Abstract
Necrotising fasciitis (NF) is a destructive invasive infection of skin, subcutaneous tissue and deep fascia. The aim of the study is to determine the causative agents of NF, its localisation, predisposing factors and comorbid conditions, duration of treatment and distribution of NF in different age groups and over the years. We conducted a retrospective study including 22 patients with NF from 2005 to 2010 in the University Clinical Center of Kosovo. The data were collected and analysed from the archives and protocols of the University Clinical Center of Kosovo. The average age of patients was 56·9 years. In eight cases or 36·4% of total patients studied, NF was caused by monobacterial agents with a predominance of Pseudomonas aeruginosa (five cases or 22·7% of total infections). Polybacterial agents were responsible for NF infection in other 14 cases (63·6%). Majority of the patients had other comorbidities like diabetes, trauma and prior history of surgical interventions. Diabetes was present in 17 patients or 77·3%. The remaining five patients (22·7%) had previous trauma and recent surgical intervention. Average length of treatment was 43 days. The hospital mortality rate in our case series was 22·6%. Early identification and diagnosis of NF significantly improves outcome and reduces mortality.
Keywords: Causative agents, Immunosuppression, Necrotising fasciitis
Introduction
In 1952, Wilson coined the term necrotising fasciitis (NF) to describe a rapidly progressive inflammation and necrosis of subcutaneous tissue and fascia. Until then, the disease had been described under various nomenclatures, such as haemolytic gangrene, acute streptococcal gangrene, gangrenous erysipelas, necrotising erysipelas, suppurative fasciitis and hospital gangrene 1, 2, 3. NF is a destructive invasive infection of skin, subcutaneous tissue and deep fascia (4). Advanced age, trauma, diabetes, immunosuppression and chronic systemic diseases (hypertension, atherosclerosis and renal failure) are considered as predisposing factors. NF has multiple causes and multiple bacteria are often involved 5, 6, 7, 8. Case‐fatality rates for NF may exceed 30% and have remained high despite advances in the care of these patients. Optimal management depends on prompt diagnosis with identification of the causative organisms and appropriate therapy in association with surgical debridement (9).
Treatment includes early surgical debridement, parenteral antibiotics and nutritional support. Given the atypical presentation of this disease, there is a delay in diagnosis and appropriate treatment. NF is easily and very often confused with other soft tissue infections, and this can also contribute to increased morbidity and mortality rates 10, 11, 12.
Objective
The objective of this study is to understand the role of the causative agents of NF, the localisation, predisposing factors, comorbid conditions, duration of treatment and distribution of NF in 22 patients in a 6‐year period.
Materials and methods
This is a retrospective study that included 22 patients with clinical signs of NF during the period of 2005 to 2010 at the Department of Plastic and Reconstructive Surgery, University Clinical Center of Kosovo. The data were collected and analysed from the archives and protocols of the University Clinical Center of Kosovo.
Results
We found that diabetes mellitus, trauma and surgery were the most important predisposing factors. NF was predominant in female patients with 86·4% and only 13·6% in male patients (Table 1)
Table 1.
General characteristics of patients with necrotising fasciitis
| Gender | No | % |
|---|---|---|
| Male | 3 | 13·6 |
| Female | 19 | 86·4 |
| Age groups | ||
| 30–39 | 2 | 9·1 |
| 40–49 | 4 | 18·2 |
| 50–59 | 6 | 27·3 |
| 60+ | 10 | 45·5 |
| Mean ± SD | 56·9 ± 10·0 | – |
Regarding the age group, the highest rate of infection was found to be in the age group over 60 with 45·5%, followed by the age group from 51 to 60 years with 27·3%, 41 to 50 years 18·2% and 31 to 40 years 9·1%. There were no documented cases under the age of 30. The average age of patients was 56·9 years (Table 1).
In eight cases (36·4%) NF was caused by monobacterial agents with a predominance of Pseudomonas aeruginosa (five cases or 22·7% of total patients), whereas in 14 patients the cause was found to be polybacterial (63·6%) (2, 3).
Table 2.
Commonly identified bacteria
| Monobacterial | Polibacterial | ||||
|---|---|---|---|---|---|
| Name | No | % | Name | No | % |
| Pseudomonas | 5 | 22·7 | Staphylococcus aureus | 9 | 40·9 |
| Escherichia coli | 3 | 13·6 | Pseudomonas | 6 | 27·2 |
| Staphylococcus aureus | 1 | 4·5 | Klebsiella | 5 | 22·7 |
| Escherichia coli | 2 | 9 | |||
| Proteus | 2 | 9 | |||
| Enterobacter | 2 | 9 | |||
| Citrobacter | 2 | 9 | |||
Table 3.
Localisation, cause, accompanying factors of necrotising fasciitis
| Localisation by the regions | No | % |
|---|---|---|
| Inguinal region | 12 | 54·5 |
| Abdomen region | 4 | 18·2 |
| Lower extremities | 4 | 18·2 |
| Cervical region | 1 | 4·5 |
| Upper extremities | 1 | 4·5 |
| Cause | ||
| Monobacterial | 8 | 36·4 |
| Polybacterial | 14 | 63·6 |
| Accompanying factors | ||
| Diabetes | 17 | 77·3 |
| Trauma and surgical intervention | 5 | 22·7 |
Accompanying chronic systemic diseases such as diabetes were present in 17 cases (77·3%), while in other 5 cases (22·7%) the predisposing factors were previous trauma and surgical interventions (Table 3).
Duration of treatment in nine cases (40·9%) was over 40 days, with the maximum duration of hospitalisation up to 118 days, whereas in six cases (27·2%) duration was only 20 days. In seven cases (31·8%), hospitalisation lasted up to 40 days. The average hospitalisation was 43 days (Table 4). Twenty cases (90·9%) underwent surgical intervention (debridement, necrectomy followed by reconstruction of the defects) up to five times, while only two patients (9·1%) were operated over five times (1, 2, 3, 4). On average these patients had three surgical interventions each (Table 4).
Table 4.
Duration of hospitalisation and number of surgical interventions
| Duration of hospitalisation | % | |
|---|---|---|
| <20 day | 6 | 27·3 |
| 20–40 days | 7 | 31·8 |
| >40 days | 9 | 40·9 |
| Range | 15–118 days | |
| Number of surgical interventions | ||
| 5 times | 20 | 90·9 |
| Over 5 times | 2 | 9·1 |
| Mean | 3 times | – |
Figure 1.
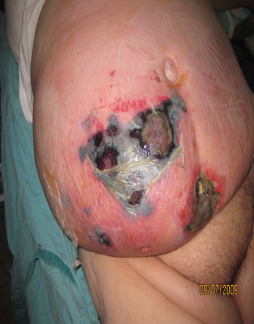
A 35‐year old female with necrotising fasciitis of abdominal region, suffering from diabetes.
Figure 2.
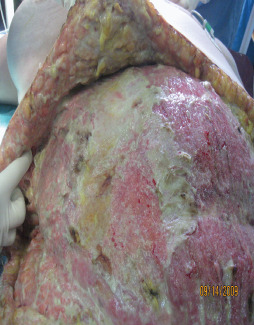
Wound after radical debridement.
Figure 3.
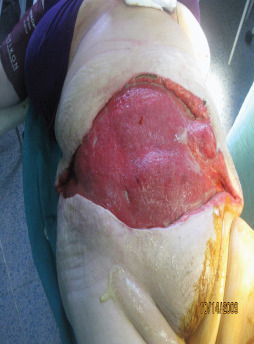
Wound 1 month after topical treatment.
Figure 4.
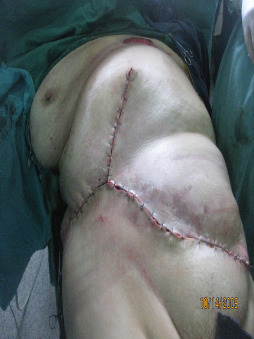
Direct closure of the wound after the surgery where the patient survived the disease.
The most frequent location was the inguinal region in which the infection often propagated up to the abdominal wall and below to the femoral region, and down to the level of the knee in 12 cases (54·5%). Other localisations were rare and had the following distribution: the region of the abdomen in four cases (18·2%), the lower extremities in four cases (18·2%), the cervical region in one case (4·5%) and upper extremities in one case (4·5%) (Table 3).
Distribution of NF across the years was as follows: 2010 was the year where the incidence of NF was the highest with seven cases (31·8%), 2009 with six cases (27·2%), 2008 with three cases (13·6%), 2007 with two cases (9·1%), 2006 with four cases (18·1%) and 2005 with no cases.
Mortality over the years was two cases in 2006 (9·1%), in 2007, 2008 and 2010 with one case each (4·5%).
Discussion
NF is a rare but very serious disease with monomicrobial and polymicrobial causes involving subcutaneous tissue and fascia in patients who suffer from diabetes, systemic chronic diseases and immunosuppression. NF is a soft tissue gangrenous infection that is optimally treated by early diagnosis, radical surgical debridement of all involved necrotic tissue, broad spectrum antibiotics and aggressive nutritional support (10).
Similar to other studies, the most common presentations in our series were swelling (89%), pain (82%) and erythema (72%).
The most important finding which was not noted in other studies is the predominance of female gender with 19 cases (86·4%).
Majority of the patients included in our study are women living in rural areas. Detailed history taking and physical examination showed that the most common localisation of the infection (primary focus) is the inguinal area (12 cases or 54·5%), from which the infection often spread towards the abdominal wall and towards the femoral region down to the knee level. Most of these patients were diagnosed late in the course. There are a couple of factors that contributed to this. First of all patients were very hesitant to seek medical attention as their intimate parts of the body were affected and they did not want to expose themselves to the medical staff, so there was a delay in diagnosing the disease. This goes along with the socio‐economic and cultural factors that dominate these rural areas of the country. The second major factor that contributed to this delay in diagnosing and treatment of NF is delayed referral by primary care services. Most of these cases were misdiagnosed and treated as cellulitis.
Furthermore, this discrepancy in Kosovo is probably a result of prior medical conditions and the lack of detailed analysis of patient data.
Since our Oncology Institute is not completely functional yet, women with prior surgical interventions (for malignancies), for example, cervical cancer are forced to seek radiotherapy treatment outside of this country. Tracking these patients and obtaining a complete medical history is very challenging. Another challenge is low socio‐economic level and low level of sexual education in these particular rural areas.
A similar study of 25 patients with NF by Musa et al. reported that the majority of patients (84%) were males (13). The mean age was 56·9 years with an age range between 30 to 80 years. Our data are similar to studies conducted by Rajput et al. (mean age of 57 years) (14). Another study Yeung et al. involved patients with a mean age of 60 years (15).
In 8 cases, the cause was monobacterial (36·4%) with a predominance of P. aeruginosa in 5 cases (22·7%) while in 14 cases the cause agent was polybacterial (63·6%) with a predominance of Staphylococcus aureus in 9 cases (40·9%). In other similar studies, polybacterial infections were found to be more frequent in NF. Mukhopadhyav et al. in their study found that the most frequent monobacterial cause was Streptococcus with 8% of cases, while the most frequent polybacterial cause was Escherichia coli with 52% of cases, P. aeruginosa as polybacterial cause was with 18% of cases, while the Staphylococcus was with 14% of cases (16). Accompanying chronic systemic diseases such as diabetes were seen in 17 cases (77·3%), compared with other similar studies where incidence was higher. Yu et al. in their study found that 53·8% of patients were diabetic (17).
Duration of treatment in nine cases (40·9%) was over 40 days, with the maximum length of hospitalisation 118 days, whereas in six cases (27·3%) was up to 20 days. In 7 cases (31·8%), hospitalisation lasted up to 40 days.
The duration of hospitalisation ranged from 7 to 118 days, with a mean 46 days of hospitalisation. In similar studies, we found approximately the same data. Legbo et al. in a comparative analysis of 56 cases with NF found that the mean duration of hospitalisation was 36 days, ranging from 3 to 126 days (1). Twenty cases (90·9%) underwent surgical intervention (debridement, necrectomy followed by reconstruction of the defects) up to five times, while only two patients (9·1%) were operated over five times. These patients had an average of three surgical interventions each.
The most frequent location was the inguinal region in which the infection often propagated up to the abdominal wall and below the femoral region up to the level of the knee in 12 cases (54·5%). Other localisations of NF were rare and had different distributions; the abdominal region in four cases (18·2%), the lower extremities in four cases (18·2%), the cervical region in one case (4·5%) and upper extremities in one case (4·5%).
The mortality rate reported in the literature varies widely from 9 to 70% 13, 14, 15, 16, 17, 18, 19, 20, 21. The hospital mortality rate in our case series was 22·6%, where all fatal cases suffered from diabetes. This rate is higher than that reported in other contemporary studies of NF. Schnall et al. noted an 11% mortality rate among 99 cases of NF treated at their institution in Los Angeles, California, between 1993 and 1995 (22). Our data are according to a report by Sin et al. who reported a 25% rate of mortality in 15 patients (10). The overall mortality in our study may also have been lower than other published reports. Redman et al. reported in their retrospective study a mortality rate of 33% in 12 patients with NF (23).
Conclusion
Despite antibiotic therapy, surgical intervention and multidisciplinary approach the morbidity and mortality remain high for NF patients. Early identification and diagnosis of NF significantly improve survival and reduce the mortality. In our experience, cases that were diagnosed late had high mortality rate. Our retrospective study shows that the highest incidence of NF was in patients who suffered from diabetes for a long period of time and had other chronic diseases. Also in most patients the cause was polybacterial. On the basis of our own experience as well as from the vast literature search, we have come to the conclusion that early diagnosis, surgical radical debridement and administration of appropriate antibiotics remain the only way to improve the outcome of this life‐threatening disease.
Acknowledgements
We thank the archives department and all the staff of the Department of Plastic Surgery for their technical support. HA designed the manuscript. VZ, HA, VI and AM analysed and interpreted the data. SD and YZ analysed the data and were major contributors in writing the manuscript. MG reviewed and statistically analysed the data. All authors declare no financial and personal relationships with other people or organisations that could inappropriately influence (bias) their work. All authors read and approved the final manuscript.
References
- 1. Legbo J, Shehu B. Necrotizing fasciitis: a comparative analysis of 56 cases. J Natl Med Assoc 2005;97:1692–7. [PMC free article] [PubMed] [Google Scholar]
- 2. Howard RJ. Necrotizing soft tissue infections. In: Schwartz SI, Shires TG, Spencer FC, Daly JM, Fischer JE, Galloway AC, editors. Principles of surgery, 7th edn. New York: McGraw–Hill, 1999:Chapter 5 (CD‐ROM). [Google Scholar]
- 3. Jones J. Investigation upon the nature, causes and treatment of Hospital Gangrene as it prevailed in the confederate armies. New York: Sanitary Commission, Surgical Memoirs of the War of Rebellion, 1871:1861–5. [Google Scholar]
- 4. Nagoba BS, Gandhi RC, Wadher BJ, Gandhi SP, Selkar SP. Citric acid treatment of necrotizing fasciitis: a report of two cases. Int Wound J 2010;7:536–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang VCW, Chen C, Wu H‐H. Necrotizing fasciitis. J Med Sci 2008;28:175–8. [Google Scholar]
- 6. Borchel G, Rees R. Necrotizing soft tissue infections and spider bites. In: Achauer BM, Errisson E, editors. Plastic surgery. 1st edn. Vol. 1. Mosby, 2000:401–4. [Google Scholar]
- 7. Muqim R. Necrotizing fasciitis: management and outcome. I Coll Physicians Surg Pak 2003;13:711–4. [PubMed] [Google Scholar]
- 8. Angelici AM, Nasti AG, Montesano G. Necrotizing fasciitis: our experience. G Chir 2004;25:167–70. [PubMed] [Google Scholar]
- 9. Audard V, Pardon A, Claude O, Jablonski M, Remy P, Desvaux D, Lantieri L, Lang P, Grimbert P. Necrotizing fasciitis during de novo minimal change nephrotic syndrome in a kidney transplant recipient. Transpl Infect Dis 2005;7:89–92. [DOI] [PubMed] [Google Scholar]
- 10. Sin FP, Yuen MC, Lam KW, Wu CW, Tung WK. A retrospective review of patients with necrotizing fasciitis presenting to an emergency department in Hong Kong. Hong Kong J Emerg Med 2002;9: 10–7. [Google Scholar]
- 11. Majeski J, Majeski E. Necrotizing fasciitis: improved survival with early recognition by tissue biopsy and aggressive surgical treatment. South Med J 1997;90:1065–8. [DOI] [PubMed] [Google Scholar]
- 12. Wang KC, Shih CH. Necrotizing fasciitis of the extremities. J Trauma 1992;32:179–82. [DOI] [PubMed] [Google Scholar]
- 13. Musa MT, Khair RZA, Ibrahim EHM. Necrotizing Fasciitis in Khartoum Teaching Hospital. Khartoum Med J 2009:2:211–4. [Google Scholar]
- 14. Rajput A, Waseem, Samad A, Khanzada TW, Shaikh GM, Channa GA. Mortality in necrotizing fasciitis. J Ayub Med Coll Abbottabad 2008;20:96–8. [PubMed] [Google Scholar]
- 15. Yeung YK, Ho ST, Yen CH, Ho PC, Tse WL, Lau YK, Choi KY, Choi ST, Lam MMY, Cheng SHS, Wong TC. Factors affecting mortality in Hong Kong patients with upper limb necrotizing fasciitis. Hong Kong Med J 2011;17:96–104. [PubMed] [Google Scholar]
- 16. Mukhopadhyay M, Saha A, Biswas R, Biswas S. A clinicopathological study of necrotizing fasciitis. AJMS Al Ame en J Med Sci 2011;4:6–13. [Google Scholar]
- 17. Yu C‐M, Huang W‐C, Tung K‐Y, Hsiao H‐T. Necrotizing Fasciitis Risk Factors in Elderly Taiwan Patientsy. Hsiao Int J Gerontol 2011;5:41–4. [Google Scholar]
- 18. Demirag B, Tirelioglu AO, Sarisozen B, Durak K. Necrotizing fasciitis in the lower extremity secondary to the diabetic wounds. Acta Orthop Traumatol Turc 2004;38:195–9. [PubMed] [Google Scholar]
- 19. Wong CH, Wang YS. The diagnosis of necrotizing fasciitis. Curr Opin Infect Dis 2005;18:101–6. [DOI] [PubMed] [Google Scholar]
- 20. Peer SM, Rodrigues G, Kumar S, Khan SA. A clinicopathological study of necrotizing fasciitis – an institutional experience. JCPSP 2007;17:257–60. [PubMed] [Google Scholar]
- 21. Burge TS. Necrotizing fasciitis–the hazards of delay. J R Soc Med 1995;88:342–3. [PMC free article] [PubMed] [Google Scholar]
- 22. Schnall SB. Necrotizing fasciitis; clinical presentation, microbiology, and determinants of mortality. J Bone Joint SurgAm 2004;86A:869. [PubMed] [Google Scholar]
- 23. Redman DP, Friedman B, Law E, Still JM. Experience with necrotizing fasciitis at a burn care center. South Med J 2003;96:868–70. [DOI] [PubMed] [Google Scholar]


