Abstract
A prospective, randomised, controlled, parallel group, multi‐centre clinical trial was conducted at three sites to compare the healing effectiveness of treatment of chronic lower extremity diabetic ulcers with either weekly applications of Apligraf® (Organogenesis, Inc., Canton, MA), EpiFix ® (MiMedx Group, Inc., Marietta, GA), or standard wound care with collagen‐alginate dressing. The primary study outcome was the percent change in complete wound healing after 4 and 6 weeks of treatment. Secondary outcomes included percent change in wound area per week, velocity of wound closure and a calculation of the amount and cost of Apligraf or EpiFix used. A total of 65 subjects entered the 2‐week run‐in period and 60 were randomised (20 per group). The proportion of patients in the EpiFix group achieving complete wound closure within 4 and 6 weeks was 85% and 95%, significantly higher (all adjusted P‐values ≤ 0·003) than for patients receiving Apligraf (35% and 45%), or standard care (30% and 35%). After 1 week, wounds treated with EpiFix had reduced in area by 83·5% compared with 53·1% for wounds treated with Apligraf. Median time to healing was significantly faster (all adjusted P‐values ≤0·001) with EpiFix (13 days) compared to Apligraf (49 days) or standard care (49 days). The mean number of grafts used and the graft cost per patient were lower in the EpiFix group campared to the Apligraf group, at 2·15 grafts at a cost of $1669 versus 6·2 grafts at a cost of $9216, respectively. The results of this study demonstrate the clinical and resource utilisation superiority of EpiFix compared to Apligraf or standard of care, for the treatment of diabetic ulcers of the lower extremities.
Keywords: Advanced wound care, Amniotic membrane, Comparative effectiveness, Cost effectiveness, Diabetic ulcers
Introduction
Diabetes and its associated morbidities are a growing problem, negatively impacting populations throughout the world and imposing severe financial burdens on healthcare resources. Worldwide, 285 million people or approximately 6·4% of the world's population is estimated to have diabetes and these numbers are expected to increase to 7·7% and 439 million adults by 2030 1. In 2012, more than 22·3 million people in the USA had a diagnosis of diabetes, with an attendant cost of approximately $245 billion, including $176 billion in direct medical cost and $69 billion in lost productivity 2.
Approximately 25% of people with diabetes will develop a lower extremity ulcer over their lifetime 3. These wounds are often slow to heal and frequently reoccur. Contributing to the slow healing rates and high rates of recidivism are concomitant conditions associated with diabetes, such as peripheral vascular disease, neuropathy and poor blood glucose control. The delayed healing of ulcers increases the risk for infection and the need for amputation, which in turn increases morbidity and healthcare costs while at the same time reducing an individual's productivity and quality of life. Diabetic ulcers precede 85% of lower extremity amputations, and it is estimated that up to 85% of these amputations may be preventable 4.
The desired goal of diabetic ulcer treatment is to promote rapid and complete healing in order to reduce the risk for infection and its limb‐ or even life‐threatening complications. Moist dressings, debridement, wound offloading and infection control are standard in the management of lower extremity ulcers, yet even with the best conservative care, these wounds are often notoriously slow to heal, requiring many months of treatment 5. The Wound Healing Society guidelines recommend consideration of advanced wound therapies if a diabetic ulcer does not reduce in size by 40% or more after 4 weeks of standard therapy 6. Therapies that promote rapid and complete healing, thus reducing the risk for infection and amputation, can substantially improve quality of life while decreasing financial burdens to the individual and society overall 7. Advanced therapies such as bioengineered skin grafts have been shown to promote wound closure, resulting in a more consistent and faster healing of diabetic ulcers compared with standard therapy 8. Because these advanced therapies are expensive, in clinical practice they are often reserved for use in patients with the most recalcitrant wounds. This type of patient is also likely to have multiple comorbidities that complicate medical management. Although the use of these advanced wound products may increase short‐term expenditures, net cost savings may be achieved through increased healing rates, faster time to healing and reduced incidence of infection and amputation.
Randomised controlled clinical trials have demonstrated that both bioengineered skin substitutes and dehydrated human amnion/chorion membrane (dHACM) promote wound closure, resulting in a more consistent and faster healing of chronic diabetic ulcers when compared to standard therapy, yet there is little data available with which to assess differences in clinical usefulness and cost effectiveness among commercially available products 8, 9, 10, 11. A retrospective analysis of data collected in separate randomised trials suggests that dHACM may be superior to several products in promoting rapid healing 12. The objective of this randomised prospective study was to directly compare rates of healing, time to wound closure, product cost, and efficiency of product utilisation in the treatment of chronic diabetic lower extremity wounds with either standard wound care, dHACM, or a commonly used tissue‐engineered skin substitute.
Methods
We conducted a prospective, randomised, controlled, parallel group, multi‐centre clinical trial to examine healing outcomes in diabetic patients with chronic lower extremity ulcers treated with weekly application of a tissue‐engineered skin substitute (Apligraf®, Organogenesis, Canton, MA), weekly application of dHACM (EpiFix®, MiMedx Group, Inc., Marietta, GA), or standard wound care. The study population consisted of patients with diabetes receiving care from clinicians specialising in wound care at three outpatient centres in the state of Virginia (USA). The study was conducted under the direction of a principal investigator. Consent was obtained prior to any study‐related procedures and all patients signed an Investigational Review Board (IRB)‐approved informed consent form, in compliance with applicable regulatory requirements and adhering to Good Clinical Practice (GCP). This study was conducted in accordance with the provisions of the Declaration of Helsinki. In addition, all products used in this study were manufactured, handled and stored in accordance with applicable Good Manufacturing Practices (GMP) or Good Tissue Practices (GTP) as appropriate. The study was reviewed and approved by Western IRB (WIRB) and pre‐registered in ClinicalTrials.gov (NCT01921491). Confidentiality was maintained with respect to all patient records. Records were retained in locked files at each study site to which only study coordinators and investigators had access. Only minimally necessary data were collected and subject identifiers were limited.
Product descriptions
Apligraf is classified as a Class III medical device supplied as a living, allogeneic bi‐layered cultured skin substitute derived from donated human neonatal male foreskin tissue. The epidermal layer is formed by human keratinocytes and has a well‐differentiated stratum corneum; the dermal layer is composed of human fibroblasts in a bovine Type I collagen lattice 13. Apligraf is supplied sealed in a heavy gauge polyethylene bag with a 10% CO2/air atmosphere and in an agarose nutrient medium. Apligraf must be ordered from the manufacturer at least 1–2 business days prior to the scheduled application and it has an expiration date of 15 days after initial packaging. The product is supplied ready for use and each 44 cm2 single‐use disc is intended for one‐time application on a single patient. To maintain cell viability, Apligraf must be kept in the shipping container and in the sealed poly bag at 68°F–73°F (20°C–23°C) until use 13. Apligraf cannot be reused, frozen or sterilised and should be used within 15 minutes after opening the poly bag.
EpiFix is an allograft regulated by the FDA as a human cells, tissues, and cellular and tissue‐based product (HCT/P, 21CFR 1271) and by Section 361 of the Public Health Service Act, and comprises dHACM. The allograft consists of layers of the amniotic sac, including an epithelial lining, amnion and chorion, which contain important biological molecules including collagen, connective tissue, cytokines and growth factors 14, 15. The EpiFix allografts do not require any special pre‐ordering, shipping or storage procedures as they are supplied ready for use in a sterile package that is stored under ambient conditions and has a 5‐year shelf life. Allografts are available in multiple sizes ranging from 1·5 to 49 cm2 and each graft is embossed to aid in identification of proper orientation for placement on the wound 11, 16, 17.
Patient screening and eligibility
The study population comprised Type 1 or Type 2 diabetic patients presenting for treatment of a lower extremity ulcer. Patients willing to participate in the clinical study and agreeing to comply with the weekly visits and follow‐up regimen were eligible for study inclusion. Study inclusion and exclusion criteria listed in Table 1 were used to determine patients eligible to enter the 2‐week study run‐in period prior to study enrolment and randomisation. The run‐in period was designed to determine whether the study ulcer was indolent to healing with conservative wound care, and was used to identify subjects who were eligible to proceed to the treatment phase of the study. During the 2‐week run‐in period, patients were instructed regarding the proper techniques for daily dressing changes and offloading. They were provided with collagen‐alginate dressings, gauze and an offloading cast walker (Royce Medical Active Offloading Walker, Royce Medical, Inc., Camarillo, CA). During the run‐in period, patients were seen every week for sharp debridement and wound measurements. At the conclusion of the run‐in period (week 2), those patients whose wounds had reduced in size by more than 20% were excluded.
Table 1.
Major inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Treatment phase of study
Patients who showed a reduction in wound size of 20% or less after the 2‐week run‐in period and who still met all study inclusion/exclusion criteria were enrolled in the treatment phase of the study, and were randomised to one of 3 study groups (Apligraf, EpiFix, or Standard Care) in a 1:1:1: ratio. In order to ensure allocation concealment, 60 opaque envelopes, 20 for EpiFix, 20 for Apligraf and 20 for standard care controls, each containing a slip of paper designating the study group, were used. The envelopes were randomly shuffled and labelled 01‐60. When a patient was scheduled for randomisation, the appropriate envelope was delivered to the study site and the sequential envelope was opened in the presence of the patient, who then signed the numerical envelope and paper slip inside acknowledging his or her group assignment. Because of the different handling requirements of the products used, blinding the treating physician or patient to group assignment was not possible, but the study adjudicators who examined photographic images for validation of healing at the completion of the study were blinded as to group assignment.
Patients were seen by the investigator at the study site at least once every 7 days (±3 days) for up to 12 weeks, or until 1 week after complete healing, whichever occurred first. Patients whose wounds failed to reduce by at least 50% after 6 weeks of study enrolment were exited from the study to seek alternative treatment. Procedures conducted at each study visit included ulcer debridement if required and cleansing with a sterile normal saline solution, ulcer measurement and photography, assessment for adverse events and wound dressing. Wound surface area was calculated by width × length, and depth, and an acetate tracing of the wound was also performed. All measurements, tracings and photographic images were taken after debridement. For subjects enrolled in the Apligraf or EpiFix groups, grafts were applied every week after debridement. A non‐adherent dressing (Adaptic Touch, or an equivalent), a moisture‐retentive dressing (NuGel, or an equivalent) and a compressive dressing were then applied. Patients randomised to the standard care control group had their wounds debrided weekly as necessary and were instructed to change their wound dressing daily using the provided collagen‐alginate and gauze dressing supplies. All wounds were similarly offloaded during both the run‐in and study periods through the use of an offloading diabetic cam walker.
Validation of healing
A healed wound was defined as the complete reepithelialisation of the wound without drainage or need for dressing. Wound healing was confirmed by the primary investigator at a follow‐up visit prior to study exit, 1 week after a 100% reepithelialisation was determined by the site investigator. At the completion of the study, photographic images, blinded to group assignment, were reviewed by three independent physicians specialising in wound care, including one vascular surgeon and one plastic surgeon, who acted as adjudicators in validating that wounds had achieved complete reepithelialisation.
Study outcomes
The primary objective of this study was to compare, across the treatment arms, the percentage of wounds that had healed completely after 4 and 6 weeks of treatment with Apligraf, EpiFix or standard care. Secondary objectives were to examine the percent change in the size of the wound area per week, velocity of wound closure and differences in amount and cost of the advanced wound products used. As the actual costs of Apligraf and EpiFix are variable because of contractual prices, we estimated the differences in cost based on the allowable charges for each product from the Centers of Medicare and Medicaid Services (CMS) product reimbursement schedule 18.
Data analysis
The null hypothesis was that the proportion of wounds that achieved complete healing within 6 weeks was the same for EpiFix‐ and Apligraf‐treated subjects. If this hypothesis was rejected, then one product would be revealed as superior to the other.
Sample size calculations (PASS 11) showed that group sample sizes of 23 in group one and 23 in group two could achieve 81% power to detect a difference of 0·4 between the group proportions. The proportion in group one (EpiFix) was assumed to be 0·3 under the null hypothesis and 0·7 under the alternative hypothesis. The proportion in group two (Apligraf) was 0·3. The test statistic used was the two‐sided Z‐test with pooled variance. The significance level of the test was targeted at 0·05; the significance level actually achieved by this design was 0·0497. Twenty standard care patients were included as a reference group.
An intent‐to‐treat analysis was used including all patients as originally allocated after randomisation. For missing observations, the last known value was carried forward. Study variables were summarised as means and standard deviations (SDs) for continuous variables and proportions or percentages for categorical variables. Parametric and non‐parametric tests were used as appropriate. Analysis of variance (ANOVA) or the Kruskal–Wallis test was used to test for differences in continuous variables. For categorical variables, χ 2 or Fisher exact tests were performed to test for statistical differences. A Kaplan–Meier analysis was conducted to compare the healing function of the three treatment groups statistically. To adjust for family‐wise error rate (FWER), P‐values were reported using the step‐up Bonferroni method of Hochberg. Adjusted two‐sided P‐values <0·05 were considered significant. SAS® 9·4 (SAS institute, Inc., Cary, NC) was used to perform the statistical testing.
Results
A total of 65 subjects were screened and enrolled in the study for the 2‐week run‐in period, between September 2013 and April 2014. At the conclusion of the run‐in period, five patients were no longer eligible for randomisation. Of these five patients, two patients had achieved wound closure of >20%, two patients no longer met the inclusion criteria because of wound infection and one patient had achieved complete wound closure during the run‐in period. Ultimately, there were 60 patients eligible for randomisation and study inclusion. Of the 60 patients enrolled in the treatment phase, 20 were randomised to receive a weekly application of Apligraf, 20 were randomised to receive a weekly application of EpiFix and 20 were randomised to receive a continuation of standard care. At study enrolment, no statistically significant differences were observed in patient characteristics, wound size or wound duration between the study groups. Patient characteristics for the study groups are presented in Table 2.
Table 2.
Clinical characteristics at study enrolment (all P > 0·05)*
| Apligraf® (n = 20) | EpiFix® (n = 20) | Standard care (n = 20) | |
|---|---|---|---|
| Mean age, in years (SD) | 65·2 (11·7) | 63·2 (13·0) | 62·2 (12·8) |
| Age ≥65 years (n, %) | 11 (55·0%) | 11 (55·0%) | 9 (45·0%) |
| Male gender | 9 (45·0%) | 10 (50·0%) | 9 (45%) |
| Race | |||
| Caucasian | 18 (90·0%) | 19 (95·0%) | 17 (85·0%) |
| African American | 2 (10·0%) | 1 (5·0%) | 3 (15·0%) |
| Ulcer location (n, %) | |||
| Forefoot | 6 (30·0%) | 7 (35·0%) | 5 (25·0%) |
| Hind foot | 5 (25·0%) | 1 (5·0%) | 3 (15·0%) |
| Mid‐foot | 4 (20·0%) | 2 (10·0%) | 3 (15·0%) |
| Toe | 3 (15·0%) | 4 (20·0%) | 5 (25·0%) |
| Other | 2 (10·0%) | 6 (30·0%) | 4 (20·0%) |
| Mean BMI (SD) | 32·7 (8·56) | 35·0 (7·5) | 35·8 (9·7) |
| Obese BMI ≥30 (n, %) | 13 (65·0%) | 14 (70·0%) | 14 (70·0%) |
| Mean HbA1c (SD) | 8·0 (1·9) | 7·4 (1·5) | 8·0 (1·8) |
| HbA1c ≥9% (n, %) | 6 (30·0%) | 2 (10·0%) | 5 (25·0%) |
| Smoker (n, %) | 3 (15%) | 5 (25%) | 5 (25%) |
| Mean duration of index ulcer, in weeks (SD) | 18·5 (13·8) | 15·6 (12·7) | 16·2 (13·5) |
| Median (Min, Max) | 13 (6, 54) | 11 (5, 54) | 9 (6, 52) |
| Mean baseline wound size, in cm2 (SD) | 2·6 (1·8) | 2·7 (2·4) | 3·3 (2·7) |
| Median (Min, Max) | 2·1 (1·0, 6·8) | 2·0 (1·0, 9·0) | 2·0 (1·0, 9·0) |
BMI, body mass index.
Data presented as mean (SD), median (minimum, maximum), or number (percent) as indicated.
Healing rates
The rates of healing at 4 and 6 weeks are presented in Figure 1. Complete healing occurred by week 4 in 35·0% (7/20) of patients receiving Apligraf, 85% (17/20) of patients receiving EpiFix, and 30% (6/20) of patients receiving standard care. Lower extremity wounds treated with EpiFix had significantly higher rates of complete healing within 4 weeks compared to wounds treated with Apligraf (Hochberg‐adjusted P‐value = 0·001) or standard care (Hochberg‐adjusted P‐value = 0·001). After 6 weeks of treatment initiation, patients treated with EpiFix continued to have the highest rates of complete healing at 95% (19/20) versus 45·0% (9/20) for patients receiving Apligraf, and 35% (7/20) for patients receiving standard care (Hochberg‐adjusted P‐values = 0·0006 and 0·0001, respectively).
Figure 1.
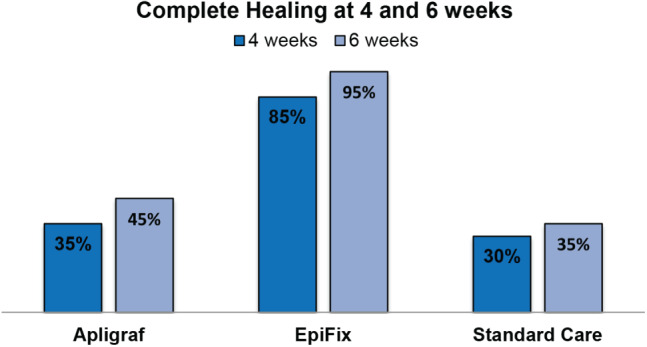
Rates of complete healing at 4 and 6 weeks for each study group.
Wound size reduction
Mean wound size reductions overall and by patient within each study group are presented in Figure 2. Overall, at each week 1 through 6, mean percent wound size reduction was greatest for those in the group receiving EpiFix. Patients in the EpiFix group showed a rapid and consistent percent reduction in wound size with less inter‐patient variation, while those in the Apligraf and standard care groups exhibited the more typical pattern of irregular wound size variation over time.
Figure 2.
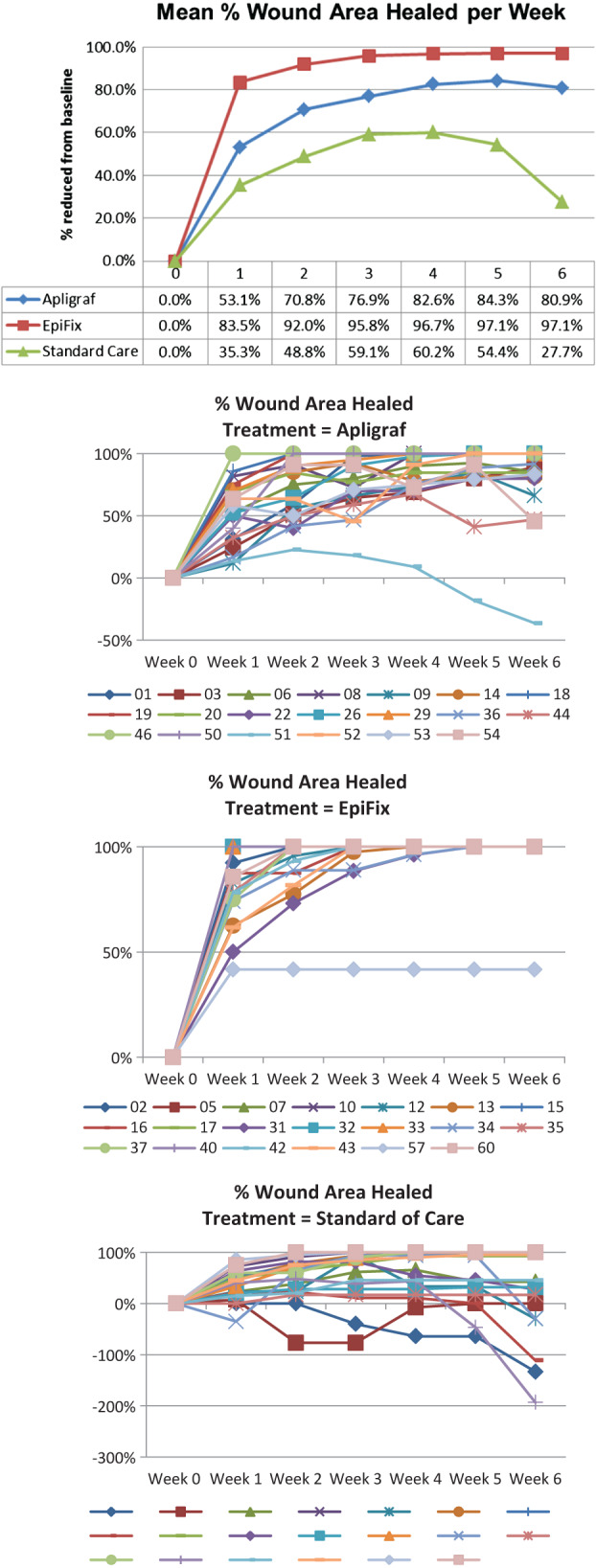
Mean percent healing overall and per patient per week.
Time to healing
A Kaplan–Meier analysis was performed to compare the time‐to‐healing performance of the three study groups. (Figure 3) The Log‐Rank test of equality of the healing function over study groups produced a χ 2 test statistic of 36·766, with a P‐value of <0·0001. When adjusting for multiple comparisons of each treatment to the other using the Hochberg method, the comparison of EpiFix to Apligraf and standard care for healing rate was significantly in favour of EpiFix (P‐value ≤0·0001). Based on the Kaplan–Meier analysis for those patients that healed completely, the estimated median healing time was 49 days (95% CI 28–63 days) for the group treated with Apligraf, 13 days for those receiving EpiFix (95% CI 7–21 days) and 49 days for patients receiving standard care (95% CI 28–70 days).
Figure 3.
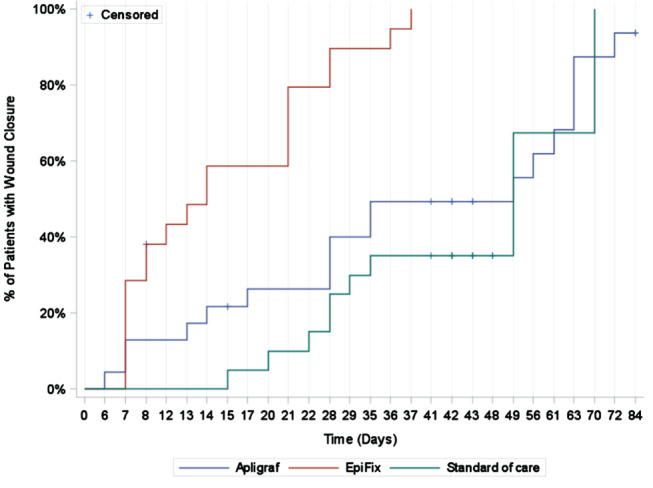
The Kaplan–Meier analysis of the time to healing by study group.
Apligraf and EpiFix product usage
Over the study period, 124 Apligraf grafts (mean = 6·2 per study patient) were used. As each graft was 44 cm2, a total of 5456 cm2 of the product was used to cover the cumulative wound area (sum of weekly wound measurements) of 158·6 cm2 for all patients in the Apligraf group. By photo‐documentation and digital measurement, 97·1% of the Apligraf product used in the study sample was discarded. Over the course of the study period, the total number of applications of the EpiFix allograft was 43 (mean 2·15 per study patient), with a total of 154 cm2 of the product used to cover a cumulative wound area of 68·2 cm2. As grafts were trimmed to wound size, 55·8% of the EpiFix product was discarded. Based on CMS average sales price data 18, the total cost of the Apligraf product used in the study was $184 315 ($9216 per study patient) compared with the total cost of $33 379 ($1669 per study patient) for EpiFix. This equates to an 81·9% lower cost for graft material used in the EpiFix group compared with the cost of graft material used in the Apligraf group.
Study completion
All 20 patients in the EpiFix group exited the study within 6 weeks. Nineteen (95%) had healed completely and one (5%) was withdrawn from the study after 1 week because of an adverse event. Four of the 20 (20%) Apligraf patients exited the 12‐week study unhealed. Of these, three (15%) were withdrawn after 6 weeks with <50% healing and one (5%) exited the study after 12 weeks unhealed. For those patients receiving standard care, 11 were withdrawn after 6 weeks failing to heal by at least 50%.
Safety evaluation
Five adverse events were documented. One patient in the EpiFix group developed cellulitis and infection on the affected foot. The patient was treated with sharp debridement, antibiotics and silver dressing. As other topical treatments were used on the study ulcer, the patient was withdrawn from the study. At the time of study withdrawal, the study wound had reduced in size from 10·8 to 6·3 cm2 (41·7%). Two patients in the Apligraf group were hospitalised for reasons unrelated to the study wound, one with a urinary tract infection and the other with wound infection on the non‐study foot, resulting in a trans‐metatarsal amputation. Both patients remained in the study. Two patients in the standard care group had adverse events. One developed cellulitis on the left ankle unrelated to the study wound, and was treated with antibiotics as an outpatient. She ended the study unhealed after 6 weeks. Another patient in the standard care group was hospitalised for treatment of infection in the study wound and was treated with sharp debridement and IV antibiotics. She also ended the study after 6 weeks because of incomplete wound closure. We believe that a good aseptic technique, the 3‐layered dressing and the offloading boot, as well as extensive training on dressing changes for the standard care group led to the overall small number of adverse events in the study. No adverse events were believed to be directly related to the treatments received.
Discussion
Previous studies have established that advanced wound therapies such as bioengineered skin grafts and dHACM promote wound closure, resulting in a more consistent and rapid complete healing of lower extremity diabetic ulcers 8, 9, 10, 11. Because of the complexity of chronic wounds, and the potential impact of concomitant comorbidities, there is no single intervention that can be established as superior for all patients in all clinical situations 19. Many factors contribute to failure of wound healing, and these must be taken into consideration by clinicians in determining an individual's treatment plan. Comparative effectiveness research offers an opportunity for improved clinical outcomes and quality by providing more and better information on how a product(s) or treatment plan performs, which in turn may reduce health care costs. This study is the first multi‐centre randomised comparative effectiveness study examining, side by side, the performance, outcomes and utilisation of two approved advanced wound care products (Apligraf, EpiFix) as a treatment for chronic lower extremity diabetic ulcers. In this study we have shown that lower extremity diabetic ulcers treated with EpiFix had significantly greater rates of complete healing and more rapid time to healing than wounds treated with Apligraf. In addition, EpiFix was found to be more cost effective than Apligraf.
The results of this study are consistent with a retrospective analysis that examined data on outcomes data from individual randomised trials and pivotal trials of three advanced wound care products: Apligraf, Dermagraft and EpiFix 12. In that analysis, patients treated with a weekly or biweekly application of EpiFix (n = 64) had complete healing rates of 81%, 91%, and 92% after 6, 9 and 12 weeks of treatment, compared to rates of 35%, 48% and 56% for patients treated with up to 5 weekly applications of Apligraf (n = 112), or 15%, 26% and 30% for patients treated with up to 8 weekly applications of Dermagraft (n = 130), after 6, 9 and 12 weeks of treatment, respectively. The healing rates at 6 weeks in the current prospective study are slightly higher for EpiFix (95%) and Apligraf (45%) than observed in the retrospective review. This difference may be related to improvements in contemporary management of lower extremity ulcers including more aggressive debridement and offloading, as well as differences in frequency and quantity of graft applications. The current results showing complete healing within 6 weeks in 95% (19/20) of patients treated with a weekly application of EpiFix is identical to the results of a previously published randomised trial comparing weekly to biweekly applications of the material, with 95% (19/20) of the patients receiving a weekly application of EpiFix healing within 6 weeks while 70% (14/20) of the patients receiving a biweekly application healed in the same 6‐week period 17.
The mechanisms of action behind many advanced wound care products are poorly understood. In general terms, they address defects in the normally well‐orchestrated and predictable sequence of events of wound healing that are impaired in diabetes and other diseases. The requirement for a functional reparative tissue microenvironment for successful healing is characterised by high levels of growth factors and other soluble mediators of cell signalling, functional fibroblasts, keratinocytes and vascular endothelial cells, as well as controlled levels of proteases and bacteria 20. Cell‐mediated regeneration of extracellular matrix and angiogenesis are critical processes in wound repair 20, 21. Because chronic wounds are characterised by persistent inflammation, cell senescence, growth factor deficiencies, bioburden, elevated levels of destructive proteases and stem cell deficiencies, interventions that address these conditions represent an opportunity for angiogenesis, granulation and epithelialisation 20.
Both the advanced wound care products examined in this study have been shown to be more successful in promoting wound healing than standard care alone 9, 11. The presence of both fibroblasts and keratinocytes in Apligraf are believed to result in a paracrine reaction, which contributes to epithelial stratification, greater tensile strength, modulation of cytokine and growth factor expression, and increased angiogenic properties, which are important for tissue homeostasis and wound healing 22. Although the presence of the live cells is not durable, this bi‐layered living cell therapy stimulates the chronic wound by providing a physiologic combination of growth factors and cytokines that is lacking in chronic wounds, particularly in those of diabetics 23. Furthermore, both these products have been shown to contain tissue inhibitors of metalloproteinases (TIMPs) although the levels have not been directly compared 24. Therefore, both products have the potential to modulate off‐target destruction known to occur in diabetic wounds because of excessive metalloproteinase activity. However, patients with lower extremity diabetic ulcers treated with EpiFix had higher rates of wound closure and more rapid healing than patients treated with Apligraf.
The use of natural human amniotic membrane as a wound covering has been reported in the literature for over a century 25. EpiFix is dHACM created through the proprietary PURION® process, a procedure that gently cleanses and washes the membranes to reduce bioburden with minimal tissue manipulation while maintaining structural integrity. ELISA assays performed on samples of EpiFix have shown quantifiable levels of vascular endothelial growth factor (VEGF), platelet‐derived growth factors AA and BB (PDGF‐AA and PDGF‐BB), transforming growth factors alpha and beta (TGFα and TGFβ1), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF) and granulocyte colony stimulating factor (GCSF) 14. The ELISA assays 14 also identified the presence of Interleukins 4, 6, 8 and 10, which suppress inflammation and may contribute to the allografts' immune‐privileged properties 26, and TIMPs 1, 2 and 4, that neutralise the destructive biological effects of matrix metalloproteinases (MMP) 2 and 9, which are often overexpressed in chronic wounds. The presence and amount of signalling molecules, including 14 cytokines and 10 chemokines known to regulate inflammation, and 12 cytokines known to regulate wound healing processes, have also been identified in EpiFix 27.
Recently, stem cells have been identified as having a role in wound healing. These mesenchymal or haematopoietic cells are mobilised, recruited and homed to sites of injury by soluble mediators generated by the wound repair process, raising the possibility for bona fide wound regenerative interventions 28, 29. In vitro and in vivo studies have confirmed that the dHACM allograft can act as a ‘stem cell magnet’ to stimulate the migration of mesenchymal stem cells, as well as bone marrow‐derived haematopoietic stem cells 15, 27. Diabetic wounds are known to be deficient in several factors that recruit stem cells to the wound bed, particularly SDF‐1 30. Correction of this deficiency using lentiviral gene therapy for SDF‐1 has been shown to improve healing of diabetic wounds through increased homing of stem cells to the wound bed 31. The EpiFix allografts release factors such as SDF‐1, VEGF and PDGF that recruit endogeneous stem cells, suggesting a true regenerative potential when used in wound management. EpiFix is additionally unique when compared with other amniotic membrane products in that it comprises both amnion and chorion. A recent study compared growth factor levels between single‐layered amnion products, without chorion, to multi‐layered allografts constituted of both amnion and chorion (EpiFix) 32. The total cytokine content contributed by chorion was higher than that contributed by amnion alone. Amniotic membrane allograft containing both amnion and chorion had significantly more growth factors than single‐layered amnion grafts. These higher levels of cytokines and growth factors in an amnion/chorion allograft may contribute to the rapid and complete healing rates seen with the use of EpiFix 11, 16, 17. Unlike living cellular constructs, EpiFix does not rely on the preservation of live cells, or on the rate of growth factor excretion by the cells placed in the hostile environment of the chronic wound. The healing outcomes achieved in this study are a consequence of the natural combination of growth factors, stem cell recruitment factors and architectural elements present in EpiFix, in comparison to Apligraf, which contains living cells manufactured into a bioengineered skin substitute.
Determining the cost effectiveness of any advanced therapy or product in the area of wound medicine requires the consideration of a number of variables including how many wounds heal completely and how fast wound closure results. Although advanced wound care products may be costly, the expense may be mitigated through shortened treatment periods, reduced rates of complications, fewer hospitalisations and lower rates of amputation 33. Additional factors to consider when evaluating an advanced wound care product include the ease of product storage and handling characteristics, as well as the amount and cost of the product used and the amount of unused and dispensed product discarded at each application as wastage. EpiFix has a 5‐year shelf life at ambient temperatures, requires minimal storage space and is easy to apply to the wound bed, while Apligraf must be stored sealed in a nutrient medium at 68°F–73°F and has a shelf life of 15 days.
A unique aspect of this prospective randomised study is the measurement of the actual amount of product used. Patients treated with EpiFix had a superior rate of wound healing and a more rapid rate of wound closure than patients treated with Apligraf, while utilising 65% fewer grafts and 97·1% fewer square centimetres of graft material. Although it is expected that wastage will occur when grafts are trimmed to fit the contours of a wound, the multiple sizes of EpiFix allow for the requirement of smaller grafts as a wound decreases in size, and less product wastage compared with Apligraf, where each week a 44 cm2 graft is trimmed to fit the wound regardless of wound size, resulting in the major part of the product being discarded. For every square centimetre of EpiFix wasted, approximately 61·5 cm2 of Apligraf was wasted. This resulted in a savings of 81·9% for patients treated with EpiFix compared with Apligraf, based on the average sales price of the products.
The strengths of our study include the randomised multi‐centre design, direct comparison between two advanced wound care modalities and inclusion of a cost‐effectiveness analysis, yet there are limitations that must be addressed. While the study was adequately powered to identify differences in primary outcome between the EpiFix and Apligraf groups, as well as the EpiFix and standard care groups, the study was not adequately powered to achieve statistical significance between the Apligraf group and standard care group at the 6‐week time period. Patients were followed for only 1 week after healing, and they were allowed to withdraw from the study after 6 weeks if their wound had not reduced in size by at least 50%. Therefore, we were unable to compare the rates of healing at 12 weeks, or the rates of wound recidivism in this study. In addition, this study includes a variety of lower extremity diabetic ulcers, both plantar and dorsal. The sample size was not sufficient to stratify by location, nor was it possible to perform any meaningful sub‐group analysis to determine factors influencing outcomes or speed of healing. As product cost data were obtained from a recent CMS reimbursement schedule, they do not reflect the actual cost of material in all clinical settings. We did not examine ancillary costs related to differences in product handling, storage and application procedures, which may have had a further impact on costs.
The choice of standard of care is often one of the most difficult aspects of wound study design. Ideally, the control should have been treated with identical topical therapy minus the study agent. Because we were comparing advanced wound therapies, we felt that it was important to utilise the wound therapy that is currently considered standard of care for lower extremity diabetic ulcers. Therefore, topical collagen‐alginate dressings were chosen over a hydrogel. The greater frequency of dressing changes in the control group would be expected to augment healing, so this was not felt to be a weakness. Although patients in the control group managed wound care on their own, they received instructions and oversight throughout the study period. Patients in all groups received the same type of offloading device and offloading instructions. Although compliance of offloading was not measured, the results are comparable to those of previous trials, suggesting that there was no difference in offloading between the study groups.
In summary, patients treated with EpiFix exhibited the highest rates of complete healing and their wounds healed significantly faster than those treated with Apligraf. EpiFix was more cost effective than Apligraf in this study because of the fewer number of grafts required to achieve complete healing, and the ability to use a graft that was closer in size to the wound being treated, leading to less wastage of graft material. The overall results of this comparative effectiveness study may be useful in guiding clinicians who are determining a treatment plan for diabetic patients with non‐healing lower extremity wounds, as well as for technology assessment of advanced wound care products.
Acknowledgements
This study was sponsored and funded by MiMedx Group, Inc.. We acknowledge the work of Niki Istwan, RN, an independent consultant, who contributed to the preparation and formatting of the manuscript, and Dr Donald Fetterolf, Stan Harris and Claudine Carnevale from MiMedx® for their assistance with the compilation of study data.
The authors have the following disclosures: Dr CMZ reports no conflicts of interest, although the Professional Education and Research Institute of which he is Medical Director received funding for completion of the study and his role as principal investigator. Dr TES has served as a principal investigator for MiMedx and has received research funds. Dr WWL has provided consultative services to MiMedx. Dr MJC has provided consultative services to MiMedx. All other authors have no potential conflicts to disclose.
The authors would like to thank Morgan Stepanek, BS, and Lynne Baker, RN, the clinical research coordinator and research nurse, respectively, from the Professional Education and Research Institute for their clinical and administrative support.
References
- 1. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4–14; doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association . Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boulton AJ, Armstrong DG, Albert SF, Frykberg RG, Hellman R, Kirkman MS, Lavery LA, Lemaster JW, Mills JL Sr, Mueller MJ, Sheehan P, Wukich DK, American Diabetes Association , American Association of Clinical Endocrinologists . Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the ADA, with endorsement by the AACE. Diabetes Care 2008;8:1679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Driver VR, de Leon JM. Health economic implications for wound care and limb preservation. J Manag Care Med 2008;11:13–9. [Google Scholar]
- 5. Snyder R. Wound percent area reduction and making decisions about utilizing advanced therapies. Podiatry Manage 2010;29:197–201. [Google Scholar]
- 6. Steed DL, Attinger C, Colaizzi T, Crossland M, Franz M, Harkless L, Johnson A, Moosa H, Robson M, Serena T, Sheehan P, Veves A, Wiersma‐Bryant L. Guidelines for the treatment of diabetic ulcers. Wound Repair Regen 2006;14:680–92. [DOI] [PubMed] [Google Scholar]
- 7. Albert S. Cost‐effective management of recalcitrant diabetic foot ulcers. Clin Podiatr Med Surg 2002;19:483–91. [DOI] [PubMed] [Google Scholar]
- 8. Ho C, Tran K, Hux M, Sibbald G, Campbell K. Artificial skin grafts in chronic wound care: a meta‐analysis of clinical efficacy and a review of cost‐effectiveness [Tech. Rep. No. 52]. Ottawa: Canadian Coordinating Office for Health Technology Assessment, 2005.
- 9. Veves A, Falanga V, Armstrong DG, Sabolinski ML. Apligraf diabetic foot ulcer study. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001;24:290–5. [DOI] [PubMed] [Google Scholar]
- 10. Marston WA, Hanft J, Norwood P, Pollak R, Dermagraft Diabetic Foot Ulcer Study Group . The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 2003;26:1701–5. [DOI] [PubMed] [Google Scholar]
- 11. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomized comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J 2013;10:502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fetterolf DE, Istwan NB, Stanziano GJ. An evaluation of healing metrics associated with commonly used advanced wound care products for the treatment of chronic diabetic foot ulcers. Manag Care 2014;23:31–8. [PubMed] [Google Scholar]
- 13. FDA (US Food and Drug Administration) . Apligraf, labeling, approval order, summary of safety and effectiveness. PMA # P950032, Docket # 00M‐1508; 2000. Sep 11. URL http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm [accessed on 4 September 2014]
- 14. Koob TJ, Rennert R, Zabek N, Massee M, Lim JJ, Temenoff JS, Li WW, Gurtner G. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J 2013;10:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maan ZN, Rennert RC, Koob TJ, Januszyk M, Li WW, Gurtner GC. Cell recruitment by amnion chorion grafts promotes neovascularization. J Surg Res 2014; pii: S0022‐4804(14)00803‐8; doi: 10.1016/j.jss.2014.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zelen CM. An evaluation of dehydrated human amniotic membrane allografts in patients with DFUs. J Wound Care 2013;22:347–8 350–51. [DOI] [PubMed] [Google Scholar]
- 17. Zelen CM, Serena TE, Snyder RJ. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int Wound J 2014;11:122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Centers for Medicare and Medicaid Services . Medicare Part B drug average sales price, July 2014 ASP drug pricing files 09/03/14. URL http://www.cms.gov/Medicare/Medicare‐Fee‐for‐Service‐Part‐B‐Drugs/McrPartBDrugAvgSalesPrice/2014ASPFiles.html [accessed on 24 September 2014]
- 19. Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care 2007;20:493–508, quiz 509–10. [DOI] [PubMed] [Google Scholar]
- 20. Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen 2011;19:134–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li WW, Talcott KE, Zhai AW, Kruger EA, Li VW. The role of therapeutic angiogenesis in tissue repair and regeneration. Adv Skin Wound Care 2005;18:491–500. [DOI] [PubMed] [Google Scholar]
- 22. Wojtowicz AM, Oliveira S, Carlson MW, Zawadzka A, Rousseau CF, Baksh D. The importance of both fibroblasts and keratinocytes in a bilayered living cellular construct used in wound healing. Wound Repair Regen 2014;22:246–55; doi: 10.1111/wrr.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brem H, Young J, Tomic‐Canic M, Isaacs C, Ehrlich HP. Clinical efficacy and mechanism of bilayered living human skin equivalent (HSE) in treatment of diabetic foot ulcers. Surg Technol Int 2003;11:23–31. [PubMed] [Google Scholar]
- 24. Osborne CS, Schmid P. Epidermal‐dermal interactions regulate gelatinase activity in Apligraf, a tissue‐engineered human skin equivalent. Br J Dermatol 2002;146:26–31. [DOI] [PubMed] [Google Scholar]
- 25. John T. Human amniotic membrane transplantation: past, present, and future. Ophthalmol Clin North Am 2003;16:43–65. [DOI] [PubMed] [Google Scholar]
- 26. Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater 2008;15:88–99. [DOI] [PubMed] [Google Scholar]
- 27. Koob TJ, Lim JJ, Massee M, Zabek N, Denozière G. Properties of dehydrated human amnion/chorion composite grafts: implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater 2014;102:1353–62; doi: 10.1002/jbm.b.33141. [DOI] [PubMed] [Google Scholar]
- 28. Chen Z, Wang Y, Shi C. Therapeutic implications of newly identified stem cell populations from the skin dermis. Cell Transplant 2014,[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 29. Kokai LE, Marra K, Rubin JP. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Transl Res 2014;163:399–408; doi: 10.1016/j.trsl.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 30. Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, Nedeau A, Thom SR, Velazquez OC. Diabetic impairments in NO‐mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF‐1 alpha. J Clin Invest 2007;117:1249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Badillo AT, Chung S, Zhang L, Zoltick P, Liechty KW. Lentiviral gene transfer of SDF‐1alpha to wounds improves diabetic wound healing. J Surg Res 2007;143:35–42. [DOI] [PubMed] [Google Scholar]
- 32. Koob TJ, Lim JJ, Zabek N, Massee M. Cytokines in single layer amnion allografts compared to multi‐layered amnion/chorion allografts for wound healing. J Biomed Mater Res B Appl Biomater 2014; doi: 10.1002/jbm.b.33265 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 33. Langer A, Rogowski W. Systematic review of economic evaluations of human cell‐derived wound care products for the treatment of venous leg and diabetic foot ulcers. BMC Health Serv Res 2009;9:115; doi: 10.1186/1472-6963-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]


