Abstract
Wound healing requires a proper functioning of keratinocytes that migrate, proliferate and lead to a competent wound closure. Impaired wound healing might be due to a disturbed keratinocyte function caused by the wound environment. Basically, chronic wound fluid (CWF) differs from acute wound fluid (AWF). The aim of this study was to analyse the effects of AWF and CWF on keratinocyte function. We therefore investigated keratinocyte migration and proliferation under the influence of AWF and CWF using MTT [3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide] test and scratch assay. We further measured the gene expression by qRT‐PCR regarding growth factors and matrixmetalloproteinases (MMPs) involved in regeneration processes. AWF had a positive impact on keratinocyte proliferation over time, whereas CWF had an anti‐proliferative effect. Keratinocyte migration was significantly impaired by CWF in contrast to an undisturbed wound closure under the influence of AWF. MMP‐9 expression was strongly upregulated by CWF compared with AWF. Keratinocyte function was significantly impaired by CWF. An excessive induction of MMP‐9 by CWF might lead to a permanent degradation of extracellular matrix and thereby prevent wounds from healing.
Keywords: Acute wound, Chronic wound, Gene expression, Keratinocytes, Migration, Proliferation, Wound healing, Wound fluid
Introduction
Wound healing is a multicellular process occurring in the following three stages: inflammation, new tissue formation and remodelling 1. Epithelialisation is the resurfacing of a wound to restore anatomy and function of the skin 2. Migration and proliferation of keratinocytes (KC) at the periphery of the wound play a mandatory role in this process 2, 3, 4. Proliferation of KC principally occurs in the basal lamina of the epithelium and these basal KC undergo terminal differentiation as they migrate to the surface 5. Growth factors that are known to stimulate wound healing include vascular endothelial growth factor (VEGF), basic fibroblast growth factor (b‐FGF), keratinocyte growth factor and others 4. Disorders in this well‐orchestrated repair process lead to an impaired wound healing, which often affects patients with diseases, such as diabetes mellitus and atherosclerosis 6. Of over 150 million diabetic patients worldwide, more than 15% suffer from chronic wounds 7. Thus, non‐healing wounds represent a growing medical and socioeconomic problem. However, treatment strategies remain limited and largely ineffective 8. Furthermore, these wounds are often accompanied by odour, exudation and chronic pain associated with a reduction in quality of life 9. Previous work has demonstrated significant differences between acute and chronic wound environments 4, 10. Compared with acute wound fluid (AWF), chronic wound fluid (CWF) is characterised by elevated local levels of pro‐inflammatory cytokines, such as tumour necrosis factor‐α (TNF‐α) and interleukin‐1 (IL‐1). Proteases such as matrixmetalloproteinases (MMP) and neutrophil elastase are also elevated, whereas the levels of tissue inhibitors of metalloproteinases are reduced 11. These mediators of the chronic wound environment might disturb KC function, leading to impaired wound healing 4. Protein composition of AWF may, however, stimulate KC and thereby positively influence epithelialisation. The aim of this study was to analyse the differences in KC function in vitro under the influence of AWF and CWF. We examined the effect of AWF and CWF on proliferation, migration and gene expression of KC.
Methods
Cell culture
Human adult low calcium high temperature (HaCaT) cells, an immortalised human keratinocyte cell line developed by Deutsches Krebsforschungszentrum Heidelberg (DKFZ), were purchased from Cell Lines Service (CLS, Eppelheim, Germany). They were cultured in Dulbecco's Modified Eagle's Medium (DMEM; Biochrom, Berlin, Germany) containing 10% foetal calf serum (FCS) or 2% FCS in experimental settings, respectively. HaCaT cells were used between passages 45 and 54.
Preparation of wound fluids
Ethical approval was obtained from the ethics committee of the Witten/Herdecke University (No 39/2007). Informed consent was obtained from all patients for wound fluid collection. Patient‐specific data are shown in Table 1.
Table 1.
Patient data
| Sample ID | Age (years) | Sex | BMI (kg/m2) | Diabetes | Arterial hypertension | Vessel disease | Smoking | Microbiology of wound swab |
|---|---|---|---|---|---|---|---|---|
| AWF I | 42 | f | 32·5 | − | − | − | − | Negative |
| AWF II | 34 | m | 19·2 | − | − | − | − | Negative |
| AWF III | 46 | f | 39·6 | − | − | − | − | Negative |
| AWF IV | 34 | f | 37·1 | − | + | − | − | Negative |
| AWF V | 65 | f | 26 | + | + | − | − | Negative |
| Mean | 44·2 | 30·88 | ||||||
| CWF I | 61 | f | 35·7 | + | + | PVD | + | Enterococci, pseudomonads |
| CWF II | 87 | m | 16·3 | − | − | − | + | Enterobacteria, enterococci, staphylococci |
| CWF III | 63 | f | 35·6 | + | − | − | − | Pseudomonads, staphylococci, streptococci |
| CWF IV | 60 | f | 22·6 | − | + | − | − | Enterobacteria, enterococci, pseudomonads, staphylococci |
| CWF V | 61 | f | 26·9 | − | + | − | − | Enterobacteria, enterococci |
| Mean | 66·4 | 27·42 |
f, female; m, male; AWF, acute wound fluid; CWF, chronic wound fluid; PVD, peripheral vascular disease.
Acute wound fluid
AWF was collected from five patients after elective abdominoplasty. Wound fluid subcutaneously drained during the first 8 hours after operation was discarded to exclude blood contamination. Wound fluid drained within the following 8 hours was collected. After centrifugation, the supernatant was diluted with DMEM and filter sterilised. Protein concentrations of all samples were analysed using Bradford test according to the manufacturer's instructions and pooled afterwards.
Chronic wound fluid
CWF was harvested from chronic sacral decubitus. All decubitus existed for at least 6 weeks. Patients with prior vacuum therapy were excluded. Wound fluid was collected by applying an occlusive dressing for 24 hours. After wound fluid centrifugation, the supernatant was diluted with DMEM and passed through a sterile filter to prevent cell cultures from bacterial contamination. Wound fluids from five patients were subjected to Bradford assay for protein quantification and pooled for further experiments.
To evaluate the most suitable wound fluid concentration for this study, we investigated different wound fluid concentrations with respect to their impact on HaCaT proliferation using MTT [3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide] assay. Based on these results, we chose a concentration of 2% AWF and 2% CWF.
MTT assay
MTT test was performed to assess cell proliferation. Therefore, HaCaT cells were seeded on 96‐well microplates. They were allowed to attach overnight and then incubated with 2% AWF or 2% CWF. The MTT assay was performed every 24 hours from day 1 until day 4 using MTT reagent (5 µg/ml; Sigma‐Aldrich, Hamburg, Germany) according to the manufacturer's instructions. Extinction was measured at 570 nm using the ELISA reader μQuant (Biotek, Bad Friedrichshall, Germany).
Scratch assay
HaCaT cells were plated onto six‐well microplates at a density of 700,000 cells per well. After 24 hours, cells were incubated with 10 µg/ml mitomycin C (Serva, Heidelberg, Germany) for 2 hours to inhibit cell proliferation. The HaCaT monolayer was then scratched with a plastic pipette tip in a standardised manner. Culture medium was changed by 2% AWF or 2% CWF. In vitro epithelialisation was documented by photography using Leica CTR400 microscope and Leica Application Suite V3.6 software. Wound closure was evaluated by measuring the remaining cell‐free area using Adobe Photoshop 12.0 and expressed as percentage of the initial cell‐free zone.
Quantitative real‐time RT–PCR
A total of 400,000 HaCaT were placed on the six‐well microplates and cultured according to the standard protocols. After 24 hours, they were exposed to 2% AWF or 2% CWF for 2, 12 and 24 hours or left untreated. Cells were dissolved in RLT buffer and RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. cDNA synthesis of 1 µg of total RNA was performed with the RevertAid First Strand cDNA Synthesis Kit (Fermentas, St. Leon‐Rot, Germany). qRT‐PCR was performed using the Brilliant II SYBR Green QRT‐PCR Master Mix (Agilent Technologies, Böblingen, Germany). Data were acquired with Stratagene Mx3005P QPCR System (Agilent Technologies). Primers were obtained from Biomers (Ulm, Germany) (Table S1). Expression was normalised to the housekeeping gene RPL. The comparative threshold cycle (C t) method was applied to determine relative expression differences 12.
Statistical analysis
For statistical reasons, all experiments were performed in triplets. Data are shown as mean ± standard deviation unless otherwise indicated. Continuous variables were compared using Student's t‐test. A two‐tailed P‐value <0·05 was considered significant.
Results
Protein concentration of wound fluids
Protein concentrations of AWF obtained from five different patients ranged from 35·82 to 41·72 g/l (38·77 ± 4·17 g/l), whereas the protein concentrations of CWF samples were slightly lower, varying from 28·64 to 38·25 g/l (33·45 ± 6·80 g/l) as summarised in Table 2.
Table 2.
Protein concentration of different wound fluid samples
| Sample | Protein concentration (g/l) | Sample | Protein concentration (g/l) |
|---|---|---|---|
| AWF I | 38·91 | CWF I | 31·34 |
| AWF II | 41·72 | CWF II | 35·81 |
| AWF III | 37·16 | CWF III | 34·30 |
| AWF IV | 36·17 | CWF IV | 32·26 |
| AWF V | 35·82 | CWF V | 38·25 |
| Mean ± SD | 38·77 ± 4·17 | Mean ± SD | 33·45 ± 6·80 |
AWF, acute wound fluid; CWF, chronic wound fluid.
HaCaT proliferation
After 24 hours of incubation with 2% AWF, KC proliferation decreased to 68·1% compared with the untreated control and then continuously increased to 121·1% after 4 days. Under the influence of 2% CWF, proliferation was reduced to 58·4% after 1 day and 38·3% after 2 days, followed by a slight increase to 54·9% on day 3 and 74·0% on day 4 (Figure 1).
Figure 1.

Proliferation of keratinocytes (KC) during incubation with acute wound fluid (AWF) and chronic wound fluid (CWF). Incubation with AWF is indicated as dark grey bars, with CWF as light grey bars. The proliferation is presented as percentage related to the untreated control. The results of three independent experiments plotted as mean ± SD are shown. Asterisks indicate significant differences between KC proliferation under the influence of AWF and CWF (P ≤ 0·05).
HaCaT migration
To assess KC migration without bias through proliferation, the scratch assay was performed after preincubation with mitomycin C. After 12 hours, there were only marginal differences regarding the size of the cell‐free area between all three groups (control: 70%, AWF: 68·6%, CWF: 74·9%). After 24 hours of incubation, the remaining cell‐free area decreased to 21·1% in the control group. Influenced by AWF and CWF, the cell‐free area measured 30·6% and 49·1%, respectively. Although in vitro wound closure was nearly completed after incubation with AWF within 36 hours (control: 2·5%, AWF: 4·1%), the cell‐free area was significantly bigger (30·4%, P < 0·05) with CWF. The results are shown in Figure 2.
Figure 2.
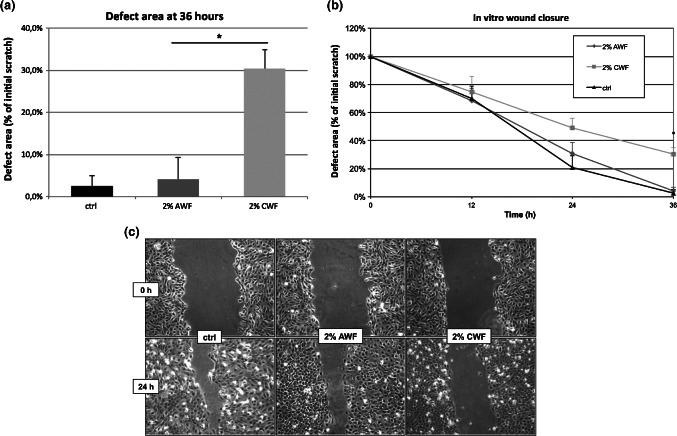
Scratch assay. Keratinocytes were plated onto microplates. After 24 hours, they were incubated with 10 µg/ml mitomycin C for 2 hours to inhibit cell proliferation. The monolayer was scratched with a plastic pipette tip. Culture medium was changed (black) or replaced by 2% acute wound fluid (dark grey) or 2% chronic wound fluid (light grey) and in vitro epithelialisation was documented. Wound closure was evaluated by measuring the remaining cell‐free area and expressed as percentage of the initial cell‐free zone. The results of three independent experiments as mean ± SD are shown. *P ≤ 0·05. (A) Remaining cell‐free area after 36 hours. (B) Time course of epithelialisation. (C) Representative photo documentation at 24 hours.
Gene expression of HaCaT
For qRT‐PCR, we focussed on two important growth factors, VEGF and b‐FGF, during the physiological wound healing. We further measured MMP‐2 and MMP‐9, which are proteases involved in extracellular matrix (ECM) degradation and allow KC to migrate to the wound bed and resurface the denuded skin. After 2 hours of treatment, VEGF expression by AWF and CWF started and increased to a fold change of nearly 3 (AWF) and 5 (CWF) after 12 hours. Gene expression was almost stagnant within the next 12 hours. Expression of b‐FGF increased after 12 hours and reached a fold change of 5 (AWF) and 6·5 (CWF) after 24 hours. MMP‐2 was only minimally regulated by both wound fluids, whereas MMP‐9 showed a strong induction. Under the influence of CWF, MMP‐9 expression increased to a fold change of 24 after 2 hours and then slowly decreased to 13 after 24 hours. Upon incubation with AWF, MMP‐9 was induced ninefold after 12 hours and then directly dropped to fourfold. Results are shown in Figure 3.
Figure 3.
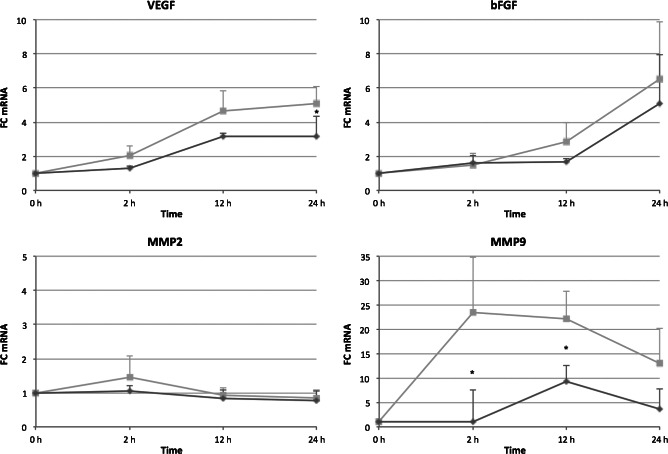
Gene expression changes by acute wound fluid (AWF) and chronic wound fluid (CWF). Keratinocytes were treated with 2% AWF (dark grey lines) or 2% CWF (light black lines) for 2, 12 and 24 hours or left untreated. Fold‐changes of gene expression were analysed by qRT–PCR and plotted as mean ± SD. Asterisks indicate significant differences in gene expression between AWF and CWF with P ≤ 0·05 (n = 3).
Discussion
Wound healing is an evolutionarily conserved process that aims the restoration of anatomic continuity and function of the skin and involves the interaction of various cell types, including KC 13, 14 Environmental conditions such as the absence or surplus of certain mediators might have an impact on KC function and thereby impair wound healing. The wound itself represents a reactive microenvironment 15, which we were able to transfer into an in vitro wound model. The wound fluids have been widely studied but rarely used in cell cultures. Previous studies have shown considerable differences in protease levels with much higher levels in CWF 16. Analysis of wound fluids regarding their growth factor content is discussed controversially in the literature. In most studies, the average level of growth factors in CWF was shown to be significantly lower than that in AWF 16, 17. However, the negative impact of the chronic wound environment can certainly not be reduced to a single factor but must be explained by a disadvantageous combination of all effectors. The competence of KC to close cutaneous defects of skin in vivo rests upon their ability to migrate and proliferate 2 To gain insight into disturbed wound healing processes, we therefore investigated the proliferation and migration capacity of KC under the influence of AWF and CWF. The proliferation assay demonstrated that initially, both AWF and CWF reduced the proliferation capacity of KC. However, AWF stimulated proliferation over time, whereas CWF impaired KC proliferation continuously. These findings fit into our understanding of a compromised KC function in the healing process of chronic wounds. The initial inhibition of proliferation mediated by AWF and CWF might be because of adaption processes to new culture conditions. CWF has previously been shown to impair proliferation of different cell types, including KC 18, 19 and the proliferation of fibroblasts and endothelial cells has been reported to be stimulated by AWF 20. A lack of distinct growth factors or the surplus of cytotoxic agents in CWF might explain these differences. Undisturbed migration of KC is an important requirement in resurfacing the wound and plays a decisive role in the very early stage of wound healing. This directed migration is critical to epithelialisation and depends on a complex balance of mediators 14. The composition of mediators in CWF might diminish KC migration and therefore impair or even prevent undisturbed wound closure. As demonstrated by the scratch assay results, wound closure was significantly delayed by CWF. As cell proliferation was inhibited by mitomycin C, these results cannot be explained by the proliferation‐inhibiting effect of CWF. They clearly demonstrate a negative impact of CWF on KC migration, which may be a relevant factor explaining the low tendency of chronic wounds to heal. Wound healing mechanisms are enhanced by a subset of growth factors secreted by various cell types 4. Resident and infiltrating cells interact with these growths factors and thereby orchestrate the wound healing process. A poor growth factor production or a lack of their availability has been shown to be a major cause of a compromised regeneration process in chronic wounds 21. Wound infection might play another important role in impaired wound healing. All chronic wounds are secondarily colonised by bacteria from the surrounding skin or the local environment 22. Lipopolysaccharides (LPS) are found in the outer membrane of Gram‐negative bacteria and act as endotoxins. Loryman and Mansbridge showed that LPS decreased keratinocyte migration in vitro 23. We analysed the gene expression profile of KC with regard to VEGF and b‐FGF under the influence of AWF and CWF. VEGF is known to have a stimulating effect on wound healing mainly through angiogenesis, but likely promotes epithelialisation and ECM deposition as well 24. As angiogenesis is a key component of the physiological repair process, diminished VEGF production and therefore angiogenesis are likely to contribute to impaired tissue regeneration 24, 25. In this study, VEGF was strongly induced by CWF than AWF, which might be because of tissue hypoxia in chronic wounds, activating the hypoxia‐inducible factor (HIF)‐1α pathway and thereby inducing VEGF expression 26. So a lacking expression of angiogenic factors by KC seems not to be responsible for an insufficient angiogenesis in chronic wounds. Deficiencies might be located in maturation and remodelling processes, which are also required to achieve a functional and stable vascular network 27, 28, 29, 30. b‐FGF is another angiogenic factor that has also been shown to stimulate KC proliferation 31. Under the influence of AWF and CWF, KC showed a significantly elevated expression of b‐FGF, but CWF had a slightly stronger effect. However, as b‐FGF activity is primarily regulated by its receptor level, the sole consideration of b‐FGF expression does not necessarily reflect its potential effects 29. MMPs are mandatory during the physiological wound healing process as they are involved in ECM remodelling and epithelialisation 32, 33. During epithelialisation, KC have to loosen their cell‐cell and cell‐ECM contacts at the wound margin 33. MMPs have been shown to lessen these contacts allowing KC to migrate across the wound 34, 35. We therefore investigated the expression of MMP‐2 and MMP‐9, which are overrepresented in CWF probably causing a constant ECM degradation in chronic wounds 17, 36, 37. In our study, MMP‐2 expression of KC was only marginally affected by AWF and CWF, respectively. The role of MMP‐2 during wound healing is discussed controversially in the literature. Although some groups relate an important role of MMP‐2 during physiological wound healing 38, others claim that MMP‐2 activity is not essential for this process 39. MMP‐9, however, was upregulated by both wound fluids with CWF having an earlier and much stronger effect. This might be explained by the higher levels of proinflammatory cytokines in CWF such as IL‐1 and TNF‐α that are well known to stimulate MMP‐9 expression 36. Although MMP‐9 is mandatory during the wound healing process 40, excessive MMP‐9 production is deleterious 41. So an excessively increased MMP‐9 expression by KC in the chronic wound environment might cause continuous self‐digestion of ECM and thereby impair wound healing. Furthermore, MMP‐9 leads to degradation of growth factors and their receptors 42, and is likely to have a negative impact on cell proliferation 33.
These results give an insight into impaired KC function in chronic wounds and identify possible targets for intervention. As proliferation and migration are the two key skills that give KC the ability to resurface a wound, impaired wound healing might partially be explained by deficiencies in these functions. Further studies are needed to identify major effectors within CWF that cause the detected effects. This understanding might then help to develop new treatment strategies that aim reactivation of impaired KC function by establishing a more physiological wound environment through inhibiting these adverse mediators.
Supporting information
Table S1. Primer sequences used for qRT‐PCR
Acknowledgements
We thank the BITOP AG Witten for financially supporting this project as well as the Witten/Herdecke University for a grant supporting the research position of the second author.
References
- 1. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835–70. [DOI] [PubMed] [Google Scholar]
- 2. Santoro MM, Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res 2005;304:274–86. [DOI] [PubMed] [Google Scholar]
- 3. Choma DP, Pumiglia K, DiPersio CM. Integrin alpha3beta1 directs the stabilization of a polarized lamellipodium in epithelial cells through activation of Rac1. J Cell Sci 2004;117(Pt 17):3947–59. [DOI] [PubMed] [Google Scholar]
- 4. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314–21. [DOI] [PubMed] [Google Scholar]
- 5. Hosoya A, Lee JM, Cho SW, Kim JY, Shinozaki N, Shibahara T, Shimono M, Jung HS. Morphological evidence of basal keratinocyte migration during the re‐epithelialization process. Histochem Cell Biol 2008;130:1165–75. [DOI] [PubMed] [Google Scholar]
- 6. Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boulton AJ, Vileikyte L, Ragnarson‐Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–24. [DOI] [PubMed] [Google Scholar]
- 8. Lin CD, Allori AC, Macklin JE, Sailon AM, Tanaka R, Levine JP, Saadeh PB, Warren SM. Topical lineage‐negative progenitor‐cell therapy for diabetic wounds. Plast Reconstr Surg 2008;122:1341–51. [DOI] [PubMed] [Google Scholar]
- 9. Gonzalez‐Consuegra RV, Verdu J. Quality of life in people with venous leg ulcers: an integrative review. J Adv Nurs 2011;67:926–44. [DOI] [PubMed] [Google Scholar]
- 10. Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clin Dermatol 2007;25:19–25. [DOI] [PubMed] [Google Scholar]
- 11. Baker EA, Leaper DJ. Proteinases, their inhibitors, and cytokine profiles in acute wound fluid. Wound Repair Regen 2000;8:392–8. [DOI] [PubMed] [Google Scholar]
- 12. Liu W, Saint DA. A new quantitative method of real time reverse transcription polymerase chain reaction assay based on simulation of polymerase chain reaction kinetics. Anal Biochem 2002;302:52–9. [DOI] [PubMed] [Google Scholar]
- 13. Ranzato E, Martinotti S, Volante A, Mazzucco L, Burlando B. Platelet lysate modulates MMP‐2 and MMP‐9 expression, matrix deposition and cell‐to‐matrix adhesion in keratinocytes and fibroblasts. Exp Dermatol 2011;20:308–13. [DOI] [PubMed] [Google Scholar]
- 14. Raja SK, Garcia MS, Isseroff RR. Wound re‐epithelialization: modulating keratinocyte migration in wound healing. Front Biosci 2007;12:2849–68. [DOI] [PubMed] [Google Scholar]
- 15. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835–70. [DOI] [PubMed] [Google Scholar]
- 16. Tarnuzzer RW, Macauley SP, Farmerie WG, Caballero S, Ghassemifar MR, Anderson JT, Robinson CP, Grant MB, Humphreys‐Beher MG, Franzen L, Peck AB, Schultz GS. Competitive RNA templates for detection and quantitation of growth factors, cytokines, extracellular matrix components and matrix metalloproteinases by RT‐PCR. Biotechniques 1996;20:670–4. [DOI] [PubMed] [Google Scholar]
- 17. Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol 1996;107:743–8. [DOI] [PubMed] [Google Scholar]
- 18. Bucalo B, Eaglstein WH, Falanga V. Inhibition of cell proliferation by chronic wound fluid. Wound Repair Regen 1993;1:181–6. [DOI] [PubMed] [Google Scholar]
- 19. Mendez MV, Raffetto JD, Phillips T, Menzoian JO, Park HY. The proliferative capacity of neonatal skin fibroblasts is reduced after exposure to venous ulcer wound fluid: a potential mechanism for senescence in venous ulcers. J Vasc Surg 1999;30:734–43. [DOI] [PubMed] [Google Scholar]
- 20. Katz MH, Alvarez AF, Kirsner RS, Eaglstein WH, Falanga V. Human wound fluid from acute wounds stimulates fibroblast and endothelial cell growth. J Am Acad Dermatol 1991;25(6 Pt 1):1054–8. [DOI] [PubMed] [Google Scholar]
- 21. Liu Y, Dulchavsky DS, Gao X, Kwon D, Chopp M, Dulchavsky S, Gautam SC. Wound repair by bone marrow stromal cells through growth factor production. J Surg Res 2006;136:336–41. [DOI] [PubMed] [Google Scholar]
- 22. Siddiqui AR, Bernstein JM. Chronic wound infection: facts and controversies. Clin Dermatol 2010;28:519–26. [DOI] [PubMed] [Google Scholar]
- 23. Loryman C, Mansbridge J. Inhibition of keratinocyte migration by lipopolysaccharide. Wound Repair Regen 2008;16:45–51. [DOI] [PubMed] [Google Scholar]
- 24. Bao P, Kodra A, Tomic‐Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res 2009;153:347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, Bunting S, Steinmetz HG, Gurtner GC. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow‐derived cells. Am J Pathol 2004;164:1935–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holzbach T, Neshkova I, Vlaskou D, Konerding MA, Gansbacher B, Biemer E, Giunta RE. Searching for the right timing of surgical delay: angiogenesis, vascular endothelial growth factor and perfusion changes in a skin‐flap model. J Plast Reconstr Aesthet Surg 2009;62:1534–42. [DOI] [PubMed] [Google Scholar]
- 27. Spanholtz TA, Theodorou P, Holzbach T, Wutzler S, Giunta RE, Machens HG. Vascular endothelial growth factor (VEGF165) plus basic fibroblast growth factor (bFGF) producing cells induce a mature and stable vascular network‐‐a future therapy for ischemically challenged tissue. J Surg Res 2011;171:329–38. [DOI] [PubMed] [Google Scholar]
- 28. Simons M. Angiogenesis: where do we stand now? Circulation 2005;111:1556–66. [DOI] [PubMed] [Google Scholar]
- 29. Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y. Angiogenic synergism, vascular stability and improvement of hind‐limb ischemia by a combination of PDGF‐BB and FGF‐2. Nat Med 2003;9:604–13. [DOI] [PubMed] [Google Scholar]
- 30. Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF‐B‐deficient mice. Science 1997;277:242–5. [DOI] [PubMed] [Google Scholar]
- 31. Ono I. Roles of cytokines in wound healing processes. Nihon Geka Gakkai Zasshi 1999;100:522–8. [PubMed] [Google Scholar]
- 32. Rayment EA, Upton Z, Shooter GK. Increased matrix metalloproteinase‐9 (MMP‐9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol 2008;158:951–61. [DOI] [PubMed] [Google Scholar]
- 33. Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol 2008;40:1334–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC. The activity of collagenase‐1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol 1997;137:1445–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McGuire JK, Li Q, Parks WC. Matrilysin (matrix metalloproteinase‐7) mediates E‐cadherin ectodomain shedding in injured lung epithelium. Am J Pathol 2003;162:1831–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, Murphy G, Schultz G. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 1999;7:442–52. [DOI] [PubMed] [Google Scholar]
- 37. Wysocki AB, Staiano‐Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP‐2 and MMP‐9. J Invest Dermatol 1993;101:64–8. [DOI] [PubMed] [Google Scholar]
- 38. Karim RB, Brito BL, Dutrieux RP, Lassance FP, Hage JJ. MMP‐2 assessment as an indicator of wound healing: A feasibility study. Adv Skin Wound Care 2006;19:324–7. [DOI] [PubMed] [Google Scholar]
- 39. Frossing S, Rono B, Hald A, Romer J, Lund LR. Skin wound healing in MMP2‐deficient and MMP2/plasminogen double‐deficient mice. Exp Dermatol 2010;19:e234–40. [DOI] [PubMed] [Google Scholar]
- 40. Parks WC. Matrix metalloproteinases in repair. Wound Repair Regen 1999;7:423–32. [DOI] [PubMed] [Google Scholar]
- 41. Muller M, Trocme C, Lardy B, Morel F, Halimi S, Benhamou PY. Matrix metalloproteinases and diabetic foot ulcers: the ratio of MMP‐1 to TIMP‐1 is a predictor of wound healing. Diabet Med 2008;25:419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase‐9 to tissue inhibitor of matrix metalloproteinase‐1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen 2002;10:26–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primer sequences used for qRT‐PCR


