Abstract
Stewart‐Bluefarb syndrome (SBS), also known as acroangiodermatitis or pseudo‐Kaposi, is a condition rarely encountered. It involves skin lesions that are clinically similar to Kaposi sarcoma but are histologically different, and are usually secondary to an underlying arteriovenous fistula. Treatment of this disease usually involves the correction of the underlying vascular abnormality, with the mainstay of therapy ranging from compression devices for venous stasis, limited oral medications (dapsone and erythromycin) and local wound care including topical steroids. Different methods of treatment showed varied success but none is ideal. We report a case of a lower extremity ulcer in a 22‐year‐old male recently diagnosed with SBS successfully treated with heparan sulphate (Cacipliq20®).
Keywords: Acroangiodermatitis, Chronic ulcer, Heparan sulphate, Pseudo‐Kaposi, Stewart‐Bluefarb syndrome
Introduction
Stewart‐Bluefarb syndrome (SBS) is a congenital disease associated with multiple lower extremity arteriovenous shunts and acroangiodermatitis 1. It is categorised under the group of ‘acroangiodermatitis’ that includes benign diseases with skin lesions clinically resembling Kaposi's sarcoma, thus the name ‘pseudo‐Kaposi's sarcoma’, but histologically different. These skin lesions can be distinguished by histology and by their expression of several markers including the CD34 antigen 2. SBS is associated with congenital and acquired arteriovenous malformations (AVMs) 1 in contradistinction to acroangiodermatitis of Mali secondary to chronic venous insufficiency of other several aetiologies 3.
The pathophysiology of SBS is not well understood. It is believed that the increase in venous pressure resulting from the multiple AVMs may stimulate proliferation of endothelial cells 4. A recent report claims that the arteriovenous steal syndrome with distal ischaemia may cause endothelial proliferation by inducing a local increase in vascular endothelial growth factor (VEGF) 5. Moreover, mast cells have been shown to play a role in the proliferation of endothelial and perivascular cells under conditions of ischaemia 6. Several other processes including partially impaired venous flow and disturbed innervations of vessels play a critical role in the pathogenesis of venous insufficiency‐induced ulcers 7.
SBS treatment is necessary to prevent further complications, including bone demineralisation, destruction of soft tissue, haemorrhage, wound infection and even heart failure 8, 9. Conservative treatment includes compression, limb elevation and care of associated ulcers, infections and other wound complications 4. The ideal treatment, however, should address the underlying vascular malformation, although this is often not possible because of the distal and multiple arteriovenous fistulas commonly present. Unfortunately, surgery correcting macroscopically detectable fistulas can lead to increased ulceration or other complications. Occasionally, limb amputation may be necessary 4.
Vascular surgery is indicated in cases involving functional impotence, refractory pain, recurrent infection, bleeding or cardiac decompensation 10, 11. Selective embolisation with different particles [Gelfoam (Pharmacia and Upjohn Company, Kalamazoo, MI), Ivalon (Ivalon Inc., San Diego, CA), acrylates, amino acids, alcohol, etc.] may be a valid alternative 12, 13, 14. Brenner and Martínez de Morentin 12 and Smiddy et al. 8 claimed that ultrasound‐guided sclerotherapy, embolisation and surgery are indicated in selected patients who have single and localised AVM. Utermann et al. 15 described successful long‐term treatment of a case of SBS with a single AV fistula using polyvinyl alcohol embolisation. Many reports, however, described temporary relief for few years with coil embolisation of the associated arteriovenous fistula after which signs of venous insufficiency recurred 16. Zutt et al. 1, Turk et al. 17 and Klode et al. 18 stated that the congenital malformation characterised by numerous small arteriovenous connections makes surgical treatment difficult. Conservative treatment with bed rest, limb elevation and compression bandages in addition to medical therapy are probably the best available therapeutic options 7.
Medical and conservative therapy with dapsonein, in combination with leg elevation and compression devices 19 or with oral erythromycin 20, has shown limited benefits in some cases with ulcer regression. We describe an easy and non invasive successful treatment of an SBS patient presenting with chronic leg ulceration with heparan sulphate (Cacipliq20®; Addmedica, Paris, France).
Case report
A 22‐year‐old male construction worker developed 6 months prior to presentation non‐traumatic ulcers over the left lower extremity at the level of the medial and lateral malleoli. The ulcers enlarged gradually to 3 × 2 cm over the medial malleolus (Figure 1A) and to 5 × 4 cm over the lateral malleolus (Figure 1B). The patient was seen by several physicians and was treated conservatively with several topical wound care preparations including silver sulphadiazine, MEBO (Julphar, Ras Al Khaimah, UAE), Promogran (Johnson & Johnson Medical, New Brunswick, NJ) as well as others to no avail. On presentation, the involved limb showed evident signs of venous insufficiency with mild hypertrophy suggestive of Klippel‐Trenaunay‐type syndrome 21. Lower limb ulcers were clean with no foul smell or excessive discharge. There was also bluish discolouration around the ankle and dorsal foot area with multiple dilated tortuous superficial veins. The skin also exhibited changes similar to dermatitis (dryness, itchiness and scaling). An arterial duplex was done that showed an aorta of normal calibre with no signs of atherosclerosis, good aortoiliac and femoropopliteal flow, in addition to normal flow in the distal vessels of the bilateral lower extremities. A venous duplex was also done that showed adequate permeability of the deep veins of the thighs reaching the femoroiliac and popliteal areas with no signs of deep venous thrombosis. Nevertheless, the venous duplex showed permeable superficial veins with mild valvular incontinence of the left great saphenous vein distal to the knee with a tortuous track and multiple varicosities at its distal part. The aforementioned studies ruled out the possibility of arterial insufficiency from the differential diagnosis. The venous insufficiency did not explain the ulcers present on the bilateral malleoli knowing that it mainly causes ulcers over the lateral malleolus. Blood studies were done and were negative for sickle cell disease and thalassaemia (both of which are associated with chronic lower extremity ulcers). Blood studies also showed no eosinophilia and normal erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP), which ruled out a parasitic aetiology of the ulcers. A wound biopsy was performed. It showed epidermal ulceration with surrounding epidermal pillars without atypia. The dermis showed noticeable fibrovascular proliferation around superficial and medial plexi accompanied by lymphoplasmocytary inflammatory infiltrate without any giant cells. There were also clusters of haemosiderin‐laden macrophages. The vessels contained a prominent endothelium with clusters of extravasated erythrocytes. All these findings are indicative of an angioendothelial reaction with arteriovenous anomalies compatible with a pseudo‐Kaposi syndrome of the Stewart‐Bluefarb type (suggested by the pathologist based only on histologic data). The patient was started on Cacipliq20 applied twice per week to both wounds for 5 minutes each and then covered with a clean moist gauze that was changed on a daily basis after cleaning the wound with soap and water. After the first application, there was a noticeable decrease in pain and tenderness of both ulcers. Two weeks later, the ulcers started to epithelialise from the edges (Figure 1C and D) and both completely healed by week 4 (Figure 1E and F). After that, the patient was maintained on skin moisturiser and compression stockings and he showed no recurrence after 3 months of follow‐up despite his return to his regular job and daily activities.
Figure 1.
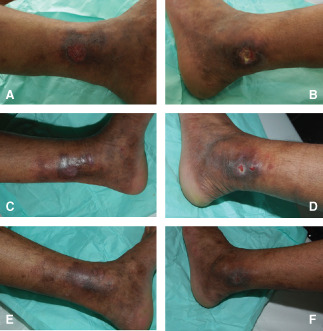
A: Medial malleolar ulcer upon presentation. B: Lateral malleolar ulcer upon presentation. C: Medial malleolar ulcer after 2 weeks of treatment with Cacipliq®. D: Lateral malleolar ulcer after 2 weeks of treatment with Cacipliq. E: Medial malleolar ulcer after 4 weeks of treatment with Cacipliq. F: Lateral malleolar ulcer after 4 weeks of treatment with Cacipliq.
Discussion
Glycosaminoglycans (GAGs) are a group of molecules that are present both intracellularly such as syndecan and glypican and extracellularly such as perlecan and argin. They are classified according to the size of the oligosaccharide component and the amount of sulphation they contain 18, 22. These GAGs have been found to be crucial for intracellular and intercellular processes including neurodegeneration 23, angiogenesis 24, inflammation 25, 26, 27, cardiovascular diseases 28, cancer 29 and infectious diseases 30, 31. Heparin and heparan sulphate are GAGs that are known to be involved in the anticoagulation process by binding antithrombin 22, 32. The activity of heparin and heparan sulphate has been recently expanded to include wound healing because of their ability to bind, activate and immobilise a variety of growth factors, chemokines and metalloproteinases 33, 34. This activity has been further confirmed by the studies that have shown that OTR4120, a molecule similar to heparin but with much less anticoagulant activity 35, can enhance wound healing in experimental animal models following peripheral nerve injuries 36, skin burns 37, chronic ulcers 38 and cutaneous wounds 39.
Among the numerous wound healing modulation strategies for the treatment of chronic non‐healing wounds, replacement of GAGs in the extracellular matrix to prevent further tissue damage may be a pertinent approach 40. Cacipliq20, a synthetic bioengineered heparan sulphate mimetic, applied topically, will replace heparin sulphate usually deficient in chronic wounds and restores extracellular matrix scaffold, thus allowing key interactions with growth factors to occur 40. Interestingly, because Cacipliq20 is resistant to endoglycosidase and by binding to heparin‐binding sites that become vacant after heparanase activation and heparin disintegration, it is an efficient agent for extracellular matrix restoration 40, 41, 42.
Earlier studies have demonstrated the effect of heparan sulphate both in vitro and in vivo 40. In vitro, it enhances angiogenesis by modulating VEGF and collagen‐type expression via fibroblast growth factor 2 and transforming growth factor β1 43, 44, 45, 46. As for in vivo, heparan sulphate was shown to promote angiogenesis in ischaemic cardiac and skeletal muscles 43, 44, improve wound healing, decrease inflammation and improve wound quality in mice with skin ulcers 46, 47. The first reported use of Cacipliq20 in humans for the treatment of 15 chronic arterial ulcers was presented in 2008 by Barritault et al. 48. One month after treatment initiation, there was a 12–100% decrease in ulcer size with net reduction in pain as reported by the patients. During the course of this study, two patients died because of their primary disease and one patient had to undergo limb amputation 48. In 2011, Groah et al. presented their experience with Cacipliq20 treatment of chronic ulcers of 2·5–10 years duration and showed that 22% of the patients participating in their study had complete healing in 1 month with significant reduction of pain 40; those who did not benefit had associated comorbid conditions such as spinal cord paralysis and were considered as high risk for recurrence. Nevertheless, they were able to show that in this high‐risk group, there was still improvement in wound healing and pain score at least for the first three sessions of treatment 40.
We have observed similar pain reduction in the treatment of chronic sickle cell ulcers with heparan sulphate mimetic (Cacipliq20). Pain significantly decreased by 80% as measured with the visual scale 2 weeks only from the first application of Calcipliq20. Groah et al. postulated that this decrease in pain level might be related to a decrease in the level of inflammatory mediators in the wound, but this hypothesis still awaits confirmation.
Because of the unsuccessful treatment of the patient ulcers with numerous wound care preparations and modalities and encouraged by our experience in treating a chronic lower extremity sickle cell ulcer with complete healing after 4 weeks, we have elected to use Cacipliq20 for the treatment of the Stewart‐Bluefarb ulcer. Although the follow‐up is short, the patient was able to regain full activity and return to his previous job with no difficulty.
To our knowledge, this is the first case report using Cacipliq20 for treating Stewart‐Bluefarb chronic ulcers. It must be noted though that heparan sulphate mimetic treatment of chronic ulcer has no guarantee against recurrence as long as the underlying disease has not been corrected; it is only a topical wound care modality successful in achieving complete wound healing where other modalities have failed.
Acknowledgements
Cacipliq20® was provided free of charge for the conduct of this study; however, there was no financial or any other benefit to any of the authors.
References
- 1. Zutt M, Emmert S, Moussa I, Haas E, Mitteldorf C, Bertsch HP, Neumann C. Acroangiodermatitis Mali resulting from arteriovenous malformation: report of a case of Stewart‐Bluefarb syndrome. Clin Exp Dermatol 2008;33:22–5. [DOI] [PubMed] [Google Scholar]
- 2. Kanitakis J, Narvaez D, Claudy A. Expression of the CD34 antigen distinguishes Kaposi's sarcoma from pseudo Kaposi's sarcoma. Br J Dermatol 2011;134:44–6. [PubMed] [Google Scholar]
- 3. Kadpagli H, Ozrutk G. Pseudokaposis sarcoma (Mali type). Int J Dermatol 1998;37:221–30. [DOI] [PubMed] [Google Scholar]
- 4. Hueso L, Llombart B, Alfaro‐Rubio A, Serra‐Guillén C, Requena C, González M, Cano B, Nagore E, Sanmartín O, Botella‐Estrada R, Guillén C. Stewart Bluefarb syndrome. Actas Dermosifiliogr 2007;98:545–8. [DOI] [PubMed] [Google Scholar]
- 5. Requena L, Farina MC, Renedo G. Intravascular and diffuse dermal reactive angioendotheliomatosis secondary to iatrogenic arteriovenous fistulas. J Cutan Pathol 1999;26:159–64. [DOI] [PubMed] [Google Scholar]
- 6. Ikeda E, Kano E, Baba S, Suzuki H. Mast cells in pseudo‐Kaposi's sarcoma lesions. J Eur Acad Dermatol Venereol 2001;15:487–9. [DOI] [PubMed] [Google Scholar]
- 7. Lai CL, Tsai CK, Tsai YT, Lin CY, Lee CY, Tsai CS. Recurrent foot ulcers in young woman: Stewart‐Bluefarb syndrome: report of one case and literature reviews. J Med Sci 2011;31:133–6. [Google Scholar]
- 8. Smiddy PF, Molloy MP, Flanagan N, Barnes L. Pseudo‐Kaposi's sarcoma: the association of arterio‐venous malformations with skin lesions resembling Kaposi's sarcoma. Australas Radiol 2001;45:225–7. [DOI] [PubMed] [Google Scholar]
- 9. Rosen T, Martin S, Stern JK. Radionuclide scanning in pseudo‐Kaposi's sarcoma. Arch Dermatol 1979;115:747–8. [PubMed] [Google Scholar]
- 10. Marcoval J, Pagerols X, Iñiguez D, Peyri J. Acroangiodermatitis asociada a malformación arteriovenosa (síndrome de Bluefarb‐Stewart). Actas Dermosifiliogr 1992;83:143–5. [Google Scholar]
- 11. Punsoda G, Ribera M, Ferrándiz C. Acroangiodermatitis por fístula arterio‐venosa. Pseudosarcoma de Kaposi tipo Bluefarb‐Stewart. Actas Dermosifiliogr 1991;82:337–40. [Google Scholar]
- 12. Brenner S, Martínez de Morentin E. What's new in pseudo‐Kaposi's sarcoma. J Eur Acad Dermatol Venereol 2001;15:382–4. [DOI] [PubMed] [Google Scholar]
- 13. Larralde M, González V, Marietti R, Nussembaum D, Peirano M, Schroh R. Pseudo‐Kaposi sarcoma with arteriovenous malformation. Pediatr Dermatol 2001;18:325–7. [DOI] [PubMed] [Google Scholar]
- 14. Agrawal S, Rizal A, Agrawal CS, Anshu A. Pseudo‐Kaposi's sarcoma (Bluefarb‐Stewart type). Int J Dermatol 2005;44:136–8. [DOI] [PubMed] [Google Scholar]
- 15. Utermann S, Kahle B, Petzold D. Successful longterm therapy of Stewart Bluefarb syndrome. Hautarzt 2000;51:336–9. [DOI] [PubMed] [Google Scholar]
- 16. Díaz‐Nido J, Wandosell F, Avila J. Glycosaminoglycans and b‐amyloid, prion and tau peptides in neurodegenerative diseases. Peptides 2002;23:1323–32. [DOI] [PubMed] [Google Scholar]
- 17. Turk BG, Turk UO, Alioglu E, Akalin T, Dereli T. Stewart‐Bluefarb syndrome: a case report with angiographic findings. J Dermatol 2009;36:415–8. [DOI] [PubMed] [Google Scholar]
- 18. Klode J, Kröger K, Grabbe S, Dissemond J. Ulcers associated with arteriovenous fistula within a Stewart‐Bluefarb syndrome: arterial and/or venous therapy? Vasa 2007;36:134–7. [DOI] [PubMed] [Google Scholar]
- 19. Rashkovsky I, Gilead L, Leibovici V. Acroangiodermatitis: review of the literature and report of a case. Acta Derm Venereol 1995;75:475. [DOI] [PubMed] [Google Scholar]
- 20. Kim TH, Kim KH, Kang JS, Kim JH, Hwang IY. Pseudo Kaposi's sarcoma associated with acquired arteriovenous fistula. J Dermatol 1997;24:28. [DOI] [PubMed] [Google Scholar]
- 21. Atiyeh BS, Musharrafieh RS. Klippel‐Trenaunay‐type syndrome: an eponym for various expressions of the same entity. J Med 1995;26 (5‐6):253–60. [PubMed] [Google Scholar]
- 22. Cole CL, Hansen SU, Barath M, Rushton G, Gardiner GM, Avizienyte E, Jayson GC. Synthetic heparan sulfate oligosaccharides inhibit endothelial cell functions essential for angiogenesis. PLoS One 2010;5:11644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gandhi NS, Mancera RL. Heparin/heparan sulphate‐based drugs. Drug Discov Today 2010;15:1058–69. [DOI] [PubMed] [Google Scholar]
- 24. Iozzo RV, San Antonio JD. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J Clin Invest 2001;108:349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lever R, Smailbegovic A, Page C. Role of glycosaminoglycans in inflammation. Inflammopharmacology 2001;9:165–9. [Google Scholar]
- 26. Parish CR. The role of heparan sulphate in inflammation. Nat Rev Immunol 2006;6:633–43. [DOI] [PubMed] [Google Scholar]
- 27. Parish CR. Heparan sulfate and inflammation. Nat Immunol 2005;6:861–2. [DOI] [PubMed] [Google Scholar]
- 28. Rosenberg RD, Shworak NW, Liu J, Schwartz JJ, Zhang L. Heparan sulfate proteoglycans of the cardiovascular system. Specific structures emerge but how is synthesis regulated? J Clin Invest 1997;99:2062–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yip GW, Smollich M, Gotte M. Therapeutic value of glycosaminoglycans in cancer. Mol Cancer Ther 2006;5:2139–48. [DOI] [PubMed] [Google Scholar]
- 30. Rostand KS, Esko JD. Microbial adherence to and invasion through proteoglycans. Infect Immun 1997;65:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sawitzky D. Protein–glycosaminoglycan interactions: infectiological aspects. Med Microbiol Immunol 1996;184:155–61. [DOI] [PubMed] [Google Scholar]
- 32. Gandhi NS, Mancera RL. The structure of glycosaminoglycans and their interactions with proteins. Chem Biol Drug Des 2008;72:455–82. [DOI] [PubMed] [Google Scholar]
- 33. Gesslbauer B, Kungl AJ. Glycomic approaches toward drug development: therapeutically exploring the glycosaminoglycanome. Curr Opin Mol Ther 2006;8:521–8. [PubMed] [Google Scholar]
- 34. Volpi N. Therapeutic applications of glycosaminoglycans. Curr Med Chem 2006;13:1799–810. [DOI] [PubMed] [Google Scholar]
- 35. Aamiri A, Mobarek A, Carpentier G, Barritault D, Gautron G. Effect of a substituted dextran on reinnervation during regeneration of adult rat skeletal muscle. C R Acad Sci III 1995;318:1037–44. [PubMed] [Google Scholar]
- 36. Zuijdendorp HM, Smit X, Blok JH, Caruelle JP, Barritault D, Hovius SE, van Neck JW. Significant reduction in neural adhesions after administration of the regenerating agent OTR4120, a synthetic glycosaminoglycan mimetic, after peripheral nerve injury in rats. J Neurosurg 2008;109:967–73. [DOI] [PubMed] [Google Scholar]
- 37. Garcia‐Filipe S, Barbier‐Chassefière V, Alexakis C, Huet E, Ledoux D, Kerros ME, Petit E, Barritault D, Caruelle JP, Kern P. RGTA OTR4120, a heparan sulfate mimetic is a possible long‐term active agent to heal burned skin. J Biomed Mater Res A 2007;80:75–84. [DOI] [PubMed] [Google Scholar]
- 38. Barbier‐Chassefière V, Garcia‐Filipe S, Yue XL, Kerros ME, Petit E, Kern P, Saffar JL, Papy‐Garcia D, Caruelle JP, Barritault D. Matrix therapy in regenerative medicine, a new approach to chronic wound healing. J Biomed Mater Res A 2009;90:641–7. [DOI] [PubMed] [Google Scholar]
- 39. Tong M, Tuk B, Hekking IM, Vermeij M, Barritault D, van Neck JW. Stimulated neovascularization, inflammation resolution and collagen maturation in healing rat cutaneous wounds by a heparan sulfate glycosaminoglycan mimetic, OTR4120. Wound Repair Regen 2009;17:840–52. [DOI] [PubMed] [Google Scholar]
- 40. Groah SL, Libin A, Spungen M, Nguyen KL, Woods E, Nabili M, Ramella‐Roman J, Barritault D. Regenerating matrix‐based therapy for chronic wound healing: a prospective within‐subject pilot study. Int Wound J 2011;8:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Agren MS, Werthén M. The extracellular matrix in wound healing: a closer look at therapeutics for chronic wounds. Int J Low Extrem Wounds 2007;6:82–97. [DOI] [PubMed] [Google Scholar]
- 42. Hodde JP, Johnson CE. Extracellular matrix as a strategy for treating chronic wounds. Am J Clin Dermatol 2007;8:61–6. [DOI] [PubMed] [Google Scholar]
- 43. Desgranges P, Barbaud C, Caruelle JP, Barritault D, Gautron J. A substituted dextran enhances muscle fiber survival and regeneration in ischemic and denervated rat EDL muscle. FASEB J 1999;13:761–6. [DOI] [PubMed] [Google Scholar]
- 44. Yamauchi H, Desgranges P, Lecerf L, Papy‐Garcia D, Tournaire MC, Moczar M, Loisance D, Barritault D. New agents for the treatment of infarcted myocardium. FASEB J 2000;14:2133–4. [DOI] [PubMed] [Google Scholar]
- 45. Rouet V, Hamma‐Kourbali Y, Petit E, Panagopoulou P, Katsoris P, Barritault D, Caruelle JP, Courty J. A synthetic glycosaminoglycan mimetic binds vascular endothelial growth factor and modulates angiogenesis. J Biol Chem 2005;280:32792–800. [DOI] [PubMed] [Google Scholar]
- 46. Alexakis C, Mestries P, Garcia S, Petit E, Barbier V, Papy‐Garcia D, Sagot MA, Barritault D, Caruelle JP, Kern P. Structurally different RGTAs modulate collagen‐type expression by cultured aortic smooth muscle cells via different pathways involving fibroblast growth factor‐2 or transforming growth factor‐beta1. FASEB J 2004;18:1147–9. [DOI] [PubMed] [Google Scholar]
- 47. Rudolph R, Suzuki M, Luce JK. Experimental skin necrosis produced by adriamycin. Cancer Treat Rep 1979;63:529–37. [PubMed] [Google Scholar]
- 48. Barritault D, Allaire E, Louissaint T, Kichenin K, Godeau B, Becquemin JP, Desgranges P. Matrix therapy in vascular pathology: first pilot study to evaluate RGTA Cacipliq 20. Abstract at Toronto 2nd World Union of Wound Healing Society Meeting; 2008; Toronto, Canada. URL http://www.wuwhs.com/congress2008/abstracts/abstract PW193.html [accessed on 15 August 2011].


