Abstract
Non healing wounds present a significant social and economic burden. Chronic non healing wounds are estimated to affect as many as 1–2% of individuals during their lifetime, and account for billions of dollars of expense annually on both a national and global basis. Our purpose is to describe the use of a novel dehydrated amniotic membrane allograft (EpiFix ®; MiMedx Group, Inc., Kennesaw, GA) for the treatment of chronic non healing wounds. We describe the results of EpiFix treatment in four patients who had not achieved wound closure with both conservative and advanced measures, and had been referred for a definitive plastic surgery procedure. Healing was observed in a variety of wounds with one to three applications of the dehydrated amniotic membrane material. The material was well tolerated by patients. Healed wounds did not recur in long‐term follow‐up. Further investigation of the use of dehydrated amniotic membrane in broader application to various types of dermal wounds should be considered.
Keywords: Allograft, Amniotic membrane, Wound treatment
Introduction
Chronic non healing wounds are significantly burdensome to patients and healthcare providers. Annually the treatment of non healing wounds consumes billions of dollars worldwide 1, 2.
Chronic non healing wounds appear in various types of patients. Diabetic patients have a 15–25% lifetime risk of developing foot ulcers, with annual treatment costs estimated to be as much as $30 000 per year 3, 4. Venous stasis ulcers account for over half of all lower extremity wounds and can be present for as long as 5 years 5. Post‐traumatic and surgical wounds can also present issues with delayed closure and the development of chronicity. Factors such as age, gender, obesity, immobility, infection and smoking also increase the risk for non healing wounds 1.
For clients such as those described above, clinicians frequently use both intensive preventive strategies to keep wounds from forming as well as focused and evidence‐based treatments using algorithms designed to promote healing and limit limb loss 6, 7. With financial resource constraints becoming increasingly common, the efficient allocation of resources in caring for these patients is becoming more closely related to the efficacy of various treatment modalities.
Human amniotic membrane has been used in the treatment of wounds for over a century 8. Amniotic membrane with its natural tissue planes, anti‐inflammatory and antibacterial properties make it a natural choice for potential wound management, which was confirmed with the application of natural membrane in various clinical situations earlier in the last century 8. It has been applied in treatment of burns, ulcers and various types of integumentary wounds 8. Historically, however widespread use of human amniotic membrane has been limited because of issues related to tissue preparation, stabilisation and storage. Derived from placentas, human amniotic membranes have been understandably difficult to obtain, prepare and store, particularly for use in locations not in close proximity to labour and delivery floors. With recent development of a system for stabilisation and preservation, dehydrated human amniotic membrane allograft (dHAM) has now been approved for distribution in the USA as a tissue allograft. Over 45 000 dHAM grafts have been used in eye surgery, periodontal surgery, treating wounds, burns, chemical burns and conjunctival repair in both adults and children.
Recent efforts in wound healing centres focus on concentration and optimisation of effective strategies in dealing with non healing wounds. Specialty referral to these centres of excellence for the treatment of chronic, non healing wounds presents a cost‐effective methodology for these patients. This paper illustrates the effectiveness of dHAM allografts (EpiFix®; MiMedx Group, Inc., Kennesaw, GA) as a treatment modality for successful healing in a variety of chronic wound types. The chronic cases presented were found to be otherwise intractable to several advanced wound healing therapies including negative pressure therapy (NPT), multiple debridements, hyperbaric oxygen and other commercially available skin substitutes.
Methods
Study setting and patient selection
Patients reviewed in this case series were selected from qualified referrals to a formally organised wound healing centre focusing on chronic wounds. The wound care centre is affiliated with the Diversified Clinical Services, Inc. and adheres to proprietary wound care treatment algorithms developed by the organisation around evidence‐based information in medical literature. Treatment regimens include sharp debridement to remove non viable tissues, weekly observational visits and therapies and the sequential application of increasingly focused treatments. Adjunctive elements include use of moist dressings, antibiotic ointment, honey‐based therapies, NPT and when appropriate, compression, splinting and full‐contact casting for pressure distribution. More advanced therapies including biologically based skin substitutes or allografts are also used in patients who had initially failed four or more weeks of conservative therapy. Hyperbaric oxygen therapy is used as adjunctive therapy only after there are no measurable signs of healing after at least 30 days of treatment with standard wound therapy. The concept of the ‘reconstructive ladder’ is used to guide decision making for plastic surgery reconstruction 9. In guidance with the reconstructive ladder, the more problematic and difficult the wound is the treatment of choice is higher up the rungs of the ladder. Lower rungs include dressings and primary closure; middle rungs include delayed closure, grafts and tissue expansion; while the highest rungs and most difficult wounds use various types of flap reconstruction. Patients presented herein, having failed other treatments, were being considered for flap reconstruction surgery. At this point, and before proceeding with surgical flap reconstruction, the intractable wounds were treated with dHAM application. We report the results of all patients treated by a single, non blinded reconstructive plastic surgeon who received the dHAM product in the time period from January through July 2010.
Discussion was held with patients regarding the use of dHAM versus autologous tissue reconstruction. Initial assessment of patients included completion of a thorough medical history and physical exam, and appropriate assessment of vascular and metabolic status. After following standard wound care at the wound centre, including sharp debridement, dHAM was applied in saline‐moistened form with a non adherent dressing. Prior to preparation of this retrospective case series, written consent was obtained from the patients allowing for the use of their de‐identified personal health information and photographs.
Results
Case 1
Case 1 is a right‐hand dominant 53‐year‐old female smoker with polyarticular rheumatoid and osteoarthritis who is dependent upon forearm crutches for ambulation. After undergoing left olecranon bursa excision and subsequent use of forearm‐based crutches, she developed postoperative wound dehiscence at 3 weeks as illustrated in Figure 1.
Figure 1.
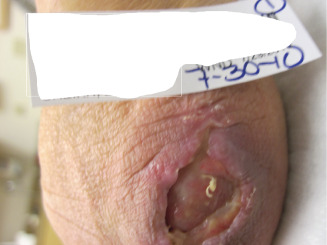
A 3‐week‐old wound showing a postoperative dehiscence after left olecranon bursa excision.
This was initially treated with in‐office wound debridement, topical medicinal honey dressings and elbow splinting. Initial wound measured 2·7 × 1·8 × 0·1 cm prior to initial debridement and 2·8 × 2·8 × 0·3 cm thereafter. No joint exposure was noted. At 5 weeks postoperatively, the wound had retracted but remained open and NPT was begun to enhance secondary intention closure of the wound.
Wound healing progressed, only to plateau again despite 8 weeks of therapy. The wound was then addressed with the dHAM application at 8 weeks with concomitant termination of negative pressure application. A single application of dHAM was used, resulting in wound closure at 3 weeks post‐application (Figure 2). A non adherent dressing over the membrane was replaced after 1 week. The patient missed her second‐week follow‐up appointment. At the third‐week time point, after single dHAM application, the wound was noted to be healed with reepithelialisation. The patient was discharged. No recurrence was reported at 1‐ and 6‐month follow‐up over phone.
Figure 2.
A 10‐week‐old wound at 3 weeks post single application of dHAM.
Both surface area and wound volume were measured during the healing process, and a graph of the wound healing time course is shown in Figure 3.
Figure 3.
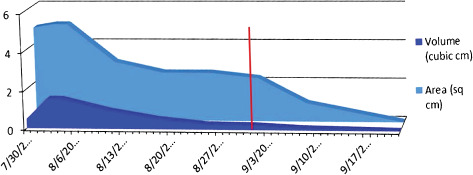
A graph plotting wound size over time (red line represents application of dHAM on 1 September 2010). Area is in square cm, volume is in cubic cm. Both parameters improved until resolution from the plateau seen before dHAM application.
Case 2
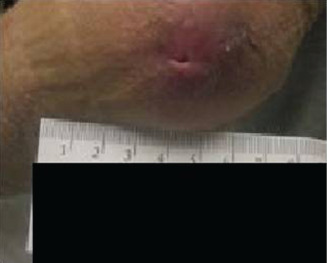
Case 2 is a 77‐year‐old male with traumatic articular fracture and a history of right hip and knee fusion during childhood. His wound was a result of striking his knee against a toilet seat, incurring full‐thickness loss of skin over the anterior aspect of the fused knee (Figure 4).
Figure 4.
Initial wound of the anterior knee.
The patient was initially treated with topical debridement ointment for 5 weeks and a Manuka honey application for an additional 3 weeks. At 9 weeks post‐injury, the wound had regressed to 1·6 × 3·2 × 0·2 cm from 1·3 × 3·0 × 0·1 cm. At this point, a porcine xenograft skin substitute was serially applied. Despite this, inadequate wound progression was noted at 14 weeks after injury with the wound remaining at 1·3 × 3·0 × 0·1 cm and dHAM was applied (Figure 5).
Figure 5.
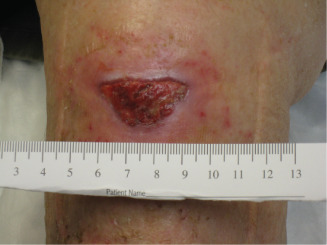
Image showing dHAM application on wound, 14 weeks post‐injury.
In an attempt to optimise the healing rate of this refractory wound, the dHAM was applied more frequently based on clinical examination for any residual product remaining. When healing was noted to stop progressing, additional dHAM was applied. The patient received a total of three dHAM applications at 14, 20 and 25 weeks.
Graphical representation of the rate of healing is shown in Figure 6. Progression of wound healing 25 weeks post‐injury after three applications of dHAM is illustrated in Figure 7.
Figure 6.
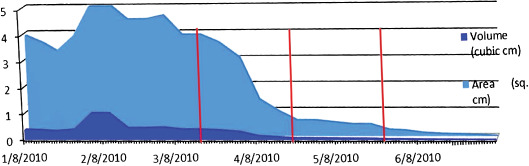
Healing rate in case 2. Amniotic membrane was applied three times at 14 weeks (17 March), 20 weeks (22 April) and 25 weeks (27 May) (red line represents application of dHAM). Area is in square cm, volume is in cubic cm. Both parameters improved until resolution from the plateau seen before dHAM application.
Figure 7.
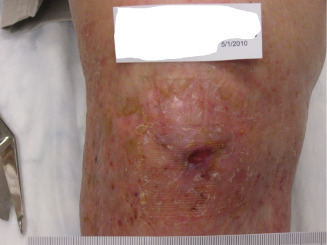
Image showing 25 weeks post‐injury, after three applications of dHAM.
The wound progressively improved and the patient was discharged with full healing at 31 weeks. No report of recurrence was noted upon 1‐ and 6‐month telephone follow‐up.
Case 3
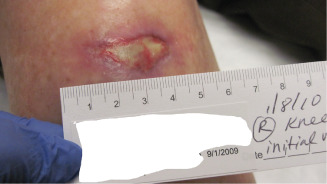
Case 3 is a 65‐year‐old diabetic male smoker who underwent dorsal wrist ganglion cyst excision of the dominant hand by a hand surgeon. Postoperatively, wound dehiscence was treated with wet‐to‐dry dressing changes. At 4 weeks, debridement and collagen‐based wound dressing was applied (Figure 8). Negative pressure dressing was initiated at 5 weeks. Autologous skin was grafted at 9 weeks. This graft was lost at 11 weeks, and at this point, serial applications of porcine xenograft‐based skin substitute were used.
Figure 8.
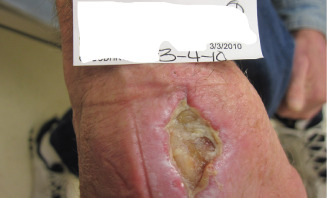
Image showing 4 weeks (4 March 2010) postoperative wound.
However, persistent tendon exposure was noted and discussions held with the patient regarding reconstructive flap procedure. To avoid the risk and complexity of this major surgical intervention and to avoid delay in care for a newly diagnosed urologic malignancy, amniotic membrane application was offered. Amniotic membrane allografts were applied at 15 (Figure 9), 17 (Figure 10) and 20 weeks, based on maintaining grossly visible product on serial examination.
Figure 9.
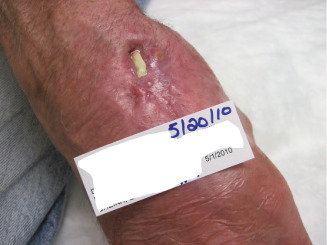
Amniotic membrane application initiated at 15 weeks. Note the exposed tendon present.
Figure 10.
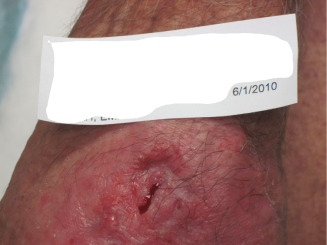
A 17‐week wound (1 June 2010) 2 weeks after application of dHAM.
The patient missed subsequent follow‐up appointments but at the 23rd week, wound closure was confirmed. Healing rate is illustrated in Figure 11. The patient was discharged with 1‐ and 6‐month telephone follow‐up showing no recurrence.
Figure 11.
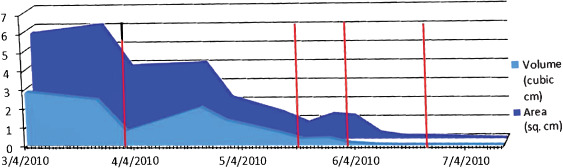
Healing rate graph for wound in case 3 (red line represents application of skin graft on 1 April, and dHAM on 20 May, 2 June and 23 June). Area is in square cm, volume is in cubic cm.
Case 4
Case 4 is a 59‐year‐old female, status post motor vehicle accident with open left ankle fracture treated with open reduction and internal fixation (ORIF) with exposed bone, which was treated with subsequent intravenous antibiotics, hardware removal, NPT, bone stimulator and hyperbaric oxygen treatment. Also, porcine allograft‐based skin substitute application was unsuccessful in achieving wound closure. The condition of wound 4 months after ankle ORIF is shown in Figure 12.
Figure 12.
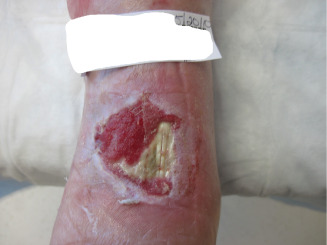
Image showing a wound 4 months after ankle ORIF.
With persistent wound and patient refusal to proceed with microvascular flap reconstruction, amniotic membrane was offered. Because of lack of insurance coverage, only a single application of dHAM was performed at 7 months (Figure 13). With continued follow‐up and wound care by protocol, eventual closure was achieved over the next 2 months (Figure 14). Healing rate is illustrated in Figure 15.
Figure 13.
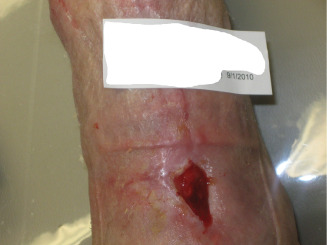
Wound treated with dHAM application as on 1 September 2010.
Figure 14.
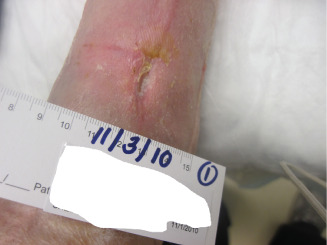
Image showing a wound 8 weeks after dHAM application.
Figure 15.
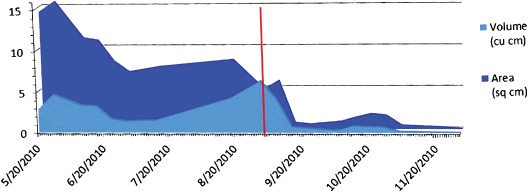
Healing rate graph for wound in case 4 (red line represents application of dHAM on 1 September 2010). Area is in square cm, volume is in cubic cm.
Discussion
The use of human amniotic membrane to facilitate wound healing has been well established since the early 1900s at which time Davis did a review of cases at the Johns Hopkins Hospital in 1910 8, 10. Stern and Sabella reported using foetal membranes to treat burned and ulcerated skin surfaces in the early 1900s 11, 12. Early adopters of this material noted that the human amniotic membrane improved control of pain, infection and healing, properties that were later confirmed in other studies and were believed to be related to the underlying molecular biology of the material. Amniotic membranes in the latter half of the 20th century have been used for a variety of clinical applications, including neurovascular leg ulcers, eye injuries, orthopaedic applications, neurorrhaphy and a variety of other applications 8, 11, 12, 13, 14, 15, 16, 17.
Numerous beneficial properties have been attributed to human amniotic membrane including creation of a natural biological barrier, promotion of increased healing, reduction of inflammation and reduction of scar tissue formation 13. Human amniotic membrane also has antibacterial and pain reduction properties, is self‐signalling, and mediates wound repair via the contained growth factors. The underlying mechanism of action of the material is believed to be resonant in the rich biological construction of the amnion and chorion membranes, which include layers of basement membranes and a variety of intrinsic factors. The basement membrane contains types I, III, IV, V collagen, lamanin‐1, lamanin‐5 and fibronectin, which play an important role in cell proliferation and differentiation 13. It has been reported that amniotic epithelial cells secrete angiogenic factors such as vascular endothelial growth factors, interleukin‐8 (IL‐8), angiogenin, interferon‐γ, IL‐6, basic fibroblastic growth factor, epidermal growth factor and platelet‐derived growth factor 18.
In this paper, we have described the results of dHAM treatment in a group of patients who had not achieved wound closure despite both conservative and advanced measures. The patients were offered dHAM as an option before advancing on the ‘reconstructive ladder’ to a surgical flap or graft procedure. In fact, the third patient had already had an unsuccessful autologous surgical graft surgery. Successful healing was observed in a variety of wounds after the use of the dHAM material (Table 1). These chronic cases were otherwise intractable to several advanced wound therapies, including other skin substitutes, topical agents, hyperbaric oxygen treatment, NPT and multiple debridements. In one case, the dHAM was applied directly to exposed tendon without resultant scar tissue formation, thereby permitting acceptable tendon glide, and resulted in wound closure. In fact, while the dimensions of the wound were improved with prior autologous skin graft, the central portion with exposed tendon was devoid of healing. Therefore, decision was made by the patient to proceed with dHAM application in lieu of fasciocutaneous flap reconstruction.
Table 1.
Summary of cases
| Case no. | Wound location | Time point (weeks) at initial dHAM application | No. of dHAM allografts | Weeks to closure after initial dHAM |
|---|---|---|---|---|
| 1 | Elbow | 8 | 1 | 3 |
| 2 | Knee | 14 | 3 | 17 |
| 3 | Hand | 15 | 3 | 8 |
| 4 | Ankle | 28 | 1 | 8 |
dHAM, dehydrated human amniotic membrane.
Gaps and potential flaws
It remains unclear as to the optimal frequency of dHAM application. In the current cases, physician judgment was used to determine frequency of dHAM application based on wound history and type, comorbid conditions and visual inspections of the wounds. It was noted that each of the patients experienced a precipitous drop in wound size after the initial application of dHAM. Additional dHAM applications were made when plateaus were noted with wound healing in two of the four cases presented. In cases 1 and 4, only one dHAM allograft was used. Wound duration, location of wound, comorbidities and other reasons may all play a role in number and frequency of dHAM applications required. We found it encouraging that none of the patients required more than three applications of the allograft material to obtain healing. All of the patients were followed up over time and as of 30 November 2011, none had any evidence of breakdown at the treated sites or wound recurrences. None have shown evidence of clinical rejection of the allografts.
The International Committee on Wound Management has published guidelines regarding the types of direct and indirect costs that may be considered in economic analyses in wound care. The Panel of Cost‐Effectiveness in Health and Medicine, convened by the US Public Health Service, has standardised methods to estimate these costs 19, 20. The potential cost‐effectiveness of dHAM over a more expensive plastic surgery procedure is one component, although not the deciding factor, in the selection of dHAM for patients. The favourable outcomes in these patients suggest dHAM may be a successful alternative to the more expensive surgical approach. Given the relative effectiveness of the material and both the direct and indirect costs of alternative reconstructive procedures, the choice of dHAM as a wound healing agent presents a significant treatment strategy for these types of patients.
Quicker wound closure, while avoiding complex plastic flap reconstruction with its prolonged recovery and morbidity, may ultimately lead towards better cost‐effective and cost‐contained wound healing. With effective closure of wounds specifically intractable to conventional wound treatments as well as more advanced, approved techniques, an argument can be advanced that the dHAM may be superior for more routine wound healing as well. In this series, patients had chronic wounds from a number of aetiologies that had otherwise reached a higher level on the plastic surgery treatment ladder. The application of the dHAM material resulted in wound closure without the need for this surgery, thus strengthening a lower rung on the reconstructive ladder for these types of patients. The wound dimension graphs of these patients show that the response after dHAM application was graded. With an initial response lasting 2–4weeks, the progress thereafter was noted to be continued but at a slower pace. Consequently, reapplication of dHAM every 2–4 weeks may be considered to optimise the rate of healing until closure.
Conclusion
We have used EpiFix®, a new dHAM‐derived allograft to enhance wound healing in a series of patients who were previously found to be refractory to both conservative measures and the application of more advanced biological tissue substitutes. The dHAM allograft was applied according to manufacturer's specifications to wounds, which were prepared using sharp dissection and were without contraindications to the use of allograft placement. The material was well tolerated by patients. It facilitated complete healing within one to three applications. Wound sites remained healed without recurrence in longer‐term follow‐up. This material warrants further investigation for consideration in broader application to various types of dermal wounds.
References
- 1. Fonder MA, Lazarus GS, Cowan DA, Aronson‐Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressing. J Am Acad Dermatol 2008;58:185–206. [DOI] [PubMed] [Google Scholar]
- 2. Miller H, Delozier J. Cost implications of the pressure ulcer treatment guideline. Columbia: Center for Health Policy Studies, 1994. (Study commissioned by AHRQ). [Google Scholar]
- 3. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28. [DOI] [PubMed] [Google Scholar]
- 4. Grey JE, Harding KG, Enoch S. Venous and arterial leg ulcers. BMJ 2006;332:347–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bergan JJ, Schmid‐Schönbein GW, Coleridge Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Mechanisms of disease: chronic venous disease. N Engl J Med 2006;355:488–98. [DOI] [PubMed] [Google Scholar]
- 6. Boulton AJM, Kirsner RS, Vileikyte L. Neuropathic diabetic foot ulcers. N Engl J Med 2004;351:48–55. [DOI] [PubMed] [Google Scholar]
- 7. Boulton AJ. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care 2008;31:1679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. John T. Human amniotic membrane transplantation: past, present, and future. Ophthal Clin N Am 2003;16:43–65. [DOI] [PubMed] [Google Scholar]
- 9. Mathes S, Nahai F. Reconstructive surgery: principles, anatomy, & technique. St Louis: Quality Medical Publishing, 2006. [Google Scholar]
- 10. Davis JW. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med J 1910;15:307–96. [Google Scholar]
- 11. Stern M. The grafting of preserved amniotic membrane to burned and ulcerated surfaces, substituting skin grafts. JAMA 1913;60:973. [Google Scholar]
- 12. Sabella N. Use of fetal membranes in skin grafting. Med Records NY 1913;83:478–80. [Google Scholar]
- 13. Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater 2008;15:88–99. [DOI] [PubMed] [Google Scholar]
- 14. Bennett JP, Matthews R, Faulk WP. Treatment of chronic ulceration of the legs with human amnion. Lancet 1980;1:1153–6. [DOI] [PubMed] [Google Scholar]
- 15. Baradaran‐Rafii A, Aghayan H, Arjmand B, Javadi M. Amniotic membrane transplantation. Iran J Ophthalmic Res 2007;2:58–75. [Google Scholar]
- 16. Adly OA, Moghazy AM, Abbas AH, Ellabban AM, Ali OS, Mohamed BA. Assessment of amniotic and polyurethane membrane dressings in the treatment of burns. Burns 2010;36:703–10. [DOI] [PubMed] [Google Scholar]
- 17. Tao H, Fan H. Implantation of amniotic membrane to reduce postlaminectomy epidural adhesions. Eur Spine J 2009;18:1202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wolbank S, Hildner F, Redl H, van Griensven M, Gabriel C, Hennerbichler S. Impact of human amniotic membrane preparation on release of angiogenic factors. J Tissue Eng Regen Med 2009;3:651–4. [DOI] [PubMed] [Google Scholar]
- 19. Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost‐effectiveness in health and medicine. JAMA 1996;276:1253–8. [PubMed] [Google Scholar]
- 20. (ICWM) ICoWM . An overview of economic model of cost‐effective wound care. Adv Wound Care 1995;8:46. [Google Scholar]


