Abstract
Kojic acid and deferiprone are iron chelators used for skin lightening and iron‐overload treatment, respectively. As iron chelation and free radical scavenging are principal factors for wound healing, it was hypothesised that the local application of these compounds might accelerate wound healing in rats. Ointments of 3%, 6% and 9% of deferiprone and kojic acid were prepared and topical treatment was performed on in vivo wound models for 12 days twice in day for test and control groups. Topical treatment with 3%, 6% and 9% showed significant improvement in wound healing after 4 days (P < 0·001). Topical application of 3% and 6% deferiprone enhanced wound healing after 8 days (P < 0·026 and P < 0·001, respectively). Accelerated wound healing was seen using 3% and 6% deferiprone after 12 days (P = 0·003 and P < 0·001, respectively). DPPH scavenging assay was also performed to compare the antioxidant potencies of kojic acid and deferiprone. Deferiprone had more free radical scavenging power than kojic acid. Generally, deferiprone topical treatment, accelerated wound healing more than kojic acid because of its higher antioxidant and iron chelation abilities.
Keywords: Antioxidant, Deferiprone, Iron chelator, Kojic acid, Wound healing
Introduction
Wound healing progresses through the normal healing process which involves homeostasis, inflammation, formation of granulation tissue and reepithelialisation. Any perturbation in this normal pattern manifests phenotypically as a non healing wound (1). Among factors which potentially disturb this process, oxidative stress and inflammation are the major causes of delayed wound healing (2). Oxidative stress is triggered by the Fenton reaction which takes place in a wound and results in the overproduction of reactive oxygen species (ROS) 3, 4. Iron facilitates the generation of highly toxic free radicals via this reaction. This oxidative burst is mainly produced by the activated macrophages and neutrophils which are recruited to the wound site (5). Iron‐catalysed formation of ROS is proposed to be a critical mechanism related to the accelerated apoptosis in injured tissues with poor rate of wound healing. Induced heat shock proteins with accumulated inorganic iron in injured tissues partly explain induced apoptosis in these tissues (6). Wound infection exacerbates this oxidative burden especially in the case of persistent wounds (7). As part of the natural defence mechanism, the iron carrier protein lactoferrin exerts potent bacteriostatic effects through withdrawal of iron away from the bacterial access. In this way, lactoferrin is potentially capable to control possible infections (8).
Various antioxidant agents have been shown to exert beneficial effects on healing rate. The role of vitamins C and E in wound healing has been clearly discussed by MacKay and Miller (9). Examples of wound healing plant extracts rich in antioxidant compounds could be found in literature (10).
There are also reports of iron chelating agents with wound healing properties. Promoted neovascularisation and enhanced wound healing by deferoxamine, a potent iron chelating agent used in iron‐overload conditions such as β‐thalassemia, has been reported (11). As another example, antimycotic ciclopirox olamine, a lipophilic bidentate iron chelator has been reported to exert beneficial effects on wound repair (8).
As iron is an essential element for bacterial growth, its scavenging by iron chelating agents would control infection in wounds and consequently promote the wound healing process. Deferiprone (L1) has been reported to prohibit bacterial contamination of wounds (12). Wound healing properties of deferiprone could also be attributed to its antioxidant and iron scavenging properties (13). Scavenging of iron inhibits its catalytic activity in the formation of free radicals which leads to oxidative stress (13).
In terms of therapeutic applications, compounds with both radical scavenging and iron chelating capability seem to accelerate healing process more than those having only one of these properties. Phenylpropanoid glycosides (PPGs) which are found in the families of Labiateae, Asteraceae, Oleaceae and Liliaceae have wound healing properties (14). PPGs, like other plant polyphenols are powerful antioxidants (15) and chelators of transition metals, especially iron and copper (16).
Kojic acid (5‐hydroxy‐2‐hydroxymethyl‐pyran‐4‐one), is a potent natural antioxidant safely used as a skin lightener without genotoxic properties 17, 18, 19. It is a bidendate metal chelator and is considered as a potent iron chelator (20). Kojic acid is also a non toxic natural antibiotic derived from various species of Aspergillus and Penicillium in an aerobic process 21, 22. Its absorption from the skin into the circulation is slow, thus it is less likely to impose side effects at therapeutic levels (23). As kojic acid is a potent antioxidant, it is potentially capable to target oxidative stress pathways involved in the process of wound healing. Furthermore, its effects on diminishing postinflammatory hyperpigmentation, might afford a good opportunity for the achievement of better final cosmetic appearance of healed wound. Thus, kojic acid seems to be a suitable candidate to accelerate the healing of chronic non healing wounds.
We hypothesised that deferiprone and kojic acid potentially meet the above‐mentioned criteria for effective wound healing by possessing both antioxidant and iron chelation capabilities. In this study, we aimed at investigating the effect of topical kojic acid and deferiprone on wound healing using an in vivo wound model. We will also make a comparison between the efficacies of these two compounds on the wound healing process in the studied model.
Methods
Preparation of kojic acid and deferiprone topical ointment
A 3, 6 or 9 g of kojic acid or deferiprone was pulverised to get very fine powder and levitated in little propylene glycol. Obtained paste was dissolved in 40 ml of hot purified water and this solution was mixed with sterile eucerin (in sterile environment) up to 100 g of weight. These prepared ointments were kept in 2–4°C, in aseptic condition. As the negative control an ointment with all ingredients except for kojic acid or deferiprone was used.
In vivo wound healing method
Adult female white wistar rats (200–220 g) were used in this study. Each group of rats was housed in the cages that were placed in a room with natural light and air cycle and constant temperature (25 ± 2°C). The animals were anaesthetised by intraperitoneal injection of ketamine (Francotar®; Virbac do Brasil Ind Com Ltda, Sao Paulo, Brazil) in concentration of 100 mg/ml and dose of 0·5 ml/rat. After shaving the backs of the animals, a circular 1 cm in diameter incision was made over skin of the back as a standard in vivo wound model. Povidone iodine was used as a topical anti‐infection only after surgery. All the animals also received veterinary ciprofloxacilin (0·4 ml intramuscular) just immediately after surgery. Thirty‐five rats were randomly divided into seven groups: one negative control group, three groups for deferiprone and three groups for kojic acid ointment. Each test or control group received its topical treatment twice a day from the beginning of the experiment until the complete wound healing.
For the determination of wound healing, all animals were anaesthetised and photos were taken from their wounds in the same conditions (same distance and resolution) in days 4, 8 and 12 after the beginning of treatment. ImageJ 1·42q software was used for the calculation of wound's area.
The percentage of wound area and the percentage of wound healing were calculated as:
 |
DPPH scavenging assay
This assay was performed according to the method described by Blois et al. (21). Briefly, 4 ml of different concentrations of methanolic solution of standard or test compounds (25–0·012 mg/ml) was added to 2 ml of DPPH methanolic solution (60 mM). The mixture was shaken vigorously and allowed to stand for 40 minutes in a dark place. Absorbance of the resulting solutions was measured at 517 nm using a UV/vis spectrophotometer. Scavenging of DPPH free radical was calculated as:
where Ac is the absorbance of the control tube (containing all reagents except the test compound) and At is the absorbance of the test tube. Ascorbic acid and BHA were used as standards.
Statistical method
The data had a normal distribution by Kolmogorov–Smirnov test. One‐way analysis of variance was used to test the differences between the test groups and control group (1–α = 0·95). Differences were considered significant when P < 0·05 and J > 5·32.
Results
Wound healing study showed that topical application of deferiprone at concentrations of 3%, 6% and 9% for four successive days has been associated with accelerated wound healing (P < 0·001). At this period of time, 3%, 6% and 9% kojic acid showed no significant healing effect (P = 0·06, 0·580 and 0·063, respectively, NS). Table 1 provides the values of mean percentage of wound healing at the day 4.
Table 1.
Evaluation of one‐way ANOVA test and the mean of wound healing percentage (±SD), on day 4 after beginning of experiments for control animals and test animals (1–α = 0.95 and J 0·95 = 5·32)
| Groups | J constant | P‐value in one‐way ANOVA test | The mean of wound healing (%) ± SD |
|---|---|---|---|
| Control | – | – | 17·802 ± 1·603 |
| 3% Deferiprone | 377·349 | <0·001 * | 37·562 ± 2·335 |
| 6% Deferiprone | 441·113 | <0·001 * | 44·936 ± 1·674 |
| 9% Deferiprone | 185·245 | <0·001 * | 31·692 ± 1·624 |
| 3% Kojic acid | 3·330 | 0·060 | 17·126 ± 1·383 |
| 6% Kojic acid | 0·332 | 0·580 | 21·188 ± 1·762 |
| 9% Kojic acid | 4·641 | 0·063 | 15·730 ± 1·434 |
ANOVA, analysis of variance.
*Statistically significant.
Topical application of 3% and 6% of deferiprone for 8 days made a significant improvement in the process of wound healing (P = 0·026 and P < 0·001, respectively). At this period of time, 9% deferiprone was not associated with significant wound healing (P = 0·08, NS). Eight days of successive application of 3%, 6% and 9% of kojic acid was associated with significant reduction in wound healing (P = 0·003, P < 0·001 and P < 0·001, respectively). Values of the mean percentage of wound healing at the day 8 are summarised in Table 2.
Table 2.
Evaluation of one‐way ANOVA test and the mean of wound healing percentage (±SD), on day 8 after beginning of experiments for control animals and test animals (1–α = 0·95 and J 0·95 = 5·32)
| Groups | J constant | P‐value in one‐way ANOVA test | The mean of wound healing (%) ±SD |
|---|---|---|---|
| Control | – | – | 62·266 ± 2·597 |
| 3%Deferiprone | 7·487 | 0·026 * | 67·370 ± 3·501 |
| 6% Deferiprone | 52·642 | <0·001 * | 76·066 ± 2·983 |
| 9% Deferiprone | 0·067 | 0·080 | 61·840 ± 2·080 |
| 3% Kojic acid | 16·816 | 0·003 * | 56·520 ± 1·714 |
| 6% Kojic acid | 11·955 | <0·001 * | 55·789 ± 2·362 |
| 9% Kojic acid | 56·771 | <0·001 * | 55·044 ± 2·516 |
ANOVA, analysis of variance.
*Statistically significant.
After 12 days of consecutive application of topical 3% and 6% deferiprone, a significant wound healing was observed (P = 0·003 and P < 0·001, respectively). Meanwhile, 9% topical deferiprone was not effective for wound healing (P = 0·195, NS). At the same period of time, wound treated with 3% and 6% topical kojic acid showed no significant improvement (P = 0·105 and 0·092, respectively, NS). Eight days of successive application of 9% of kojic acid was associated with significant reduction in wound healing (P = 0·002). Data in Table 3 represent mean percentage of wound healing at day 12 from the beginning of the treatment. Figure 1 shows graphical comparison of the effects of topical deferiprone and kojic acid (at the concentrations of 3%, 6% and 9%) through the percentage of wound healing in the control and test animals in days 4, 8 and 12.
Table 3.
Evaluation of one‐way ANOVA test and the mean of wound healing percentage (±SD), on day 12 after beginning of experiments for control animals and test animals (1–α = 0·95 and J 0·95 = 5·32)
| Groups | J constant | P‐value in one‐way ANOVA test | The mean of wound healing (%) ±SD |
|---|---|---|---|
| Control | – | – | 77·068 ± 1·954 |
| 3% Deferiprone | 102·414 | 0·003 * | 83·916 ± 2·912 |
| 6% Deferiprone | 18·389 | <0·001 * | 93·260 ± 2·577 |
| 9% Deferiprone | 0·947 | 0·195 | 78·616 ± 2·979 |
| 3% Kojic acid | 15·196 | 0·105 | 75·206 ± 2·743 |
| 6% Kojic acid | 21·203 | 0·092 | 76·982 ± 2·839 |
| 9% Kojic acid | 19·535 | 0·012 * | 72·488 ± 2·702 |
ANOVA, analysis of variance.
*Statistically significant.
Figure 1.
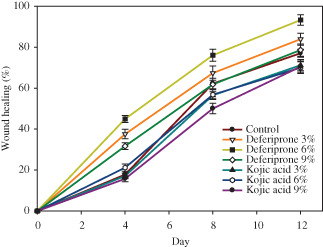
Comparison of the effects of topical deferiprone and kojic acid (the concentrations of 3, 6 and 9%) through the percentage of wound healing in control animals and test animals in days 4, 8 and 12.
Results of DPPH free radical scavenging assay are shown in Figure 2. According to this comparative antioxidant assay, kojic acid is weaker in free radical scavenging ability compared with deferiprone.
Figure 2.
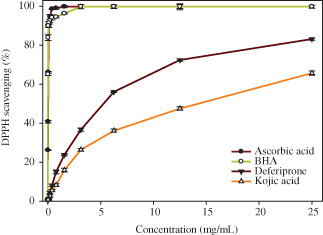
Comparison of the percent DPPH scavenging effect of deferiprone and kojic acid (ascorbic acid and BHT as standard) (BHA IC50 = 0·009 mg/ml, ascorbic acid IC50 = 0·038, deferiprone IC50 = 5·231 mg/ml, IC50 = 14·290 mg/ml).
Discussion
Currently, despite of the presence of various advanced technologies and medicines for the treatment of wounds, unhealed wounds impose a great burden on public health. Thus, search for finding agents which promote wound healing without toxic effects is continuing. Wound healing is an orchestrated process which basically requires a good healthy environment for proceeding proper healing without scar formation. ROS (including superoxide anion ·O2 −, hydroxyl radical ·OH, singlet oxygen 1O2 and hydrogen peroxide H2O2) are harmful agents involved in various human disease states like chronic non healing wounds 18, 24. Deferiprone positively influences disturbances in iron metabolism and ameliorates free radical generation leading to tissue damage as an iron chelator agent (20). Kojic acid and its derivatives, small‐molecule compounds isolated from fungus Aspergillus oryzae, are used mostly for the treatment of various skin conditions and diseases (25). Kojic acid, deferiprone and their derivatives have shown antibacterial and antifungal effects against several species such as Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Pseudomonas aeruginosa, Candida albicans, Candida krusei and Candida parapsilosis 21, 22. This antimicrobial effect has been attributed to the chelation of the metal iron required for the growth of some pathogens (26). This prevents delayed wound healing resulted from wound infection.
Deferiprone showed beneficial effects in accelerating wound healing in this in vivo study which can be attributed to its antioxidant, iron chelating and antimicrobial capabilities 27, 28. Our data implicate beneficial role of kojic acid, although less effective than deferiprone, in wound healing improvement. The stronger wound healing ability of deferiprone compared with kojic acid can be explained considering the differences in their antioxidant and iron chelating abilities.
DPPH free radical scavenging assay is a common antioxidant assay method which determines the ability of the compound to react with a free radical to give hydrogen to it and neutralise it (Figure 3). This assay was performed to have a comparison between the free radical scavenging ability of deferiprone and kojic acid. The difference between the wound healing ability of deferiprone and kojic acid can be explained by comparing the antioxidant abilities of these two compounds. Deferiprone possesses more antioxidant properties than kojic acid. This can be explained according to the differences in their chemical structures. Deferiprone has a pyridinone ring in its structure and kojic acid belongs to the pyranone ring containing compounds. Deferiprone presents more powerful antioxidant properties than kojic acid because of its greater aromatic properties compared with kojic acid. In fact, the hydroxyl in deferiprone exerts more phenolic, so acidic properties than kojic acid. This can be verified by comparing the pKa values of these compounds. Kojic acid has a pKa of 7·90–8·03 and pKa for deferiprone is 9·7. The labile hydrogen of the phenolic hydroxyl can be easily transferred to free radicals to neutralise them. The other proof for the greater wound healing efficacy of deferiprone is its stronger iron chelation ability compared with kojic acid. This comparison is made by taking a look at the cumulative affinity constants of these compounds for FeIII (log β). Log β for deferiprone is 37·2 and for kojic acid is 27 (29).
Figure 3.
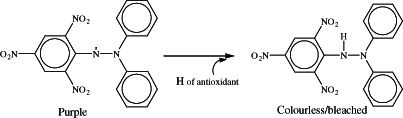
Neutralisation mechanism of DPPH with antioxidants.
In summary, it seems that antioxidant, iron chelating and antimicrobial properties make deferiprone and kojic acid deserved as potential agents for wound healing management.
References
- 1. Ramos R, Silva JP, Rodrigues AC, Costa R, Guardão L, Schmitt F, Soares R, Vilanova M, Domingues L, Gama M. Wound healing activity of the human antimicrobial peptide LL37. Peptides 2011;32:1469–76. [DOI] [PubMed] [Google Scholar]
- 2. Chaturvedi AP, Tripathi YB. Methanolic extract of leaves of Jasminum grandiflorum Linn modulates oxidative stress and inflammatory mediators. Inflammopharmacology 2011;19:273–81. [DOI] [PubMed] [Google Scholar]
- 3. Rao MC, Sudheendra AT, Nayak PG, Paul P, Kutty GN, Shenoy RR. Effect of dehydrozingerone, a half analog of curcumin on dexamethasone‐delayed wound healing in albino rats. Mol Cell Biochem 2011;355:249–56. [DOI] [PubMed] [Google Scholar]
- 4. Isaya G, O'Neill HA, Gakh O, Park S, Mantcheva R, Mooney SM. Functional studies of frataxin. Acta Paediatr Suppl 2004;93:68–73. [DOI] [PubMed] [Google Scholar]
- 5. Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkötter C, Scharffetter‐Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest 2011;121:985–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jacob AK, Hotchkiss RS, DeMeester SL, Hiramatsu M, Karl IE, Swanson PE, Cobb JP, Buchman TG. Endothelial cell apoptosis is accelerated by inorganic iron and heat via an oxygen radical dependent mechanism. Surgery 1997;122:243–54. [DOI] [PubMed] [Google Scholar]
- 7. Ekuni D, Firth JD, Nayer T, Tomofuji T, Sanbe T, Irie K, Yamamoto T, Oka T, Liu Z, Vielkind J, Putnins EE. Lipopolysaccharide‐induced epithelial monoamine oxidase mediates alveolar bone loss in a rat chronic wound model. Am J Pathol 2009;175:1398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mertz PM, Ovington LG. Wound healing microbiology. Dermatol Clin 1993;11:739–47. [PubMed] [Google Scholar]
- 9. MacKay D, Miller A. Nutritional support for wound healing. Altern Med Rev 2003;8:359–72. [PubMed] [Google Scholar]
- 10. Karodi R, Jadhav M, Rub R, Bafna A. Evaluation of the wound healing activity of a crude extract of Rubia cordifolia L. (Indian madder) in mice. Int J Appl Res Nat Prod 2009;2:12–8. [Google Scholar]
- 11. Gupta A, Singh RL, Raghubir R. Antioxidant status during cutaneous wound healing in immunocompromised rats. Mol Cell Biochem 2002;241:1–2. [DOI] [PubMed] [Google Scholar]
- 12. Ward CG. Iron and immunologic function. J Burn Care Rehabil 1987;8:487–91. [PubMed] [Google Scholar]
- 13. Farmaki K, Tzoumari I, Pappa C. Oral chelators in transfusion‐dependent thalassemia major patients may prevent or reverse iron overload complications. Blood Cells Mol Dis 2011;47:33–40. [DOI] [PubMed] [Google Scholar]
- 14. Hsu S. Green tea and the skin. J Am Acad Dermatol 2005;52:1049–59. [DOI] [PubMed] [Google Scholar]
- 15. Denisov E, Afanas'ev I. Oxidation and antioxidants in organic chemistry and biology. Boca Raton, London, New York, Singapore: CBC, Taylor & Francis Group, 2005. [Google Scholar]
- 16. Galey JB. Potential use of iron chelators against oxidative damage. Adv Pharmacol 1996;38: 167–203. [DOI] [PubMed] [Google Scholar]
- 17. Panich U, Tangsupa‐a‐nan V, Onkoksoong T, Kongtaphan K, Kasetsinsombat K, Akarasereenont P, Wongkajornsilp A. Inhibition of UVA‐mediated melanogenesis by ascorbic acid through modulation of antioxidant defense and nitric oxide system. Arch Pharm Res 2011;34:811–20. [DOI] [PubMed] [Google Scholar]
- 18. Gomes AJ, Lunardi CN, Gonzalez S, Tedesco AC. The antioxidant action of polypodium leucotomos extract and kojic acid: reactions with reactive oxygen species. Braz J Med Biol Res 2001;34:1487–94. [DOI] [PubMed] [Google Scholar]
- 19. Leyden JJ, Shergill B, Micali G, Downie J, Wallo W. Natural options for the management of hyperpigmentation. J Eur Acad Dermatol Venereol 2011;25:1140–5. [DOI] [PubMed] [Google Scholar]
- 20. Kotyzová D, Eybl V, Koutenský J, Brtko J, Glattre E. Effects of kojic acid on oxidative damage and on iron and trace element level in iron‐overloaded mice and rats. Cent Eur J Public Health 2004;12:S41–4. [PubMed] [Google Scholar]
- 21. Aytemir MD, Hider RC, Erol DD, Ozalp M, Ekizoglu M. Synthesis of new antimicrobial agents; amide derivatives of pyranones and pyridinones. Turk J Chem 2003;27:445–52. [Google Scholar]
- 22. Aytemir MD, Erol DD, Hider RC, Ozalp M. Synthesis and evaluation of antimicrobial activity of new 3‐hydroxy‐6‐methyl‐4‐oxo‐4H ‐pyran‐2‐carboxamide derivatives. Turk J Chem 2003;27:757–64. [Google Scholar]
- 23. Burnett CL, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, Marks JG Jr, Shank RC, Slaga TJ, Snyder PW, Andersen FA. Final report of the safety assessment of kojic acid as used in cosmetics. Int J Toxicol 2010;29:244S–73S. [DOI] [PubMed] [Google Scholar]
- 24. Ou J, Walczysko P, Kucerova R, Rajnicek AM, McCaig CD, Zhao M, Collinson JM. Chronic wound state exacerbated by oxidative stress in Pax6+/‐ aniridia‐related keratopathy. J Pathol 2008;215:421–30. [DOI] [PubMed] [Google Scholar]
- 25. Rodrigues AP, Carvalho AS, Santos AS, Alves CN, do Nascimento JL, Silva EO. Kojic acid, a secondary metabolite from Aspergillus sp., acts as an inducer of macrophage activation. Cell Biol Int 2011;35:335–43. [DOI] [PubMed] [Google Scholar]
- 26. Fassihi A, Abedi D, Saghaie L, Sabet R, Fazeli H, Bostaki G, Deilami O, Sadinpour H. Synthesis, antimicrobial evaluation and QSAR study of some 3‐hydroxypyridine‐4‐one and 3‐hydroxypyran‐4‐one derivatives. Eur J Med Chem 2009;44:2145–57. [DOI] [PubMed] [Google Scholar]
- 27. Kim CM, Shin SH. Effect of iron‐chelator deferiprone on the in vitro growth of staphylococci. J Korean Med Sci 2009;24:289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ibrahim AS, Edwards JE Jr, Fu Y, Spellberg B. Deferiprone iron chelation as a novel therapy for experimental mucormycosis. J Antimicrob Chemother 2006;58:1070–3. [DOI] [PubMed] [Google Scholar]
- 29. Shunmugaperumal T. Polymer‐based antimicrobial delivery carriers in: biofilm eradication and prevention: a pharmaceutical approach to medical device infections. Hoboken, NJ: Wiley, 2010:393. [Google Scholar]


