Abstract
Necrotising fasciitis (NF) is characterised by rapidly spreading necrosis of the soft tissue and fascia. It is rare but can be fatal when not managed properly. The aim of this study is to evaluate the diagnosis, treatment and results such as mortality, morbidity and reconstructive options of NF localised in the central part of the body. The main goal is to emphasise upon the clinical symptoms for early diagnosis which is the most important factor in saving the lives of these patients. Between January 2000 and December 2010, 30 patients with NF localised in central parts of the body were treated. Six of the patients were female (20%) and the others were male (80%). The mean age was 54·03 years (ranged between 26 and 83 years). The average time from the onset of symptoms to diagnosis was 6 days, ranging from 2 to 11 days. The localisation of NF was perineum in 24 patients (80%); inguinal and thigh region in 5 patients (16·7); and back in 1 patient (3·3%). The hospitalisation time was varying between 17 and 32 days (mean 23 days). Six patients (20%) died and 24 patients (80%) survived. All non‐survivors had risk factors and secondary comorbidities such as immunosuppression, chronic cardiac failure, and diabetes with high glucose level. Survivors also underwent repeated debridement operation 2–4 times. Reconstructive procedures were split‐thickness skin graft (STSG) in eight patients (33·3%), fasciocutaneous flaps in four patients (16·6%), fasciocutaneous flap + STSG in six patients (25%), scrotal flap + STSG in two patients (6·6%), scrotal flap in two patients (6·6%) and musculocutaneous flap + STSG in one patient (3·3%). There was no major complication such as flap and graft loss, after reconstructive procedures. Early diagnosis of NF may be the lifesaving factor. Amuputation can save the patient's life in the case of NF in the extremities; however, this is not an option for NF in central parts of the body. In these cases, when NF is suspected, early debridement of necrotic tissues should be performed. As soon as the infection and the spread of the necrosis are controlled, reconstruction should be considered.
Keywords: Fournier gangrene, Necrotising fasciitis, Perineum
Introduction
Necrotising fasciitis (NF) is a rare soft‐tissue infection with the incidence of 0·4 cases per 100·000 1, 2. It is characterised by rapidly spreading necrosis of the soft tissue and fascia. It is also a potentially fatal infection. In 1883, Fournier described the pathology of NF affecting the external genitalia and perineum. Meleney identified haemolytic streptococcus as the aetiologic agent of the disease. Although NF was first named by Wilson in 1952, NF had other names such as Phagedena gangrenosum, progressive bacterial synergistic gangrene and non‐clostridial gas gangrene 3, 4, 5. Wilson also described the cardinal features of the disease, which are inflammation and necrosis of the subcutaneous fat tissue and deep fascia with sparing of the muscles (3).
Typically, a history of minor injury to the involved site is followed by NF. Minor injuries may include insect bites, surgical incisions, cuts, abrasions, contusions, injection sites, cutaneous ulcers, perirectal abscesses, incarcerated hernia, burns, splinters, childbirth, penetrating injury and muscle strains 3, 6, 7. If NF occurs as a result of known aetiology, it is classified as secondary NF. In 45% of the cases, no definitive causative factor can be found and these NFs are also classified as primary or idiopathic NF 8, 9. NF commonly affects the extremities, but other parts of the body are also involved (10). When the extremities are involved, amputation of the affected part is possible as a lifesaving option. NF of the central part of the body (trunk and perineal regions) has a higher mortality rate than that of extremities because amputation is not feasible 3, 10.
Some risk factors are defined for NF. Immunocompromised state which is caused by any host condition is a risk for NF. The most common comorbidity of NF is diabetes mellitus (DM) which is present in 18–60% of the patients (6). The other risk factors are obesity, peripheral vascular disease, intravenous drug use, alcohol abuse, smoking, chronic cardiac disease, chronic corticosteroid therapy, chronic immune suppression, cancer and age 11, 12, 13, 14, 15, 16. Although there have been many previously identified risk factors, half of NF cases occur in healthy individuals (6).
Early diagnosis is important for treatment and lifesaving of the patient. The diagnosis is primarily based on the clinical findings. Clinical characteristics of NF are classified in three stages by Wong and Wang 17, 18 (Table 1). The early stage of NF may not be distinguished clinically from other soft‐tissue infections such as erysipelas and cellulites, but there are other helpful clinical features such as poorly defined and indistinct margins, and tenderness beyond the involved area (18). Although NF may present with classical skin changes of bullae, vesicles and necrosis, in 47% of the patients, the most sensitive symptom is disproportionately severe pain, which is noted in nearly all the patients (19) (1, 2).
Table 1.
| Stage | Clinical features |
|---|---|
| I (early) | Tenderness to palpation (extending beyond the apparent area of skin involvement), erythema, swelling, calor (warm skin) |
| II (intermediate) | Blister or bullae formation (serous fluid), skin fluctuance |
| III (late) | Crepitus, skin anesthesia, crepitation, skin necrosis with dusky discoloration, tissue necrosis progressing to gangrene |
Figure 1.
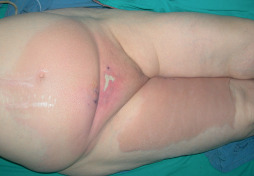
Eighty‐two‐year‐old female patient. Erythema starting from the pubic region. She had pain and rapid progression of the erythema to the thigh and genitalia.
Figure 2.
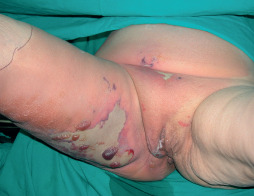
The eighty‐two‐year‐old female patient had also bullous erythema and fluid discharge. (stage II NF).
Laboratory findings may also be helpful in the diagnosis of NF. Wall et al. reported that white blood cell (WBC) >15 400 cells/mm3 or serum sodium level <135 mmol/l was associated with NF. The combination of both factors increases the risk of NF (20). Wall's method is a very sensitive tool, with a negative predictive value of 99%, but not very specific with a positive predictive value of only 26%. This means that it is good for ruling out NF but it is not as good for confirming presence of NF (21). Wong et al. developed a laboratory risk indicator for necrotising fasciitis score (LRINEC) (Table 2) (22). They identified six independent laboratory parameters associated with NF. According to this method, patients were categorised as low (<5), intermediate 6, 7 or high (>8) based on the LRINEC score points and the probability of NF was also 50, 50–75 and >75%, respectively (21).
Table 2.
Laboratory risk indicator for necrotising fasciitis (LRINEC): variables and scoring system
| Value | LRINEC score, points |
|---|---|
| C‐reactive protein, mg/l | |
| <150 | 0 |
| >150 | 4 |
| WBC count, cells/mm3 | |
| <15 | 0 |
| 15–25 | 1 |
| >25 | 2 |
| Haemoglobin level, g/dl | |
| >13·5 | 0 |
| 11–13·5 | 1 |
| <11 | 2 |
| Sodium level, mmol/l | |
| ≥135 | 0 |
| <135 | 2 |
| Creatinin level, mg/dl | |
| ≤1·6 | 0 |
| >1·6 | 2 |
| Glucose level, mg/dl | |
| ≤180 | 0 |
| >180 | 1 |
WBC, white blood cell.
Radiologic evaluation has also been used in the diagnosis of NF. Plain radiography can help detecting the subcutaneous gas; however, soft‐tissue gas is never present in most NF patients (4). Computed tomography (CT) scans can also be used. An increased attenuation of the subcutaneous fat with stranding and fascial thickening may be described (23). Magnetic resonance imaging (MRI) has a high sensitivity (93–100%) in diagnosing NF. Liquefactive tissue necrosis and fascial fluid which are caused by the inflammatory oedema can be detected by using MRI 19, 24, 25.
There are many reports about NF of the extremities; however, regarding the literature there is no sufficient data focused on NF of the central part of the body. The aim of this study is to evaluate the diagnosis, treatment and results such as mortality, morbidity and reconstructive options of NF localised in the central part of the body. The results were also compared with that of related literature.
Materials and methods
Between January 2000 and December 2010, 30 patients with NF localised in central parts of the body were treated at the Department of Plastic Reconstructive and Aesthetic Surgery of Dokuz Eylul University Faculty of Medicine. Six of the patients were female (20%) and the others were male (80%). The mean age was 54·03 years (ranged between 26 and 83 years). There was a history of an injury to the involved site in 11 (36·6%) of the patients. The injuries were injection in two patients (18·1%), surgical incisions in five patients (45·6%) and localised abscesses in four patients (36·3%). The risk factors of the patients are shown in Table 3. The major risk factor was diabetes; on the other hand, there was no defined risk factor in three patients (10%). The average time from the onset of symptoms to diagnosis was 6 days, ranging from 2 to 11 days. Table 4 summarises the clinical staging of the patients. The localisation of NF was perineum in 24 patients (80%); inguinal and thigh region in 5 patients (16·7); and back in 1 patient (3·3%).
Table 3.
Risk factors of the patients
| Risk factor | Number of cases | Percentage (%) |
|---|---|---|
| Diabetes mellitus (DM) | 8 | 26·6 |
| DM + smoking | 4 | 13·3 |
| DM + obesity | 2 | 6·6 |
| Chronic cardiac disease | 4 | 13·3 |
| Smoking | 3 | 10 |
| Alcohol abuse + smoking | 1 | 3·3 |
| Cancer and chemotherapy | 2 | 6·6 |
| Chronic corticosteroid therapy | 2 | 6·6 |
| Peripheral vascular disease | 1 | 3·3 |
| Not defined risk factor | 3 | 10 |
Table 4.
Clinical staging of the patients at the time of initial diagnosis
| Stage | Number of cases |
|---|---|
| I (early) | 14 |
| II (intermediate) | 9 |
| III (late) | 7 |
The laboratory findings were also examined and evaluated according to the LRINEC scores at the time of admittance to the hospital. LRINEC scores were ≥8 for 13 patients; 6–7 for 11 patients and ≤5 for 6 patients. Radiologic imaging studies employed were CT in 8 patients and MRI in 11 patients. Common findings were fascial thickening, fluid accumulation and oedema.
A wide spectrum of organisms was recovered. The most common isolated microorganisms were Escherichia coli, Enterococci, Pseudomonas aeruginosa, Streptococcus species, Clostridium species, Acinetobacter and Staphylococcus species. Seven patients (23%) had monomicrobial infections and the rest of them (77%) had polymicrobial infections. The isolated microorganisms in monomicrobial cases were Escherichia coli, Enterococci and Clostridium species.
Treatment
Empirical broad spectrum antimicrobial therapy started soon after the diagnosis of NF. According to the recommendation of infectious disease specialist, antibiotics with coverage for gram negative, gram positive and anaerobic microorganisms were selected. Antibiotic therapy was changed later based on the outcome of culture results. All the patients underwent surgical exploration and debridement as soon as possible (3, 4). NF is mainly addressed to the subcutaneous tissue level and the superficial skin may not be involved in some cases. However, it is very difficult to distinguish the true margins of the infection. The skin overlying the infected subcutaneous tissue should also be debrided. All affected components including skin, subcutaneous tissue, fascia and muscle were removed. Tissue biopsy cultures were obtained from the most affected area. We performed the first debridement procedure very extensively. Debridement was carried out until healthy bleeding was obtained. In certain areas such as perineal region, scrotum and penis, much attention should be made to preserve non‐affected tissues. After debridement, the wound was left open and followed‐up either with standard wound care or vacuum‐assisted closure (VAC). According to the appearance of the wound, patients were operated for further debridement every 16–48 hours until removal of all the necrotic tissues. Fourteen patients (46·7%) underwent debridement twice while the rest of the patients had three to four debridement procedures. The urinary catheterisation was performed for all patients. On top of that, rectal tubing was introduced to control bowel issues. The management of all patients was carried out in the intensive care unit with aggressive resuscitation after first debridement procedure. After the infection was controlled with healthy granulation tissue, closure of the open wound was considered with primary suture, skin grafts and/or flaps depending on the defect (Figure 5). Primary suture was used only for one patient (3·3%). The rest of the surviving patients' wounds were reconstructed by using split‐thickness skin grafts (STSG) and/or flaps (fasciocutaneous or musculocutaneous flaps). Colostomy was performed in only three patients (10%) who had extensive perineal defect after final debridement and prior to definitive reconstructive procedure.
Figure 3.
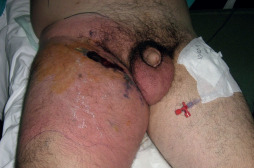
Forty‐five‐year‐old male patient. He had a history of localised abscess on lower end of the inguinal crease. He had been treated with antibiotics, but erythema had progressed to the thigh and abdominal regions. The margins of the erythema were marked and rapid progression during the hours was encountered.
Figure 4.
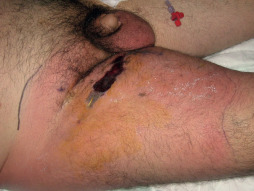
The lateral view of the 45‐year‐old male patient.
Figure 5.
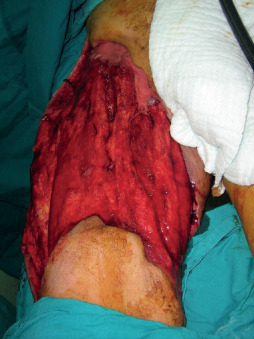
The intraoperative view of the 45‐year‐old male patient after the final debridement procedure.
Results
Six patients (20%) died and 24 patients (80%) survived. All non‐survivors had risk factors and secondary comorbidities due to their risk factors such as immunosuppression, chronic cardiac failure, and diabetes with high glucose level. Four of the non‐surviving patients were at stage 3 and the others were at stage 2. Their culture results were polymicrobial. Although surgical debridement of necrotic tissues was performed, they all were lost within 1‐week of the diagnosis of NF.
Survivors had also undergone repeated debridement operations two to four times. After obtaining healthy granulation tissue, reconstructive procedures such as STSG and/or flap procedures were performed. Reconstructive procedures were STSG in eight patients (33·3%), fasciocutaneous flaps in four patients (16·6%), fasciocutaneous flap + STSG in six patients (25%), scrotal flap + STSG in two patients (6·6%), scrotal flap in two patients (6·6%) and musculocutaneous flap + STSG in one patient (3·3%) (6, 7, 8, 9). There was no major complication such as flap and graft loss, after reconstructive procedures. The hospitalisation time varied between 17 and 32 days (mean 23 days).
Figure 6.
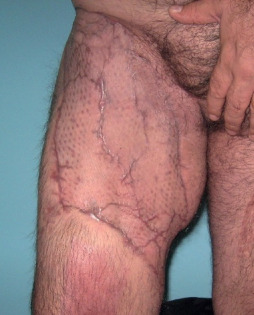
The latest postoperative view of the 45‐year‐old male patient. The abdominal region was closed primarily and STSG was applied for the thigh region.
Figure 7.
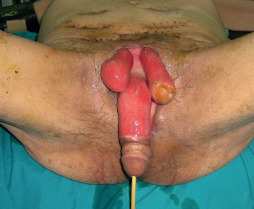
Sixty‐seven‐year‐old male patient. He had serial debridement for the NF in the genitalia.
Figure 8.
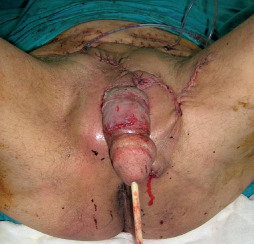
Fasciocutaneous flaps were used for the coverage of the testicles and STSG was applied for the penile shaft. The intraoperative view of the 67‐year‐old male patient.
Figure 9.

The late postoperative view of the 67‐year‐old male patient.
Discussion
NF is a potentially fatal infection, which is characterised by rapidly spreading necrosis of the soft tissue and fascia. Because NF commonly affects the extremities, most of the studies in the literature are focused on NF of the extremities. The aim of this study is to evaluate NF in the central parts of the body.
Most patients with NF (55%) have secondary NF which occurs as a result of known aetiology 8, 9. In our study, only 11 patients (36·6%) had known aetiology such as surgical incisions, injection and localised abscesses. The other patients (63·4%) had primary or idiopathic NF. The rate of primary NF is as high as 45% in the literature; however, this rate mainly represents NF of the extremities 8, 9. Risk factors are also important for the formation and treatment of the NF. In 27 patients (90%) risk factors were detected. The most common risk factor was DM which was found in 14 patients (47%). However, the other risk factors, such as chronic cardiac disease, cancer and chemotherapy and combined factors (DM + obesity) showed more effective treatment results and mortality rate than DM alone. NF should be suspected in patients who have skin infection and those who possess risk factors for NF and history of minor trauma.
Early diagnosis is the most important factor in the treatment and lifesaving operations of the patient. The diagnosis is primarily based on the clinical findings. Clinical stages of NF as described by Wong and Wang are useful for clinical evaluation 17, 18. The most important factor is the high degree of suspicion about NF, because it is difficult to distinguish it from other soft‐tissue infections characterised by erythema. But all the patients in this study had poorly defined and indistinct margins, and disproportionate pain and tenderness beyond the involved area. One other important clinical finding was rapid progression of the clinical findings even though they were given antibiotics. The margin of the erythema should be defined with skin marker and progression must be observed closely. If there was a rapid onset of progression in hours, NF must be strongly suspected and the diagnosis should be supported with laboratory and radiological studies as soon as possible. LRINEC scoring system which was developed by Wong et al., including six independent laboratory parameters for NF are very useful in diagnosis (22). LRINEC scores were >6 in 24 patients in our study. CT and MRI findings were not specific and could not be used in isolation for the definitive diagnosis.
In our study, the average time from the onset of symptoms to diagnosis was 6 days, ranging from 2 to 11 days. According to the related literature, the average time of diagnosis may vary from 2 days to several weeks (4). The analysis of our cases showed that the patients who had late diagnosis were referred from other medical centres. The early signs of NF may not be distinguished from other soft‐tissue infections. When these patients are first evaluated by inexperienced medical staff, time for true diagnosis may be prolonged. We believe that the most important factor for early diagnosis is to be aware of NF in the differential diagnosis of soft‐tissue infections. We advise that NF should be strongly considered when the rapid onset of the infection is not controlled within 48 hours of antibiotherapy.
Early surgical debridement of all the affected tissues is the only treatment option in controlling the source of infection. We believe that early surgical exploration can be lifesaving when NF is suspected according to carefully evaluated clinical findings and rapid onset of the infection. Debridement must be carried out until healthy bleeding tissues. Aggressive and complete debridement is advised during the first operation by some of the authors in the literature. They have shown that mortality rate was significantly lower with early and complete debridement 26, 27, 28. Although we believe that aggressive strategy is useful for lifesaving and in the final outcome of the patient, debridement procedure must be limited only to the necrotic tissues. The preservation of healthy tissues is especially necessary for the debridement of NF localised to the central part of the body such as trunk and perineum. Unnecessary extended debridement may also increase the possibility of functional and aesthetic deformities. For this reason, removal of necrotic tissues must be performed only by an experienced surgeon. Close monitoring of the patient physiology and the wound and then redebridement procedure must be performed if it is necessary. In our study all patients required redebridement. Redebridements at interval of 24–48 hours has been advised in the literature; however, there is no standardised interval (21). If there is progression of necrosis and additional physiologic derangement in the patient, it can be performed earlier than this time interval. Debridement procedure can be used more aggressively for the extremities and amputation possibility can be considered if it is necessary as a lifesaving option for the patient. However, amputation is not an option for NF of the central parts of the body. Early diagnosis and immediate debridement are more important in such patients.
The wound must be covered against secondary infections. Wound‐care products such as hydrogel and alginate dressings can be used to encourage the formation of granulation tissue and absorb the inflammatory exudates. VAC (negative pressure therapy) can also be used to reduce oedema and stimulate the granulation tissue.
After the infection is resolved and healthy granulation tissue is present, reconstruction of the defect must be considered. If the remaining skin and subcutaneous tissue is sufficient, primary closure can be performed. However, most of the wounds cannot be closed by primary suture. We used primary suture for only one patient. The reconstructive procedures after debridement for NF have not been discussed thoroughly in the literature; however, it is a very important aspect of NF treatment for survivors. If vital structures such as testicles and main neurovascular structures are not exposed and the granulation tissue is sufficient, STSG should be applied. STSG was also the most frequently used reconstructive method in our study. On the other hand, if these vital structures are exposed, if the wound is in close relationship with anus, and if a dead space occurs after the debridement, coverage with flaps should be considered. Local flaps are the first choice for these patients. The fasciocutaneous and scrotal flaps were also used in six patients. But the local flaps may not be enough to cover all the defects. In these circumstances, flap and STSG can be used together. We used this combination in nine cases. The reconstructive method should be planned according to the final defect. Wound closure should not be the only goal of the reconstruction; the functional and aesthetic outcomes should also be considered.
The mortality rate of NF varies between 6% and 76% in the literature (22). The extremities are most commonly involved and many studies have focused on NF in the extremities. Hence, the mortality rates of related literature refer to NF in the extremities. While there was no definitive comparative study, most of the authors believe that mortality rate is higher in centrally localised NF than that in the extremities (22). The mortality rate was 20% in our study, which was not higher than the rate for NF in the extremities. We believe that early diagnosis and debridement of necrotic tissues are the most important lifesaving factors for those with NF. The localisation of NF may have secondary importance in mortality rate.
Conclusion
NF is a rapidly spreading necrosis of the soft tissue and fascia. It has a high mortality rate when it is not properly diagnosed and managed. Thus, early diagnosis of NF may be lifesaving. Amputation can save the patient's life in NF of the extremities; however, this is not an option for NF in central parts of the body. We believe that the localisation of NF may be very important; however, early diagnosis is the most important issue regarding the mortality rate. In these cases, when NF is suspected, serial debridement of necrotic tissues should be performed. Because of the existence of vital and functional structures in the central parts of the body, early diagnosis and management of NF in these areas can also diminish the final defect which is more important than the other parts of the body. Wound management is more difficult in the perineal region due to contamination with bowel and urinary tracts. Urinary and bowel diversion may be considered in extensive wounds.
Acknowledgement
The authors have declared that no conflict of interest exists.
References
- 1. Trent JT, Kirsner RS. Diagnosing necrotizing fasciitis. Adv Skin Wound Care 2002;15:135–8. [DOI] [PubMed] [Google Scholar]
- 2. File TM Jr, Tan JS, DiPersio JR. Diagnosing and treating the “flesh‐eating bacteria syndrome”. Cleve Clin J Med 1998;65:241–9. [DOI] [PubMed] [Google Scholar]
- 3. Carter PS, Banwell PE. Necrotising fasciitis: a new management algorithm based on clinical classification. Int Wound J 2004;1: 189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bellapianta JM, Ljungquist K, Tobin E, Uhl R. Necrotizing fasciitis. J Am Acad Orthop Surg 2009;17:174–82. [DOI] [PubMed] [Google Scholar]
- 5. Meleney FL. Hemolytic streptococcus gangrene. Arch Surg 1924;9: 317–64. [Google Scholar]
- 6. Dufel S, Martino M. Simple cellulitis or a more serious infection? J Fam Pract 2006;55:396–400. [PubMed] [Google Scholar]
- 7. Bisno AL, Stevens DL. Streptococcal infections of skin and soft tissues. N Engl J Med 1996;334:240–5. [DOI] [PubMed] [Google Scholar]
- 8. Mulla ZD. Treatment options in the management of necrotising fasciitis caused by group A streptococcus. Expert Opin Pharmacother 2004;5:1695–700. [DOI] [PubMed] [Google Scholar]
- 9. Taviloglu K, Yanar H. Necrotizing fasciitis: strategies for diagnosis and management. World J Emerg Surg 2007;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levine EG, Manders SM. Life threatening necrotizing fasciitis. Clin Dermatol 2005;23:144–7. [DOI] [PubMed] [Google Scholar]
- 11. Sudarsky LA, Laschinger JC, Coppa GF, Spencer FC. Improved results from a standardized approach in treating patients with necrotizing fasciitis. Ann Surg 1987;206:661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Childers BJ, Potyondy LD, Nachreiner R, Rogers FR, Childers ER, Oberg KC, Hendricks DL, Hardesty RA. Necrotizing fasciitis: a fourteen‐year retrospective study of 163 consecutive patients. Am Surg 2002;68:109–16. [PubMed] [Google Scholar]
- 13. Lamagni TL, Neal S, Keshishian C, Alhaddad N, George R, Duckworth G, Vuopio‐Varkila J, Efstratiou A. Severe Streptococcus pyogenes infections, United Kingdom, 2003–2004. Emerg Infect Dis 2008;14:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharkawy A, Low D, Saginur R, Gregson D, Schwartz B, Jessamine P, Green K, McGeer A. Severe group A streptococcal soft‐tissue infections in Ontario: 1992–1996. Clin Infect Dis 2002;34: 454–60. [DOI] [PubMed] [Google Scholar]
- 15. Geusens E, Pans S, Van Breuseghem I, Knockaert D. Necrotizing fasciitis of the leg presenting with chest wall emphysema. Eur J Emerg Med 2004;11:49–51. [DOI] [PubMed] [Google Scholar]
- 16. Cox NH. Streptococcal necrotizing fasciitis and the dermatologist. Br J Dermatol 1999;141:613–4. [DOI] [PubMed] [Google Scholar]
- 17. Wong CH, Wang YS. The diagnosis of necrotizing fasciitis. Curr Opin Infect Dis 2005;18:101–6. [DOI] [PubMed] [Google Scholar]
- 18. Wang YS, Wong CH, Tay YK. Staging of necrotizing fasciitis based on the evolving cutaneous features. Int J Dermatol 2007;46:1036–41. [DOI] [PubMed] [Google Scholar]
- 19. Young MH, Aronoff DM, Engleberg NC. Necrotizing fasciitis: pathogenesis and treatment. Expert Rev Anti Infect Ther 2005;3:279–94. [DOI] [PubMed] [Google Scholar]
- 20. Wall DB, Klein SR, Black S, de Virgilio C. A simple model to help distinguish necrotizing fasciitis from nonnecrotizing soft tissue infection. J Am Coll Surg 2000;191:227–31. [DOI] [PubMed] [Google Scholar]
- 21. Anaya DA, Dellinger EP. Necrotizing soft‐tissue infection: diagnosis and management. Clin Infect Dis 2007;44:705–10 [PubMed]. [DOI] [PubMed] [Google Scholar]
- 22. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (laboratory risk indicator for necrotizing fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med 2004;32:1535–41. [DOI] [PubMed] [Google Scholar]
- 23. Wysoki MG, Santora TA, Shah RM, Friedman AC. Necrotizing fasciitis: CT characteristics. Radiology 1997;203:859–63. [DOI] [PubMed] [Google Scholar]
- 24. Arslan A, Pierre‐Jerome C, Borthne A. Necrotizing fasciitis: unreliable MRI findings in the preoperative diagnosis. Eur J Radiol 2000;36:139–43. [DOI] [PubMed] [Google Scholar]
- 25. Brothers TE, Tagge DU, Stutley JE, Conway WF, Del Schutte H Jr, Byrne TK. Magnetic resonance imaging differentiates between necrotizing and non‐necrotizing fasciitis of the lower extremity. J Am Coll Surg 1998;187:416–21. [DOI] [PubMed] [Google Scholar]
- 26. McHenry CR, Piotrowski JJ, Petrinic D, Malangoni MA. Determinants of mortality for necrotizing soft‐tissue infections. Ann Surg 1995;221:558–63; discussion 563–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bilton BD, Zibari GB, McMillan RW, Aultman DF, Dunn G, McDonald JC. Aggressive surgical management of necrotizing fasciitis serves to decrease mortality: a retrospective study. Am Surg 1998;64:397–400; discussion 400–1. [PubMed] [Google Scholar]
- 28. Lille ST, Sato TT, Engrav LH, Foy H, Jurkovich GJ. Necrotizing soft tissue infections: obstacles in diagnosis. J Am Coll Surg 1996;182:7–11. [PubMed] [Google Scholar]


