Abstract
Silver nanoparticles are gaining importance as an antimicrobial agent in wound dressings. Chitin is a biopolymer envisioned to promote rapid dermal regeneration and accelerate wound healing. This study was focused on the evaluation of chitin membranes containing silver nanoparticles for use as an antimicrobial wound dressing. Silver nanoparticles were synthesised by gamma irradiation at doses of 50 kGy in the presence of sodium alginate as stabiliser. The UV–Vis absorption spectra of nanoparticles exhibited an absorption band at 415–420 nm, which is the typical plasmon resonance band of silver nanoparticles. The peaks in the X‐ray diffraction (XRD) pattern are in agreement with the standard values of the face‐centred cubic silver. Transmission electron microscopy (TEM) images indicate silver nanoparticles with spherical morphology and small particle size in the range of 3–13 nm. In vitro antimicrobial tests were performed using Pseudomonas aeruginosa and Staphylococcus aureus to determine the antimicrobial efficiency of the chitin membranes containing 30, 50, 70 and 100 ppm nanosilver. No viable counts for P. aeruginosa were detected with 70 ppm silver nanoparticles dressing after 1‐hour exposure. A 2‐log reduction in viable cell count was observed for S. aureus after 1 hour and a 4‐log reduction after 6 hours with 100 ppm nanosilver chitin membranes. This study demonstrates the antimicrobial capability of chitin membranes containing silver nanoparticles. The chitin membranes with 100 ppm nanosilver showed promising antimicrobial activity against common wound pathogens.
Keywords: Antimicrobial activity; Chitin; Silver nanoparticles; Wound dressing
Introduction
Wounds often provide a favourable environment for the colonisation of micro‐organisms. High bacterial levels interfere with the progression of wound healing (1). Infection is a leading cause of morbidity and mortality in extensive burn injuries, traumatic injuries and surgical procedures. The control of infection remains a major challenge in wound management. In order to improve the opportunity for wound healing, it is important to create conditions that are unfavourable to micro‐organisms and favourable for the host repair mechanisms. Consequently, broad‐spectrum antimicrobial agents are required to control infection and facilitate the healing process.
Silver has been used for centuries in the form of metallic silver, silver nitrate and silver sulfadiazine for the treatment of burns, wounds and several bacterial infections (2). The increasing level of bacterial resistance to traditional antibiotics and the isolation of organisms with minimal antibiotic sensitivity are driving the current interest in silver products for chronic wound care. Ionic silver is a potent antimicrobial agent and its use in health care has increased in recent years as a consequence of escalating bacterial resistance to antibiotics. The proven antimicrobial activity of ionic silver includes its broad‐spectrum activity against Gram‐positive and Gram‐negative micro‐organisms 3, 4 and also its antibacterial effects on antibiotic‐resistant bacteria, such as methicillin‐resistant Staphylococcus aureus (MRSA) and vancomycin‐resistant enterococci (VRE) 1, 5. In addition to the recognised antimicrobial properties, silver is also reported to promote wound healing (6).
The emergence of nanotechnology has provided a new therapeutic modality of silver nanoparticles for use in wounds. Silver nanoparticles are reported to possess antibacterial (7), antifungal (8), antiviral (9), anti‐inflammatory (10), antiangiogenesis (11) and antiplatelet activity (12). Nanoparticles play a crucial role in inhibiting microbial growth because of their high reactivity due to the large surface to volume ratio. Several mechanisms have been postulated for the antimicrobial activity of silver nanoparticles. The adhesion of nanoparticles to the surface causes deformation of the membrane, which leads to an increase in membrane permeability. The nanosilver particles penetration into the bacterial cell results in the DNA damages. The dissolution of nanosilver releases antimicrobial silver ions. The free silver ion bind to the thiol (SH) groups of cellular components, such as protein causing inactivation of bacteria 13, 14. Nanocrystalline technology gives the highest, sustained release of silver to a wound without clear risk of toxicity (15). The silver ion is released into the wound for long‐lasting bactericidal effect in a controlled fashion. Hence, nanosilver has found tremendous applications in the field of antimicrobials and therapeutics (16). Nanosilver dressings can play an important role in the healing of wounds due to their antimicrobial effects. This study focuses on the preparation and evaluation of chitin membranes containing silver nanoparticles for use as an antimicrobial wound dressing.
Materials and methods
Preparation of chitin–nanosilver dressing
Silver nanoparticles were synthesised by gamma irradiation using 10 mM AgNO3. Sodium alginate as stabiliser and isopropanol as a scavenger of hydroxyl radicals were used (17). The solution was irradiated with 60Co Gamma rays to 50 kGy under ambient conditions. UV–Vis spectroscopy, X‐ray diffraction (XRD) and transmission electron microscopy (TEM) was used to characterise the silver nanoparticles. UV‐Visible absorption spectra were recorded using SPECORD S600 UV–Vis Spectrophotometer (Analytik Jena AG, Jena, Germany). XRD patterns were recorded on a Panalytical, X'pertPRO Diffractometer with a Cu Kα source. TEM was used to characterise the morphology of the silver nanoparticles. TEM images were taken with a JEOL 2100 HRTEM (JEOL Ltd., Akishima, Japan) operating at 200kV. 30, 50, 70 and 100 ppm nanosilver was added to the chitin in 5% lithium chloride and dimethylacetamide solvent system. Dressing films were caste on flat glass plates.
Antimicrobial assay of chitin–nanosilver dressing
The antimicrobial activity of nanosilver dressings was tested quantitatively by viable cell count method. Two clinically relevant bacteria–Gram‐negative Pseudomonas aeruginosa (ATCC No. 9027) and Gram‐positive S. aureus (ATCC No. 6538) were used. The bacteria were grown in nutrient broth at 32°C for 18 hours. The inocula were prepared as a suspension representing 105–106 CFU/ml. The nanosilver dressings were immersed in Tryptone Soya Broth inoculated with 104–105 CFU/ml of the test organism. The broths were incubated and samples were withdrawn at periodic intervals. The solutions were serially diluted and plated for viable counts using Soyabean Casein Digest Agar medium. The number of surviving bacteria was determined in the presence of chitin membranes containing 0, 30, 50, 70 and 100 ppm nanosilver and without any membrane.
Statistical analysis
Results of antimicrobial assay were represented as mean ± SD (N = 4). Software SPSS 16.0 (SPSS Inc., Chicago, IL) was used for statistical analysis. One‐way ANOVA followed by Duncan post hoc test was used to identify differences between groups.
Results
Silver nanoparticles were prepared by gamma irradiation method. The formation of the silver nanoparticles was monitored using UV–Vis absorption spectroscopy. The UV–Vis absorption spectra of silver nanoparticles prepared by gamma irradiation at doses of 50 kGy and stabilised by sodium alginate is presented in Figure 1. The absorption spectrum shows the formation of silver nanopartícles by exhibiting the typical surface plasmon absorption maxima at 415–420 nm. XRD analysis was also performed to confirm the crystal phase of silver nanoparticles. Figure 2 shows XRD pattern of the silver nanoparticles supported on glass substrate. The reflection peaks can be indexed to face‐centred cubic silver, as indicated by diffraction peaks (111), (200), (220) and (311). The average size and morphology of silver nanoparticles were determined by TEM. TEM photograph (Figure 3A) indicates that the nanoparticles with spherical morphology are well dispersed. Histograms of size distribution were calculated from the TEM images by measuring the diameters of 100 particles. The particle size histograms of silver particles (Figure 3B) show that the particles range in size from 3–13 nm.
Figure 1.
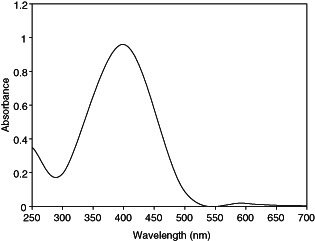
UV–Vis absorption spectrum of silver nanoparticles prepared by gamma radiation.
Figure 2.
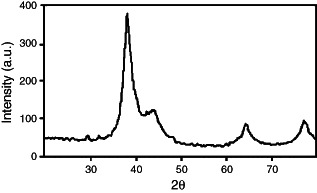
XRD pattern of silver nanoparticles prepared by gamma radiation.
Figure 3.
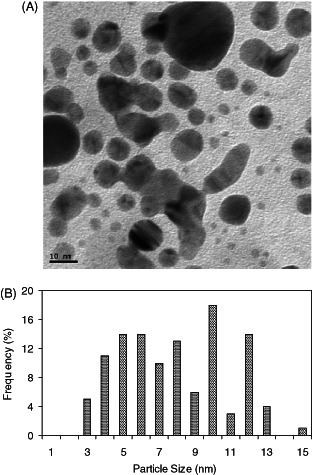
(A) TEM image of silver nanoparticles, (B) histogram of particle size distribution.
The antimicrobial efficacy of the chitin membranes containing nanosilver against Gram‐negative bacteria, P. aeruginosa is presented in Figure 4. The initial counts of P. aeruginosa in the broth was 5·16 ± 0·25 log CFU/ml. About 1‐log reduction in the counts was observed in the presence of 30 ppm nanosilver dressing after 1 hour of exposure. With increasing exposure time, the counts were significantly reduced (P < 0·01) and no viable counts were detected after 4 hours. About 4‐log reduction in the counts were observed with 50 ppm nanosilver dressing after 1 hour and complete killing was observed at 3 hours. No viable counts for P. aeruginosa were detected at 70 and 100 ppm nanosilver dressing after 1‐hour exposure. The counts in the broth after 24 hours in the absence of nanosilver dressings were 7·53 ± 0·27 log CFU/ml. However, no counts were detected (P < 0·001) in the presence of chitin membranes containing 30–100 ppm nanosilver after 24 hours.
Figure 4.
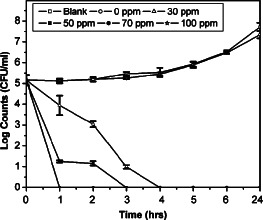
Effect of chitin membranes containing nanosilver on Pseudomonas aeruginosa. Data presented are mean ± SD, N = 4.
The effect of chitin nanosilver membranes on Gram‐positive bacteria S. aureus is presented in Figure 5. Bactericidal effect was significant (P < 0·01) in the presence of chitin nanosilver dressings, whereas the counts progressively increased in the absence of nanosilver dressings. About 3‐log reduction in cell counts was observed with 30 ppm nanosilver dressing after 6 hours as compared with counts in the absence of chitin nanosilver membranes. Maximum bactericidal effect was observed with 100 ppm nanosilver dressing. A 2‐log reduction in viable cell count was observed for S. aureus after 1 hour and a 4‐log reduction after 6 hours with 100 ppm nanosilver membranes. More than 99·9% reduction (P < 0·001) in the viable cell counts was observed after 24 hours in the presence of 100 ppm nanosilver dressings. Comparison of the microbial reduction rates in this study shows the presence of Gram‐negative bacteria P. aeruginosa to be more susceptible to the antimicrobial effects of nanosilver than Gram‐positive S. aureus.
Figure 5.
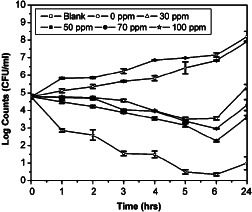
Effect of chitin membranes containing nanosilver on Staphylococcus aureus. Data presented are mean ± SD, N = 4.
Discussion
Antimicrobial dressings reduce wound bioburden, decrease the risk of infection and create an environment that supports the normal healing process 1, 18. Silver is commonly used in wound dressings and topical formulations to assist in the management of wounds that are infected or at the risk of infection. They provide potent broad‐spectrum antimicrobial activity 4, 19. Percival et al. (19) have demonstrated the efficacy of silver alginate and silver carboxymethyl cellulose dressing against planktonic S. aureus and P. aeruginosa. Silver dressings are used to remove or reduce an increasing bioburden in burns and open wounds, or to act as a barrier against cross contamination of resistant organisms such as MRSA. Silver‐releasing dressings are reported to have a positive effect on tissue management, control of infection and inflammation, moisture balance, epithelial advancement and are cost‐effective (20). Nanotechnology is gaining tremendous impetus in the present century due to its capability of modulating metals into their nanosize, which drastically changes the chemical, physical and optical properties of metals. Silver nanoparticles have high reactivity due to the large surface to volume ratio and have emerged up with diverse medical applications including wound dressings.
Silver nanoparticles were synthesised by gamma radiation in the presence of sodium alginate as stabiliser. A number of chemical and physical approaches are available for the synthesis of silver nanoparticles. The radiation technique offers many advantages and has proven to be a convenient method to prepare size‐controllable metal nanoparticles 21, 22. Hydrated electrons generated over the course of irradiation reduce metal ions to zero‐valent metal particles. The growth of silver nanoparticles by reduction of Ag+ to Ag is stepwise (23). Silver atoms primarily reduced by hydrated electrons rapidly combine with silver ions to form dimmer clusters. Continued reduction of the Ag+ solution causes the aggregation of tetramer clusters into nanoparticles. The metallic clusters and the nanoparticles formed via gamma irradiation are capped with the sodium alginate chain. Sodium alginate is a natural polymer with excellent biocompatibility. The sodium alginate stabilised Ag nanoparticles, therefore, offer benefits of compatibility for biomedical applications. The nanoparticles were examined using UV‐Visible Spectroscopy, XRD and TEM. The UV–Vis absorption spectra of silver nanoparticles exhibited an absorption band at 415–420nm, which is the typical plasmon resonance band of silver nanoparticles (24). The narrow band range indicates a narrow size distribution of the silver nanoparticles. The synthesised nanoparticles were characterised by XRD. The peaks in the XRD pattern are in agreement with the standard values of the face‐centred cubic silver. Strong reflections around 38°, 44°, 65° and 78° are characteristic of (111) (200) (220) and (311) planes of face centred cubic (FCC) of the silver nanoparticles (25). The wide peaks indicate the small size of the silver nanoparticles (26). According to the Scherer equation, the average size of the silver particles calculated is 3 nm. TEM studies also suggest the formation of silver nanoparticles of size 3–13 nm.
Wound dressings containing silver have been in widespread use for many years. Topical silver creams and solutions, and other topical antimicrobial preparations require frequent application, are care intensive to apply and remove and are sometimes painful. In contrast to the topical silver agents, the dressings control the release of silver to the wound and are required to be changed with less frequency. Therefore, wound dressings containing silver as antimicrobial agent are being developed for wound care in recent years. Silver dressings are used extensively for wound management, particularly in burn wounds, chronic leg ulcers, diabetic wounds and traumatic injuries 27, 28. Caruso et al. (27) have reported Aquacel Ag containing 1·2% w/w silver to be beneficial for the management of partial‐thickness burns. Karlsmark et al. (28) evaluated the clinical performance of sustained silver‐releasing dressing, Contreet Foam comprising of soft hydrophilic polyurethane foam. The dressing was found to be safe and performed well for the treatment of chronic exuding venous leg ulcers, combining effective antibacterial properties with excellent exudate management. These dressings vary in containing compounds of silver nitrate or sulphadiazine, to sustained silver‐ion release preparation and silver‐based crystalline nanoparticles. The dressing component also varies, as nylon, mesh, hydrocolloid or methylcellulose. Chitin is a natural polymer that possesses excellent properties that are advantageous for wound dressing (29). The repeat unit N‐acetyl‐glucosamine occurs in hyaluronic acid that is responsible for the formation of fibrous networks for protein attachment during wound healing. Chitin has been found to have an accelerating effect on the wound‐healing process (30). The incorporation of silver nanoparticles in biopolymer chitin is therefore envisioned to play an important role in the wound management. In the present investigation, nanosilver‐incorporated chitin dressings were prepared and evaluated for their antimicrobial activity. Chitin dressing containing 100 ppm nanosilver completely inactivated viable cells of P. aeruginosa within 1 hour. S. aureus was reduced by about 2 log units within the same period. The difference of Gram‐positive and Gram‐negative bacteria in terms of antibacterial activity of nanosilver can be attributed to the cell wall content. The cell walls of Gram‐positive bacteria are known to contain 3–20 times more peptidoglycan than Gram‐negative bacteria (31). As peptidoglycans are negatively charged, they probably bind to some portion of silver ion. Therefore, silver is supposed to exhibit better antibacterial activity against Gram‐negative bacteria (32).
The results of this study demonstrate the potential of the chitin membranes containing 100 ppm silver nanoparticles for use as an antimicrobial wound dressing. The chitin dressings containing silver nanoparticles demonstrated in vitro antimicrobial effect against wound‐related micro‐organisms including Gram‐positive and Gram‐negative bacteria.
References
- 1. Parsons D, Bowler PG, Myles V, Jones S. Silver antimicrobial dressings in wound management: a comparison of antibacterial, physical, and chemical characteristics. Wound 2005;17:222–32. [Google Scholar]
- 2. Klasen HJ. Historical review of the use of silver in the treatment of burns. I. Early uses. Burns 2000;26:117–30. [DOI] [PubMed] [Google Scholar]
- 3. Percival SL, Thomas JG, Slone W, Linton S, Corum L, Okel T. The efficacy of silver dressings and antibiotics on MRSA and MSSA isolated from burn patients. Wound Repair Regen 2011;19:767–74. [DOI] [PubMed] [Google Scholar]
- 4. Singh R, Kumar D, Kumar P, Chacharkar MP. Development and evaluation of silver‐impregnated amniotic membrane as an antimicrobial burn dressing. J Burn Care Res 2008;29:64–72. [DOI] [PubMed] [Google Scholar]
- 5. Lansdown AB. Silver. I: Its antibacterial properties and mechanism of action. J Wound Care 2002;11:125–30. [DOI] [PubMed] [Google Scholar]
- 6. Beele H, Meuleneire F, Nahuys M, Percival SL. A prospective randomized open label study to evaluate the potential of a new silver alginate/carboxymethylcellulose antimicrobial wound dressing to promote wound healing. Int Wound J 2010;7:262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li WR, Xie XB, Shi QS, Zeng HY, OU‐Yang YS, Chen YB. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli . Appl Microbiol Biotechnol 2010;85:1115–22. [DOI] [PubMed] [Google Scholar]
- 8. Medina‐Ramirez I, Bashir S, Luo Z, Liu JL. Green synthesis and characterization of polymer‐stabilized silver nanoparticles. Colloids Surf B Biointerfaces 2009;73:185–91. [DOI] [PubMed] [Google Scholar]
- 9. Nadworny PL, Wang J, Tredget EE, Burrell RE. Anti‐inflammatory activity of nanocrystalline silver in a porcine contact dermatitis model. Nanomedicine 2008;4:241–51. [DOI] [PubMed] [Google Scholar]
- 10. Nadworny PL, Landry BK, Wang JF, Tredget EE, Burrell RE. Does nanocrystalline silver have a transferable effect? Wound Repair Regen 2010;18:254–65. [DOI] [PubMed] [Google Scholar]
- 11. Rogers JV, Parkinson CV, Choi YW, Speshock JL, Hussain SM. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res Lett 2008;3:129–33. [Google Scholar]
- 12. Gurunathan S, Lee KJ, Kalishwaralal K, Sheikpranbabu S, Vaidyanathan R, Eom SH. Antiangiogenic properties of silver nanoparticles. Biomaterials 2009;30:6341–50. [DOI] [PubMed] [Google Scholar]
- 13. Sedlarik V, Galya T, Sedlarikova J, Valasek P, Saha P. The effect of preparation temperature on the mechanical and antibacterial properties of poly (vinyl alcohol)/silver nitrate films. Polym Degrad Stabil 2010;95:399–404. [Google Scholar]
- 14. Lee SM, Lee BS, Byun TG, Song KC. Preparation and antibacterial activity of silver‐doped organic–inorganic hybrid coatings on glass substrates. Colloids Surf A 2010;355:167–71. [Google Scholar]
- 15. Leaper DJ. Silver dressings: their role in wound management. Int Wound J 2006;3:282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 2009;27:76–83. [DOI] [PubMed] [Google Scholar]
- 17. Liu Y, Chen S, Zhong L, Wu G. Preparation of high‐stable nanoparticle dispersion by using sodium alginate as a stabilizer under gamma radiation. Radiat Phys Chem 2009;78:251–5. [Google Scholar]
- 18. Collier M. Silver dressings: more evidence is needed to support their widespread use. J Wound Care 2009;18:77–8. [PubMed] [Google Scholar]
- 19. Percival SL, Slone W, Linton S, Okel T, Corum L, Thomas JG. Use of flow cytometry to compare the antimicrobial efficacy of silver‐containing wound dressings against planktonic Staphylococcus aureus and Pseudomonas aeruginosa . Wound Repair Regen 2011;19:436–41. [DOI] [PubMed] [Google Scholar]
- 20. Lo SF, Hayter M, Chang CJ, Hu WY, Lee LL. A systematic review of silver‐releasing dressings in the management of infected chronic wounds. J Clin Nurs 2008;17:1973–85. [DOI] [PubMed] [Google Scholar]
- 21. Kang YO, Choi SH, Gopalan A, Lee K, Kang HD, Song YS. Tuning of morphology of Ag nanoparticles in the Ag/polyaniline nanocomposites prepared by γ‐ray irradiation. J Non-Cryst Solids 2006;352:463–8. [Google Scholar]
- 22. Liu FK, Hsu YC, Tsai MH, Chu TC. Using γ‐irradiation to synthesize Ag nanoparticles. Mater Lett 2007;61:2402–5. [Google Scholar]
- 23. Mostafavi M, Dey GR, Francuois L, Belloni J. Transient and stable silver clusters induced by radiolysis in methanol. J Phys Chem A 2002;106:10184–94. [Google Scholar]
- 24. Scholes FH, Furman SA, Lau D, Rossouw CJ, Davis TJ. Fabrication of photo‐patterned microstructures in an organic–inorganic hybrid material incorporating silver nanoparticles. J Non-Cryst Solids 2004;347:93–9. [Google Scholar]
- 25. Thomas V, Murali Mohan Y, Sreedhar B, Bajpai SK. Fabrication, characterization of chitosan/ nanosilver film and its potential antibacterial application. J Biomater Sci A 2009;20:2129–44. [DOI] [PubMed] [Google Scholar]
- 26. Prasad V, D'Souza C, Yadav D, Shaikh AJ, Vigneshwaran N. Spectroscopic characterization of zinc oxide nanorods synthesized by solid‐state reaction. Spectrochim Acta A 2006;65:173–8. [DOI] [PubMed] [Google Scholar]
- 27. Caruso DM, Foster KN, Hermans MH, Rick C. Aquacel Ag in the management of partial‐thickness burns: results of a clinical trial. J Burn Care Rehabil 2004;25:89–97. [DOI] [PubMed] [Google Scholar]
- 28. Karlsmark T, Agerslev RH, Bendz SH, Larsen JR, Roed‐Petersen J, Andersen KE. Clinical performance of a new silver dressing, Contreet foam, for chronic exuding venous leg ulcers. J Wound Care 2003;12:351–4. [DOI] [PubMed] [Google Scholar]
- 29. Singh R, Chacharkar MP, Mathur AK. Chitin membrane for wound dressing application ‐ preparation, characterization and toxicological evaluation. Int Wound J 2008;5:665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yusof NL, Wee A, Lim LY, Khor E. Flexible chitin films as potential wound‐dressing materials: wound model studies. J Biomed Mater Res 2003;66:224–32. [DOI] [PubMed] [Google Scholar]
- 31. Kawahara K, Tsuruda K, Morishita M, Uchida M. Antibacterial effect of silver‐zeolite on oral bacteria under anaerobic conditions. Dent Mater 2000;16:452–5. [DOI] [PubMed] [Google Scholar]
- 32. Basri H, Ismail AF, Aziz M, Nagai K, Matsuura T, Abdullah MS, Ng BC. Silver‐filled polyethersulfone membranes for antibacterial applications ‐ effect of PVP and TAP addition on silver dispersion. Desalination 2010;261:264–71. [Google Scholar]


