis AD, Vaxevanidou A, Zacharis G, Giafis K, Kekelos S, Rogdakis A. Wound dehiscence: is still a problem in the 21th century: a retrospective study. World J Emerg Surg 2009;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Richter H, Kraft K, Kleinwechter H, Demandt N, Meincke G, Dabelstein A, Weisser B. Effects of a telephone intervention in patients with type 2 diabetes. Dtsch Med Wochenschr 2008;133:2203–8. [DOI] [PubMed] [Google Scholar]
- 27. Rucinski J, Margolis M, Panagopoulos G, Wise L. Closure of the abdominal midline fascia: meta‐analysis delineates the optimal technique. Am Surg 2001;67:421–6. [PubMed] [Google Scholar]
- 28. Hollinsky C, Sandberg S. A biomechanical study of the reinforced tension line (RTL)—a technique for abdominal wall closure and incisional hernias. Eur Surg 2007;39:122–7. [Google Scholar]
- 29. Agarwal A, Hossain Z, Das A, Chakraborty S, Nitram N, Gupta M, Ray U. Reinforced tension line suture after midline laparotomy in emergency surgery. Tropical Doctor 2011;41:193–6. [DOI] [PubMed] [Google Scholar]
- 30. Roscher C, Schumacher J, Weisser WW, Schulze ED. Genetic identity affects performance of species in grasslands of different plant diversity: an experiment with Lolium perenne cultivars. Ann Bot 2008;102:113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodriguez‐Hermosa, J.I , Codina‐Cazador, A . Ruiz, B . Girones, J . Pujadas, M . Pont, J . Aldeguer, X . Acero, D . Risk factors for acute abdominal wall dehiscence after laparotomy in adults. Cir Eps 2005;77:(5) 280–6. [DOI] [PubMed] [Google Scholar]
- 32. Losanoff JE, Richman BW, Jones JW. Disruption and infection of median sternotomy: a comprehensive review. Eur J Cardiothorac Surg 2002;21:831–9. [DOI] [PubMed] [Google Scholar]
- 33. Buja A, Zamperion A, Cavalet S, Chiffi D, Sandona P, Vinelli A, Baldovin T, Baldo V. An update review on risk factors and prediction of deep sternal wound infections. International Wound Journal 2012;9:372–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khan RJ, Fick D, Yao F, Tang K, Hurworth M, Nivbrant B, Wood D. A comparison of three methods of wound closure following arthroplasty: a prospective, randomised, controlled trial. J Bone Joint Surg Br 2006;88:238–42. [DOI] [PubMed] [Google Scholar]
- 35. Smith, T.O. Sexton, D. Mann, C. Donell, S . Sutures versus staples for skin closure in orthopaedic surgery: meta‐analysis. BMJ 2010;340:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shetty AA, Kumar VS, Morgan‐Hough C, Georgeu GA, James KD, Nicholl JE. Comparing wound complication rates following closure of hip wounds with metallic skin staples or subcuticular vicryl suture: a prospective randomised trial. J Orthop Surg (Hong Kong) 2004;12:191–3. [DOI] [PubMed] [Google Scholar]
- 37. Newman JT, Morgan SJ, Resende GV, Williams AE, Hammerberg EM, Dayton MR. Modality of wound closure after total knee replacement: are staples as safe as sutures? A retrospective study of 181 patients. Patient Saf Surg 2011;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Biancari F, Tiozzo V. Staples versus sutures for closing leg wounds after vein graft harvesting for coronary artery bypass surgery. Cochrane Database Syst Rev. 2010:CD008057. [DOI] [PubMed] [Google Scholar]
- 39. Khan MN, Naqvi AH, Irshad K, Chaudhary AR. Frequency and risk factor of abdominal wound dehiscence. J Coll Physicians Surg Pak 2004;14:355–7. [PubMed] [Google Scholar]
- 40. Leaper D, Burman‐Roy S, Palanca A, Cullen K, Worster D, Gautam‐Aitken E, Whittle M, Grp GD. Guidelines: prevention and treatment of surgical site infection: summary of NICE guidance. Br Med J 2008;1049–51. [DOI] [PubMed] [Google Scholar]
- 41. McLaws ML, Taylor PC. The Hospital Infection Standardised Surveillance (HISS) programme: analysis of a two‐year pilot. J Hosp Infect 2003;53:259–67. [DOI] [PubMed] [Google Scholar]
- 42. CARE ACSQHC . National strategy to address health care associated infections. Fourth Annual Report to the Australian Health Ministers' Conference. Canberra: Commonwealth of Australia; 2003:1–202.
- 43. Baskett RJ, MacDougall CE, Ross DB. Is mediastinitis a preventable complication? A 10‐year review. Ann Thorac Surg 1999;67:462–5. [DOI] [PubMed] [Google Scholar]
- 44. Floros P, Sawhney R, Vrtik M, Hinton‐Bayre A, Weimers P, Senewiratne S, Mundy J, Shah P. Risk factors and management approach for deep sternal wound infection after cardiac surgery at a tertiary medical centre. Heart Lung Circ 2011;20:712–7. [DOI] [PubMed] [Google Scholar]
- 45. Sharma M, Fakih MG, Berriel‐Cass D, Meisner S, Saravolatz L, Khatib R. Harvest surgical site infection following coronary artery bypass grafting: risk factors, microbiology, and outcomes. Am J Infect Control 2009;37:653–7. [DOI] [PubMed] [Google Scholar]
- 46. Schimmer C, Reents W, Berneder S, Eigel P, Sezer O, Scheld H, Sahraoui K, Gansera B, Deppert O, Rubio A, Feyrer R, Sauer C, Elert O, Leyh R. Prevention of sternal dehiscence and infection in high‐risk patients: a prospective randomized multicenter trial. Ann Thorac Surg 2008;86:1897–904. [DOI] [PubMed] [Google Scholar]
- 47. Ulicny KS Jr, Hiratzka LF. The risk factors of median sternotomy infection: a current review. J Card Surg 1991;6:338–51. [DOI] [PubMed] [Google Scholar]
- 48. Careaga Reyna G, Aguirre Baca GG, Medina Concebida LE, Borrayo Sanchez G, Prado Villegas G, Arguero Sanchez R. Risk factors for mediastinitis and sternal dehiscence after cardiac surgery. Rev Esp Cardiol 2006;59:130–5. [PubMed] [Google Scholar]
- 49. Salehi Omran A, Karimi A, Ahmadi SH, Davoodi S, Marzban M, Movahedi N, Abbasi K, Boroumand MA, Moshtaghi N. Superficial and deep sternal wound infection after more than 9000 coronary artery bypass graft (CABG): incidence, risk factors and mortality. BMC Infect Dis 2007;7:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sorensen LT. Wound healing and infection in surgery: the clinical impact of smoking and smoking cessation: a systematic review and meta‐analysis. Arch Surg 2012;147:373–83. [DOI] [PubMed] [Google Scholar]
- 51. Sorensen, L. T . Jorgensen, S . Petersen, L. J . Hemmingsen, U . Bulow, J . Loft, S . Gottrup, F . Acute Effects of Nicotine and Smoking on Blood Flow, Tissue Oxygen, and Aerobe Metabolism of the Skin and Subcutis. Journal of Surgical Research 2009;152:224–30. [DOI] [PubMed] [Google Scholar]
- 52. Kean J. The effects of smoking on the wound healing process. J Wound Care 2010;19:5–8. [DOI] [PubMed] [Google Scholar]
- 53. de Blacam C, Ogunleye AA, Momoh AO, Colakoglu S, Tobias AM, Sharma R, Houlihan MJ, Lee BT. High body mass index and smoking predict morbidity in breast cancer surgery: a multivariate analysis of 26,988 patients from the national surgical quality improvement program database. Ann Surg 2012;255:551–5. [DOI] [PubMed] [Google Scholar]
- 54. Lind J, Kramhoft M, Bodtker S. The influence of smoking on complications after primary amputations of the lower extremity. Clin Orthop Relat Res 1991;267:211–7. [PubMed] [Google Scholar]
- 55. Siana, JE Rex, S and Gottrup, F . The Effect of Cigarette‐Smoking on Wound Healing. Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery. 1989;23 (2):207–9. [DOI] [PubMed] [Google Scholar]
- 56. Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg 2003;238:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jorgensen LN, Kallehave F, Christensen E, Siana JE, Gottrup F. Less collagen production in smokers. Surgery 1998;123:450–5. [PubMed] [Google Scholar]
- 58. Hunt TK, Pai MP. The effect of varying ambient oxygen tensions on wound metabolism and collagen synthesis. Surg Gynecol Obstet 1972;135:561–7. [PubMed] [Google Scholar]
- 59. Jorgensen, LN Kallehave, F Christensen, E Siana, JE and Gottrup, F . Less collagen production in smokers. Surgery 1998;123 (4):450–5. [PubMed] [Google Scholar]
- 60. Sorensen, LT Toft, B Rygaard, J Ladelund, S Teisner, B and Gottrup, F . Smoking attenuates wound inflamation and proliferation while smoking cessation restores inflammation but not proliferation. Wound Repair and Regeneration 2010;18 (2):186–92. [DOI] [PubMed] [Google Scholar]
- 61. Sorensen, LT Karlsmark, T and Gottrup, F . Abstinence from smoking reduces incisional wound infection: A randomized controlled trial. Annals of Surgery 2003;238 (1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Leaper DJ. Risk factors for and epidemiology of surgical site infections. Surg Infect (Larchmt) 2010;11:283–7. [DOI] [PubMed] [Google Scholar]
- 63. Percival SL, Emanuel C, Cutting KF, Williams DW. Microbiology of the skin and the role of biofilms in infection. Int Wound J 2012;9:14–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wolcott R, Costerton JW, Raoult D, Cutler SJ. The polymicrobial nature of biofilm infection. Clin Microbiol Infect 2013;19:107–12. [DOI] [PubMed] [Google Scholar]
- 65. Wolcott RD, Rhoads DD, Dowd SE. Biofilms and chronic wound inflammation. J Wound Care 2008;17:333–41. [DOI] [PubMed] [Google Scholar]
- 66. Singh VA, Barbul A. Bacterial biofilms in wounds. Wound Repair Regen 2008;16:1. [DOI] [PubMed] [Google Scholar]
- 67. James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilms in chronic wounds. Wound Repair Regen 2008;16:37–44. [DOI] [PubMed] [Google Scholar]
- 68. Tekumit H, Cenal AR, Tataroglu C, Uzun K, Akinci E. Comparison of figure‐of‐eight and simple wire sternal closure techniques in patients with non‐microbial sternal dehiscence. Anadolu Kardiyol Derg 2009;9:411–6. [PubMed] [Google Scholar]
- 69. McDonald WS, Brame M, Sharp C, Eggerstedt J. Risk factors for median sternotomy dehiscence in cardiac surgery. South Med J 1989;82:1361–4. [DOI] [PubMed] [Google Scholar]
- 70. Borger MA, Rao V, Weisel RD, Ivanov J, Cohen G, Scully HE, David TE. Deep sternal wound infection: risk factors and outcomes. Ann Thorac Surg 1998;65:1050–6. [DOI] [PubMed] [Google Scholar]
- 71. Paletta CE, Huang DB, Fiore AC, Swartz MT, Rilloraza FL, Gardner JE. Major leg wound complications after saphenous vein harvest for coronary revascularization. Ann Thorac Surg 2000;70:492–7. [DOI] [PubMed] [Google Scholar]
- 72. John LCH. Modified closure technique for reducing sternal dehiscence; a clinical and in vitro assessment. Eur J Cardiothorac Surg 2008;33:769–73. [DOI] [PubMed] [Google Scholar]
- 73. Gislason H, Gronbech JE, Soreide O. Burst abdomen and incisional hernia after major gastrointestinal operations–a comparison of three closure techniques. European Journal of Sugery 1995;161:349–54. [PubMed] [Google Scholar]
- 74. Gottrup F, Melling A, Hollander DA. An overview of surgical site infections: aetiology, incidence and risk factors. World Wide Wounds 2005. http://www.worldwidewounds.com/2005/september/Gottrup/Surgical‐Site‐Infections‐Overview.html [accessed on 3 April 2012] [Google Scholar]
- 75. Singh Bijayendra MA, Mowbray S, Nunn G, Mearns S. Closure of hip wound, clips or subcuticular sutures: does it make a difference? European Journal of Orthopaedic Surgery & Traumatology 2006;16:124–9. [DOI] [PubMed] [Google Scholar]


