Abstract
The majority of the population experience successful wound‐healing outcomes; however, 1–3% of those aged over 65 years experience delayed wound healing and wound perpetuation. These hard‐to‐heal wounds contain degraded and dysfunctional extracellular matrix (ECM); yet, the integrity of this structure is critical in the processes of normal wound healing. Here, we evaluated a novel synthetic matrix protein for its ability to act as an acellular scaffold that could replace dysfunctional ECM. In this regard, the synthetic protein was subjected to adsorption and diffusion assays using collagen and human dermal tissues; evaluated for its ability to influence keratinocyte and fibroblast attachment, migration and proliferation and assessed for its ability to influence in vivo wound healing in a porcine model. Critically, these experiments demonstrate that the matrix protein adsorbed to collagen and human dermal tissue but did not diffuse through human dermal tissue within a 24‐hour observation period, and facilitated cell attachment, migration and proliferation. In a porcine wound‐healing model, significantly smaller wound areas were observed in the test group compared with the control group following the third treatment. These data provide evidence that the synthetic matrix protein has the ability to function as an acellular scaffold for wound‐healing purposes.
Keywords: Acellular scaffold, Extracellular matrix, Hard‐to‐heal wounds
Introduction
The skin is responsible for the maintenance of critical haemostatic parameters and the provision of a physical barrier between the environment and the vital organs of the body. When the integrity of the skin is compromised because of physical or other trauma, spontaneous self‐repair mechanisms are triggered. This process of wound healing involves the dynamic and successive coordination of inflammation, cell proliferation and tissue remodelling 1. During the inflammatory phase, the innate immune system is activated, which involves the infiltration of inflammatory cells, including neutrophils and macrophages, into the wound site to clear necrotic tissue and fend off infection. As this phase begins to decline, resident skin cells, including keratinocytes and fibroblasts, divide and migrate into the tissue defect eventually reconstructing a continuous stratified layer. This new tissue is subsequently remodelled to remove excess materials such as collagen and superfluous vasculature. Critical to each of these phases is the substrate by which all cells are able to access and infiltrate the wound: the extracellular matrix (ECM) 2.
The ECM is the most abundant component of the dermal skin layer 1 and is composed of a variety of extracellular proteins, proteoglycans and glycosaminoglycans 3. These biomolecules function to provide an optimal three‐dimensional environment for cells to reside in and traverse as required in normal skin. During wound healing, the ECM provides a critical role as a substrate on which cells are able to gain traction and infiltrate the wound site. Without a functional ECM, immune and skin cells do not have the means to efficiently enter the tissue defect and cannot undertake their intended functions at the required location. Consequently, wound healing cannot progress efficiently, resulting in delayed or perpetual skin defects, such as chronic or hard‐to‐heal wounds 2.
Hard‐to‐heal wounds commonly present as diabetic foot ulcers, lower extremity venous ulcers, mixed aetiology ulcers and pressure ulcers. These wounds are costly to individuals as well as health care systems and are a vast burden to society 4. Relevant to this investigation, hard‐to‐heal wounds are characterised by compromised and dysfunctional ECM. Investigations have demonstrated depleted, degraded and/or disorganised matrix components in these wound aetiologies. Examples include the low abundance of hyaluronan observed in pressure ulcers 5 and degraded fibronectin 6, 7, 8, 9, 10, vitronectin (VN) 7 and tenascin‐C 8 in other aetiologies. Atypical expression patterns of various proteoglycans have also been observed in chronic wounds 11. Furthermore, the observation of increased protease abundance 12, particularly matrix metalloproteinases and age‐related biochemical changes, has been cited as resulting in a reduction in dermal collagen. Such observations have led to the development of therapies that are able to restore structure, and consequently function, of the defective ECM present in hard‐to‐heal wounds 2.
Acellular scaffolds are ECM replacements or substitutes that recapitulate an environment reminiscent of native ECM. These materials can be permanent or transitional in the wound site and can be complex or simple 3. Complex scaffolds, for example, are manufactured from a variety of decellularised materials including animal‐derived dermis, small intestine submucosa, forestomach, pericardium and tendon and are composed of multiple biomolecular species. Several complex products derived from human dermis are also available. Simple acellular scaffolds are materials composed of a single or defined number of components such as an integral ECM protein. Xelma (Molnlycke Health Care, Gothenburg, Sweden), for instance, is a self‐assembling acellular scaffold composed of a single liquid‐phase ECM protein, amelogenin, which is applied to a wound surface to form a functional and transitional neo‐ECM 13. Acellular scaffolds are gaining in popularity as a means to promote effective wound healing by recapitulating an environment and function reminiscent of native ECM. In addition to amelogenin, other ECM components also have the potential to function as effective acellular scaffolds for the promotion of wound healing, including VN.
VN is a dermal ECM component that is vital for optimal wound healing 14. Critically, this matrix protein has been observed to be degraded in the environment of hard‐to‐heal wounds 6. Given these observations and the effectiveness of other acellular scaffolds in the promotion of wound healing, the application of VN, or a synthetic VN analogue, to the surface of hard‐to‐heal wounds could also be expected to provide a neo‐ECM with structural and functional properties capable of promoting wound healing. Herein, a pre‐clinical functional assessment of such a synthetic VN‐based matrix protein intended for use in promoting wound healing in hard‐to‐heal wounds is provided. This assessment demonstrates the ability of the matrix protein to adsorb to the wound bed, supporting a primary requirement for attachment of skin cells, and the subsequent cell functions that are dependent on this attachment, including cell proliferation and migration.
Methods
Ethics
Keratinocytes and fibroblasts were obtained from adult patients undergoing elective surgery, which resulted in surgical discard of dermal tissue. The tissue was obtained with both patient consent and institutional approval from the QUT Human Research Ethics Committee (approval number 3865H), Wesley Private Hospital (approval number 2003/46) and Saint Andrew's Private Hospital (approval number 2003/6). The porcine wound model investigations described in this study were conducted in compliance with the animal care guidelines established by the Canadian Council on Animal Care and were approved by the University of Calgary Animal Care Committee.
Acellular matrix
A VN‐based synthetic matrix protein intended for wound‐healing applications was examined throughout this investigation. The synthetic matrix protein comprises normal human VN (amino acids 1–64) tethered via a (Gly4‐Ser)4 amino acid linker to insulin‐like growth factor‐1 (amino acids 1–70) and was supplied as a 28 µg/ml solution in phosphate‐buffered saline (PBS) (Tissue Therapies Limited, Brisbane, Australia).
Isolation of human fibroblasts and keratinocytes
Keratinocytes and fibroblasts were isolated and cultured as previously described 15. In brief, human skin was incubated in 0·125% trypsin (Invitrogen, Brisbane, CA) overnight at 4°C, and keratinocytes were collected by gentle scraping of the exposed dermal and epidermal surfaces into a 10‐fold volume of Full Green's Media (FGM), consisting of Dulbecco's Modified Eagle Medium (DMEM; Gibco, Mulgrave, Australia) and Ham's F12 medium (Invitrogen) in a 3:1 ratio, supplemented with foetal calf serum (FCS; 10%), insulin (1 µg/ml; Sigma‐Aldrich, St. Louis, MO), human recombinant epidermal growth factor (10 ng/ml; Invitrogen), cholera toxin (0·1 µg/ml; Sigma‐Aldrich), non‐essential amino acid solutions [0·01% (v/v); Invitrogen], hydrocortisone (0·4 µg/ml; Sigma‐Aldrich), adenine (180 μM; Sigma‐Aldrich), transferrin (5 µg/ml; Sigma‐Aldrich), triiodothyronine (0·2 μM; Sigma‐Aldrich), l‐glutamine (2 mM), penicillin (100 U/ml) and streptomycin (100 U/ml). An irradiated murine feeder cell layer (i3T3; ATCC, Manassas, VA) was used to expand the freshly isolated keratinocytes. In brief, the i3T3 cells were maintained in DMEM supplemented with 5% FCS. The i3T3 cells were then seeded into cell culture flasks (1 × 106 cells/T75‐cm2 cell culture flask; Nunc, Rochester, NY) 2 hours before the keratinocytes were added (2 × 106 cells/T75‐cm2 cell culture flask). Keratinocytes were routinely cultured in FGM and were maintained in the standard conditions of 37°C and 5% CO2. Cell cultures were grown to 70–80% confluency before being serum‐starved for 4 hours in Stripped Green's medium (SGM; Full Green's medium without FCS, insulin or EGF) prior to trypsinisation and transfer to experimental culture plates.
For fibroblast isolation and culture, the dermis was washed in 5 ml of PBS prior to being placed in 10 ml of fibroblast growth medium [DMEM supplemented with FCS (5%; Hyclone, Thermo Fisher Scientific, Scoresby, Australia), penicillin (100 U/ml; Invitrogen), streptomycin (100 U/ml; Invitrogen) and l‐glutamine (2 × 10−3 M; Invitrogen)]. Dermal sections were then transferred into 0·05% collagenase A solution (Invitrogen) and incubated overnight at 37°C. Following digestion, the suspension was centrifuged at 400 g for 10 minutes to allow removal of excess collagenase and the resulting cell pellet was resuspended in fibroblast growth medium before being transferred into a tissue culture flask. Fibroblasts were maintained at 37°C and 5% CO2 with the culture medium being changed every 3–5 days.
De‐epidermised dermis (DED) preparation
DED was used in multiple experiments as an approximate tissue analogue of a clinically advanced wound. DED was prepared as previously described 15. In brief, human donor skin samples were incubated in 1 M NaCl at 37°C for approximately 20 hours. Epidermis and dermis were gently separated and washed for 2 hours in PBS (Invitrogen) before storage at 4°C. Native human skin samples were cut into discs before being stored in PBS at 4°C.
Dermal adsorption assay
The adsorption of the synthetic matrix protein to human skin was assessed in vitro by measuring the depletion of unbound matrix protein following incubation with DED. DED tissue from three skin donors was incubated with matrix protein (28 µg/ml) in Protein LoBind® Microcentrifuge Tubes (Eppendorf, Hamburg, Germany) with gentle shaking at room temperature for 24 hours. Protein LoBind® tubes containing synthetic matrix protein and no dermal tissue were used as a negative control. At 0, 1, 3, 10, 60 minutes, and 4 and 24 hours, samples were collected and soluble (unbound) synthetic matrix protein was quantified by enzyme‐linked immunosorbent assay (ELISA). In brief, a synthetic matrix protein standard curve was prepared in PBS and together with the test samples was dispensed into clear 96‐well plates (Nunc) in triplicate. Following incubation, samples were removed and each well was washed twice with 0·5% Tween 20/PBS (PBS‐T). The wells were then blocked with blocking buffer (5% skim milk powder/PBS‐T) for 1 hour at room temperature. Following two more washing steps with PBS‐T, the rabbit anti‐VN : insulin‐like growth factor 1 primary antisera (Mimotopes) was added in a 1:100 dilution in blocking buffer, incubated for 1 hour and the wells were washed twice with wash buffer. Secondary antibody [goat anti‐rabbit horseradish peroxidase (HRP); 1:10 000 dilution in wash buffer] was added to each well, incubated for 30 minutes and washed five times in PBS‐T. HRP substrate solution (TMB/E Single Reagent; Millipore, Kilsyth, Australia) was added to each well for 30 minutes before a stop solution (0·25 M HCl) was added and absorbance was quantified at 450 nm.
Collagen adsorption assay
To test the affinity of the synthetic matrix protein to collagen I, the wells of a 96‐well plate were first coated with collagen I (5 µg/cm2 from a 50 µg/ml solution in 0·02 M acetic acid, 1‐hour incubation). Known concentrations of synthetic matrix protein were incubated in these wells for 1 hour in PBS in the presence of 0·5% skim milk powder and 0·5% Tween 20 to block non‐specific interactions. The standard curve, blocking and detection were carried out as per the dermal adsorption assay.
Franz cell diffusion assay
A Franz Cell diffusion apparatus was used to measure transdermal permeation of synthetic matrix protein through DED under passive conditions based on standard approaches 16, 17. The test article, consisting of a single synthetic matrix protein treatment (28 µg/ml), was applied to the prepared DED (thickness of 1·5 ± 0·5 mm and cross‐sectional area of 0·79 cm2) at a loading volume of 100 µl. The lower chamber of the Franz Cell contained 3·5 ml of PBS. Samples taken from the lower chamber of the Franz Cell, representing synthetic matrix protein permeation across the DED membrane, were taken after 1‐, 2‐, 5‐ and 24‐hour incubation at ambient conditions. As a representative control for complete diffusion, 100 µl of synthetic matrix protein was diluted into 3·5 ml of PBS and stored alongside the Franz Cell for 24 hours. Samples including residual solution from the upper chamber were analysed by Western immunoblot. In brief, samples were electrophoresed on pre‐cast NuPAGE® Novex® Bis‐Tris 4–12% gradient minigels (Invitrogen), as per the manufacturer's instructions, and were transferred onto polyvinylidene fluoride (PVDF) membranes (PALL Corporation, Ann Arbor, MI). The membrane was blocked with 5% BSA and probed using an anti‐vitronectin rabbit polyclonal antibody (Merck, Darmstadt, Germany). Membranes were incubated with a rabbit anti‐goat HRP secondary antibody (R&D Systems, Minneapolis, MN) and detection was based on ECL Plus chemiluminescent (GE Healthcare, Rydalmere, Australia) development.
Transwell attachment and electron microscopy
The response of keratinocyte attachment and migration to surfaces coated with synthetic matrix protein was examined using Transwell™ plates (Corning COSTAR, Mount Martha, Australia). The Transwell membranes were pre‐coated with synthetic matrix protein by adding 28 µg/ml synthetic matrix protein diluted in SGM to the lower chamber of the Transwell for 3 hours at 37°C. Negative controls of Transwell membranes coated in SGM were included in the assay. Following incubation, unbound proteins were removed using two washes of SGM + 0·5% BSA. SGM + 0·05% BSA was then added to the lower chamber of each well. Serum‐starved keratinocytes, collected using a three‐stage trypsin digestion, were suspended in SGM containing 0·05% BSA and then seeded into the upper chamber of Transwell inserts (1 × 105 cells/well) and incubated at 37°C in 5% CO2. Following 24‐hour migration, cells remaining on inserts were fixed in a 3% glutaraldehyde buffer for 1 hour at room temperature. Fixed samples were washed twice in distilled water and then dehydrated with two washes in 50%, 70%, 90% and 100% ethanol. The samples were then chemically dried with two washes of hexamethyldisiloxane (Sigma‐Aldrich) for 30 minutes each and allowed to dry overnight. A scalpel was used to remove the Transwell membranes from the inserts, with the membranes being mounted on stubs in orientations exposing both the upper and lower surfaces. Samples were coated with gold using a sputter coater and viewed under the scanning electron microscope (SEM; FEI QUANTA200 Environmental Scanning Electron Microscope).
Proliferation assays
The proliferative response of cells to the synthetic matrix protein was measured using the WST‐1 Assay Kit (Roche Applied Science, Castle Hill, Australia). Synthetic matrix protein treatments were prepared in SGM at concentrations of 2·66 µg/ml (150 nM), 5·32 µg/ml (300 nM) and 7·98 µg/ml (450 nM) and the controls were VN at a concentration of 1 µg/ml (Promega, Madison, WI) and SGM alone. Samples were pre‐coated in 96‐well cell culture plates for 3 hours at 37°C. Following incubation, unbound proteins were removed using two washes of SGM + 0·05% BSA. Keratinocytes harvested by three‐stage trypsin digestion and fibroblasts harvested by standard trypsinisation were suspended in SGM containing 0·05% BSA and then seeded into each well at a density of 5 × 103 cells/well before incubation at 37°C in 5% CO2 for 72 hours. Following incubation, the WST‐1 assay was performed according to the manufacturer's instructions. Absorbances at a wavelength of 440 nm were measured using a Benchmark Plus Microplate Reader (Bio‐Rad, Hercules, CA). Differences in cell proliferation were evaluated using a Student's t‐test.
Fence migration assay
Keratinocytes and fibroblasts were assessed for their response to the synthetic matrix protein in a Fence‐type migration assay 18. Treatments, including synthetic matrix protein (2·66 µg/ml in SGM) and the positive (VN) and negative (SGM) controls, were pre‐coated in 24‐well plates as above. One hour prior to cell seeding, fence rings were inserted into the wells and placed into a cell incubator to allow the rings to seal. Keratinocytes and fibroblasts were seeded at a density of 4 × 104 and 1 × 104 cells/well, respectively, in SGM + 0·05% BSA, with the inclusion of 10 µg/ml mitomycin‐C to inhibit cell proliferation. The cells were incubated for 4 hours to allow attachment, after which the fence inserts were removed and the wells washed twice with SGM containing 0·05% BSA. Cells were allowed to migrate over 24 hours, then fixed in formalin and stained with 0·02% Crystal Violet. Photographs of each well were taken and the area occupied by the cells was calculated using ImageJ software (NCBI). Cell migration was calculated as a function of the negative control (SGM). Differences in cell migration were evaluated using a Student's t‐test.
Effect on healing using an in vivo porcine model
Three deep partial thickness wounds (3 × 3 × 0·4 cm deep) were created on both flanks of 16 female domestic Yorkshire pigs, giving a total of six wounds per pig. Pigs were pre‐medicated by intramuscular injection of ketamine followed by general anaesthesia induced by inhalation of isoflurane. Following wounding, a 1:10 000 dilution of epinephrine solution was sponged onto the wounds until haemostatis had occurred (∼5 minutes). After wounding, one flank (three wounds), selected at random on each pig, received topical treatment of synthetic matrix protein in sterile PBS at a concentration of 560 ng/cm2 wound area. The remaining flank (three wounds) on each pig received a control treatment of sterile PBS. Treatments were applied across each wound site and allowed to adsorb for 2 minutes before the wounds were dressed with OpSite Post‐Op® (Smith and Nephew, Mississauga, Canada) and secured by a layer of Elastoplast® (BSN Medical, Laval, Canada). Treatments were repeated on days 1, 3, 6, 8 and 10. Clinical observations of the wound with each dressing change were scored for severity of erythema (0 = normal, 1 = slight, 2 = moderate, 3 = severe and 4 = very severe) and swelling (0 = normal, 1 = slight, 2 = moderate, 3 = severe and 4 = very severe). Wounds were photographed digitally at wounding and each dressing change to record the size of the wound area. Wound area was measured using the elliptical method described by Plassmann et al. 19. Wounds were photographed alongside a ruler and the longest (a) and shortest (b) sides of the wound ellipse were measured by a blinded assessor. Area was calculated using the standard formula: π × a × b. Differences in wound area at each time point were evaluated using a Student's t‐test.
Results
Dermal adsorption
The adsorption of synthetic matrix protein to human DED was investigated over a 24‐hour incubation period and the concentrations of unbound (or soluble) synthetic matrix protein were measured by ELISA. ELISA assays were performed in triplicate, and the data from three independent adsorption experiments using skin derived from different patients were pooled. As shown in Figure 1A, the percentage of synthetic matrix protein remaining in solution showed a strongly biphasic profile, which fitted well to a sum of two exponentials:
| (1) |
Figure 1.
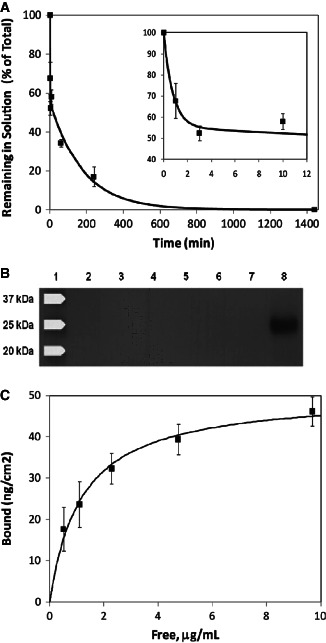
Synthetic matrix protein adsorption and permeation through human dermis. (A) Graph of synthetic matrix protein adsorption to de‐epidermised dermis (DED) following incubation for 24 hours (1440 minutes). Data are expressed as the percentage of the initial synthetic matrix protein concentration remaining in the soluble phase. Data were obtained from three independent experiments using tissues donated by three individual patients with error bars representing the standard error of the mean (SEM). Approximately 45% of the synthetic matrix protein was adsorbed in an initial rapid deposition (inset) followed by slower adsorption over the remaining time. (B) Permeation of synthetic matrix protein through DED, as measured using a Franz cell. Western immunoblot analysis of permeate samples taken at 1 hour (lane 2), 2 hours (lane 3), 5 hours (lane 4) and 24 hours (lane 5). Lane 1 represents the molecular weight control, lane 6 represents unbound residual test solution taken from the upper surface of the membrane after 24 hours, lane 7 was left blank and lane 8 is the synthetic matrix protein reference standard at a concentration that is representative of complete diffusion. (C) Adsorption of synthetic matrix protein to surface‐bound collagen I followed a typical Langmuir isotherm with a calculated affinity constant of approximately 55 nM.
where ϕ is the percentage remaining at time t, A is the percentage adsorbed in the initial rapid phase and k fast and k slow are the time constants for the fast and slow adsorption phases, respectively. The best‐fit parameters were A = 45%, k fast = 1·3 minute−1 and k slow = 5·5 × 10−3 minute−1. This is consistent with the presence of high‐affinity binding sites for the synthetic matrix protein both on and within the DED: the surface‐accessible sites are rapidly saturated, while saturation of the internal sites is controlled by the slow diffusion of the synthetic matrix protein through the DED matrix. The synthetic matrix protein was undetectable in solution at the 24‐hour time point; the adsorption profile suggests that >98% adsorption would occur within 10 hours.
The permeation of synthetic matrix protein through a 1·5‐mm DED membrane was also investigated using a Franz Cell approach where the presence of synthetic matrix protein in the permeate was monitored by Western immunoblotting (Figure 1B). The synthetic matrix protein was applied to the upper surface of the DED membrane at the standard concentration of 28 µg/ml. Because of the cross‐sectional area of the DED membrane, this dose represents 3·6 µg/cm2 and is significantly greater (∼6·4‐fold) than that tested on the porcine partial thickness wound model (560 ng/cm2). Synthetic matrix protein diluted to a concentration that is representative of complete diffusion across the DED membrane was included as a control. This analysis shows that the synthetic matrix protein did not diffuse across the DED membrane over the 24‐hour incubation period. In addition, no residual synthetic matrix protein was detected in the test solution on the upper surface of the membrane following the 24‐hour incubation. These permeation and adsorption studies confirm that the synthetic matrix protein adheres to the DED obtained from human skin samples and is unable to permeate through the 1·5‐mm DED membrane.
Collagen adsorption
We tested the affinity of the synthetic matrix protein for purified collagen I by assessing its adsorption to collagen‐coated tissue culture plastic in the presence of 0·5% skim milk powder to prevent non‐specific adsorption (Figure 1C). Collagen‐bound and‐free synthetic matrix protein were calculated using the reasonable assumption that direct adsorption to bare tissue culture plastic in the standard curve was quantitative and irreversible. Adsorption of synthetic matrix protein as a function of concentration was fitted to a Langmuir isotherm model:
where Γ and Γmax represent the current and maximum surface‐bound concentration of synthetic matrix protein, respectively, K d is the dissociation constant between the synthetic matrix protein and collagen I and C is the concentration of synthetic matrix protein in solution. The best‐fit parameters were Γmax = 51 ng/cm2 and K d = 1·2 × 103 ng/ml (55 nM).
Attachment assay
The attachment of keratinocytes on synthetic matrix protein‐coated Transwell membranes was investigated using SEM. Cells were applied to the synthetic matrix protein‐ or SGM‐ negative control‐coated membranes with each test being performed in triplicate. Each sample was photographed at 800× magnification (Figure 2). Keratinocytes that had migrated and attached to the bottom surface of the membrane were observed in samples treated with synthetic matrix protein but not in samples treated with SGM.
Figure 2.
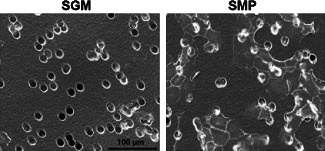
Keratinocyte attachment to synthetic matrix protein‐coated surfaces. Scanning electron microscopy was used to investigate keratinocyte attachment to Transwell™ membranes coated with Stripped Green's medium (SGM) or synthetic matrix protein (SMP). Membranes coated with SGM were used as a negative control. Images were taken on the bottom of the microporous Transwell membrane (pore sizes at 12 µm) insert at 800× magnification. Scale bar indicates 100 µm.
Functional assays
The effect of the synthetic matrix protein on cellular processes important to wound healing, namely cell migration and cell proliferation, was evaluated. Synthetic matrix protein was compared with VN alone and the serum‐free media control using keratinocytes and fibroblasts (Figure 3). The cell migration results, expressed as the area above that observed in the SGM control, indicated that the synthetic matrix protein facilitated keratinocyte and fibroblast migration to levels that were equivalent to that observed with VN. Similarly, keratinocyte and fibroblast proliferation was equivalent to the responses observed with VN.
Figure 3.
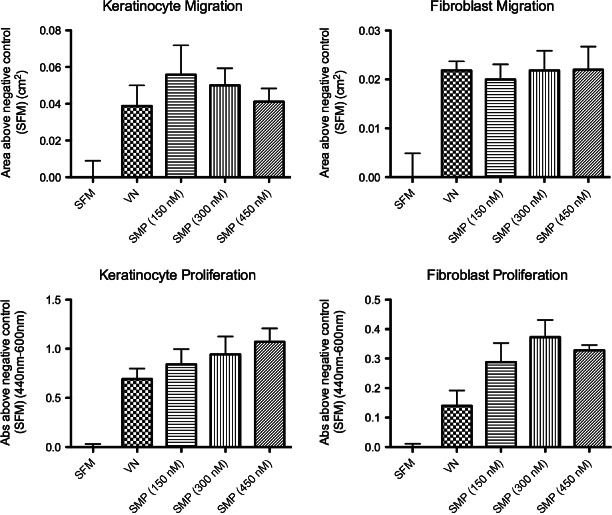
Keratinocyte and fibroblast migration and proliferation on synthetic matrix protein (SMP)‐coated surfaces. Keratinocyte and fibroblast migration, as determined using the Fence assay, and proliferation, as measured by the WST‐1 assay at different SMP concentrations. Data represent three independent determinations using cells derived from two different donors (n = 6) and are expressed as an average response above serum‐free media (SFM). Vitronectin (VN) represents the positive control and error bars indicate the mean ± standard error of the mean (SEM).
Effect on healing using an in vivo porcine model
To evaluate the effects of synthetic matrix protein on wound closure, we performed a pilot study using an in vivo porcine deep partial thickness wound model. We elected to use this model as pig skin is known to be anatomically and physiologically most similar to human skin and is claimed to be the most reliable model in terms of ultimate translation of wound healing therapies to patients 20. The mean results obtained for the synthetic matrix protein and control groups are shown in Figure 4A. The measurements and images obtained for Pig 15 are also depicted in Figure 4A and B, respectively. Across the mid‐stages of treatment, healing progressed more rapidly in the synthetic matrix protein‐treated group than the control group. This was particularly evident in the mean results and those obtained for Pig 15 at day 8. Wounds from both synthetic matrix protein and the control group healed within the 13‐day observation period. No significant differences in erythema, swelling or granulation tissue formation were observed between the control and treatment groups (data not included).
Figure 4.
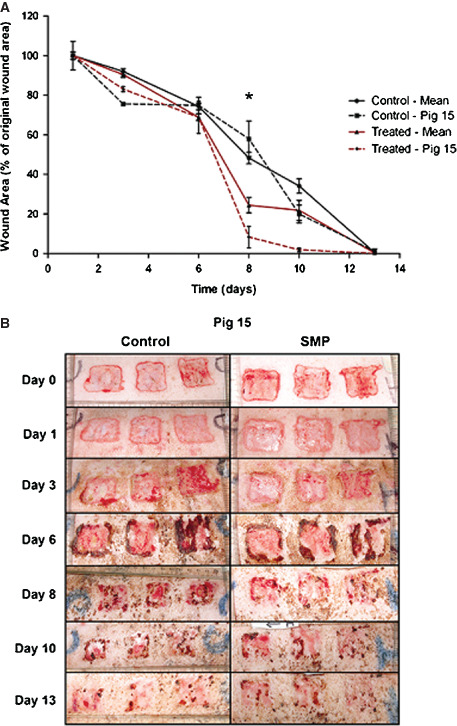
Topical application of synthetic matrix protein (SMP) using an in vivo porcine deep partial thickness wound model. Wound areas were photographed digitally for surface area calculation. (A) Wound‐healing response (% original wound area) of one representative pig 15 and overall mean (n = 16) are shown. Values shown for the overall mean include mean ± standard error of the mean (SEM). Significant differences (*) are illustrated, where P < 0·05 between the treated group and untreated control. (B) Corresponding photos obtained for Pig 15.
Discussion
Dermal wound healing is the process by which skin regenerates following mechanical or other trauma. While most instances of dermal wounding will result in the spontaneous activation and successful completion of the phases of wound healing, a portion of the population's wounds will fail to heal 21. These hard‐to‐heal wounds generally affect the elderly and comprise diabetic foot ulcers, lower extremity venous ulcers, mixed aetiology ulcers and pressure ulcers. These wounds have been observed to exhibit compromised ECM components and consequently, dysfunctional ECM 7. This dysfunctional ECM is proposed to be unable to provide the traction required for immune and skin cells to migrate into the wound site and undertake their requisite tasks, eventually leading to delayed wound healing or perpetuation. Therefore, the defective ECM requires repair in order to restore the wound‐healing process and promote effective wound healing 2.
Acellular scaffolds provide a means to promote wound healing in hard‐to‐heal wounds by replacing defective ECM 3. These materials offer an intact scaffold reminiscent of that found in the native wound environment, which cells can effectively use to attach and migrate to the site of a dermal defect. Once there, these cells can drive wound healing to completion through the coordination of migration and proliferation. Thus, the integral requirements of an acellular scaffold for use in clinical practice are to form a neo‐ECM and to facilitate wound‐healing cellular processes.
This study demonstrates the ability of a synthetic VN‐based matrix protein to function as an acellular scaffold for wound‐healing purposes. The synthetic matrix protein was initially shown to rapidly deplete from the liquid phase and adsorb to DED (Figure 1A). This tissue was used to replicate a worst‐case scenario from a clinical perspective, thus representing a wound with no evident epithelia or indeed other cells. The specific mechanism of this adsorption may be explained by the collagen‐binding action of VN identified by Izumi et al. 22. Through the use of monoclonal antibodies, Izumi et al. demonstrated that the N‐terminal portion of VN, present in the synthetic matrix protein, will bind and interact with collagens. This high‐affinity interaction of the synthetic matrix protein with collagen I was confirmed by a solid plate binding assay (Figure 1C). Furthermore, VN has been demonstrated to bind cells 6 through a cell‐binding domain located towards the N‐terminal of the protein 23. This capacity to bind both cells and components of the ECM may contribute to the ability of the synthetic matrix protein to significantly enhance in vivo wound healing as observed in a porcine wound model. Permeation analysis then established that the adsorption to DED was localised to the superficial layer of the tissue (Figure 1B). This result may also be attributed to the expected interaction of the synthetic matrix protein with ECM components of the DED rather than a simple diffusion process taking place.
Given the required adsorption and localisation of the matrix protein to the dermal surface, the resulting neo‐ECM was investigated for its ability to support wound‐healing cellular functions. Specifically, the matrix protein was shown to facilitate cell attachment, and the attachment‐dependent functions of migration and proliferation. The ability of the adsorbed protein scaffold to support cell attachment was first confirmed by SEM (Figure 2). These data showed that primary keratinocytes were able to adhere to matrix‐coated plates even in the absence of serum, which would normally lead to minimal cell attachment, as observed in the SGM control (Figure 3). Cell migration was then examined by a keratinocyte fence cell migration assay. This assay demonstrated cell migration in both the synthetic matrix protein‐ and VN‐positive control‐coated plates but not in the serum‐free control (SGM) (Figure 3). These data demonstrate that the synthetic matrix protein can facilitate the adsorption and cellular processes necessary to function as an acellular scaffold for wound‐healing purposes. This potential was tested in an in vivo porcine wound‐healing model, which is generally regarded as the industry‐standard wound‐healing model 24, 25. Six deep partial thickness wounds were induced in 16 pigs with one block of three wounds per animal selected at random for treatment with the synthetic matrix protein. The acute porcine model used in this study was based on healthy animals with no induced delay in the healing rate. The advantage of this model is that it is ideal for use in the detection of any potential negative influences associated with the application of the synthetic matrix protein. Conversely, because of the rapid healing rates associated with deep partial thickness wounds in healthy animals, any strong positive influences of the synthetic matrix protein will be difficult to detect. While the loss of significant difference in wound area between the control and the treatment at day 13 may be attributed to the rapid healing rate of this model, our study clearly demonstrates different healing trajectories between the synthetic matrix protein and the control. At day 8, there is a clear significant difference in wound area, indicating that the treatment has a positive effect by accelerating wound healing up to this time point. This outcome supports the in vitro cell function data and is an encouraging indication that the ECM replacement therapy may have similar success in a clinical environment.
Wound‐healing literature regarding hard‐to‐heal wounds has for some time highlighted the importance of the ECM for successful repair. This view has been underpinned by numerous articles demonstrating that the ECM is compromised, dysfunctional or disorganised in hard‐to‐heal wounds 5, 6, 7, 8, 10, 11 and has additionally spurred the development of therapeutics that seek to correct this situation 3. These therapeutic approaches encompass xenografts, allografts and synthetic dermal replacements. The primary objectives of these materials are to provide a matrix for cell attachment and facilitate the cellular functions required for successful wound healing. Existing materials such as Xelma and INTEGRA have met these requisite conditions and are now used in clinical practice 3, 13. Additionally, the use of such treatments is economically comparable to conventional treatments such as split‐thickness skin grafts, suggesting that these therapies may become more common in clinical practice 26. When considered in combination, the experiments described herein demonstrate the ability of a novel synthetic matrix protein to function like a scaffold, which is then able to support the cellular functions essential for the progression of wound healing. Critically, in vivo assessment based on the industry‐standard animal model of wound healing provides support for the evaluation of this acellular matrix therapy in the treatment of hard‐to‐heal wounds in the clinical setting.
Acknowledgements
The authors would like to acknowledge Dr Anthony Kane and his staff, and the patients for their time and generous donations of skin samples. We would also like to thank Merle Olson, DVM, MSc, and his staff at Innovotech Inc., Edmonton, Canada, for performing the porcine wound‐healing study.
References
- 1. Schultz GS, Ladwig G, Wysocki A. Extracellular matrix: review of its role in acute and chronic wounds. World Wide Wounds 2005;1:1–16. [Google Scholar]
- 2. Hodde JP, Johnson CE. Extracellular matrix as a strategy for treating chronic wounds. Am J Clin Dermatol 2007;8:61–6. [DOI] [PubMed] [Google Scholar]
- 3. Harding KG, Kirsner R, Lee D, Mulder G, Serena T. International consensus: acellular matrices for the treatment of wounds. An expert working group review. London: Wounds International, 2010. [Google Scholar]
- 4. Harding K, Queen D. Chronic wounds and their management and prevention is a significant public health issue. Int Wound J 2010;7:125–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dechert TA, Ducale AE, Ward SI, Yager DR. Hyaluronan in human acute and chronic dermal wounds. Wound Repair Regen 2006;14:252–8. [DOI] [PubMed] [Google Scholar]
- 6. Grinnell F, Ho CH, Wysocki A. Degradation of fibronectin and vitronectin in chronic wound fluid: analysis by cell blotting, immunoblotting, and cell adhesion assays. J Invest Dermatol 1992;98:410–6. [DOI] [PubMed] [Google Scholar]
- 7. Grinnell F, Zhu M. Fibronectin degradation in chronic wounds depends on the relative levels of elastase, alpha1‐proteinase inhibitor, and alpha2‐macroglobulin. J Invest Dermatol 1996;106:335–41. [DOI] [PubMed] [Google Scholar]
- 8. Latijnhouwers MA, Bergers M, Veenhuis RT, Beekman B, Ankersmit‐Ter Horst MF, Schalkwijk J. Tenascin‐C degradation in chronic wounds is dependent on serine proteinase activity. Arch Dermatol Res 1998;290:490–6. [DOI] [PubMed] [Google Scholar]
- 9. Palolahti M, Lauharanta J, Stephens RW, Kuusela P, Vaheri A. Proteolytic activity in leg ulcer exudate. Exp Dermatol 1993;2:29–37. [DOI] [PubMed] [Google Scholar]
- 10. Schmidtchen A. Degradation of antiproteinases, complement and fibronectin in chronic leg ulcers. Acta Derm Venereol 2000;80:179–84. [DOI] [PubMed] [Google Scholar]
- 11. Lundqvist K, Schmidtchen A. Immunohistochemical studies on proteoglycan expression in normal skin and chronic ulcers. Br J Dermatol 2001;144:254–9. [DOI] [PubMed] [Google Scholar]
- 12. Rayment EA, Upton Z. Finding the culprit: a review of the influences of proteases on the chronic wound environment. Int J Low Extrem Wounds 2009;8:19–27. [DOI] [PubMed] [Google Scholar]
- 13. Vowden P, Romanelli M, Peter R, Bostrom A, Josefsson A, Stege H. The effect of amelogenins (Xelma) on hard‐to‐heal venous leg ulcers. Wound Repair Regen 2006;14:240–6. [DOI] [PubMed] [Google Scholar]
- 14. Jang YC, Tsou R, Gibran NS, Isik FF. Vitronectin deficiency is associated with increased wound fibrinolysis and decreased microvascular angiogenesis in mice. Surgery 2000;127:696–704. [DOI] [PubMed] [Google Scholar]
- 15. Xie Y, Rizzi SC, Dawson R, Lynam E, Richards S, Leavesley DI, Upton Z. Development of a three‐dimensional human skin equivalent wound model for investigating novel wound healing therapies. Tissue Eng Part C Methods 2010;16:1111–23. [DOI] [PubMed] [Google Scholar]
- 16. Addicks WJ, Flynn GL, Weiner N. Validation of a flow‐through diffusion cell for use in transdermal research. Pharm Res 1987;4:337–41. [DOI] [PubMed] [Google Scholar]
- 17. Franz TJ. Percutaneous absorption on the relevance of in vitro data. J Invest Dermatol 1975;64:190–5. [DOI] [PubMed] [Google Scholar]
- 18. Pratt BM, Harris AS, Morrow JS, Madri JA. Mechanisms of cytoskeletal regulation. Modulation of aortic endothelial cell spectrin by the extracellular matrix. Am J Pathol 1984;117:349–54. [PMC free article] [PubMed] [Google Scholar]
- 19. Plassmann P, Melhuish JM, Harding KG. Methods of measuring wound size: a comparative study. Ostomy Wound Manage 1994;40:50–2 54, 56–60. [PubMed] [Google Scholar]
- 20. Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen 2001;9:66–76. [DOI] [PubMed] [Google Scholar]
- 21. Posnett J, Franks PJ. The burden of chronic wounds in the UK. Nurs Times 2008;104:44–5. [PubMed] [Google Scholar]
- 22. Izumi M, Shimo‐Oka T, Morishita N, Ii I, Hayashi M. Identification of the collagen‐binding domain of vitronectin using monoclonal antibodies. Cell Struct Funct 1988;13:217–25. [DOI] [PubMed] [Google Scholar]
- 23. Schvartz I, Seger D, Shaltiel S. Vitronectin. Int J Biochem Cell Biol 1999;31:539–44. [DOI] [PubMed] [Google Scholar]
- 24. Meyer W, Schwarz R, Neurand K. The skin of domestic mammals as a model for the human skin, with special reference to the domestic pig. Curr Probl Dermatol 1978;7:39–52. [DOI] [PubMed] [Google Scholar]
- 25. Vardaxis NJ, Brans TA, Boon ME, Kreis RW, Marres LM. Confocal laser scanning microscopy of porcine skin: implications for human wound healing studies. J Anat 1997;190(pt 4):601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Voigt DW, Paul CN, Edwards P, Metz P. Economic study of collagen‐glycosaminoglycan biodegradable matrix for chronic wounds. Wounds 2006;18:1–7. [Google Scholar]


